Translate this page into:
Marine invertebrates’ proteins: A recent update on functional property
⁎Corresponding authors. manojsaravanaguru@gmail.com (Manoj Saravana Guru Mohanram), bala.m.k@sejong.ac.kr (Balamuralikrishnan Balasubramanian) geneticsmurali@gmail.com (Balamuralikrishnan Balasubramanian)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The marine invertebrates are vast species in the animal kingdom which consists abundant source of novel functional biopolymers like proteins, lipid and polysaccharides that possess numerous biological activities. These biopolymers had been used for multiple application and served as a functional food for health perspective. In recent times, marine organisms were effectively investigated for potential pharmaceuticals and natural drugs. Besides, marine invertebrate proteins including peptides served as a traditional food and effective alternative medicine for infectious disease. This review focuses on antioxidant, anticancer, antimicrobial activities of peptides and protein including collage and gelatin, were critically analysed with global market status of protein and peptides from marine invertebrates. Hence, this would give more insight on functional property of marine invertebrate, and their applications in biomedical and food industrial application.
Keywords
Marine invertebrates
Functional protein
Collagen
Gelatin
Molecular weight
Biological activity
1 Introduction
The marine environment serves as a significant reservoir of biodiversity with the richest source of primary and secondary metabolites. Marine invertebrates are found to be a diverse group widely distributed in the intertidal zone to deep ocean ecosystem. These are classified into different taxonomic groups such as Porifera (sponges), Cnidaria (corals, jellyfish), Annelida (marine worms), molluscs (oysters, squids, mussels, prawns and crayfish), and echinoderms (starfish, sea cucumbers and sea urchin). There is a long history of dietary habits and medicinal practices of using marine invertebrates among the coastal communities (Sudhakar and Nazeer, 2015). Therefore, this raising the aquaculture production in last few decades which had annual growth rate of 5.8% during the period of 2000–2016 (FAO, 2018). The growth of aquaculture contribution about 73.4 million tonnes which are almost 44% of total food fish production and from the marine and brackish environment (mariculture) it contributes 27.6 million tonnes (37.6%). About 60% of mariculture income from the molluscs and crustaceans farms. The economic important species are salmon, seabass, seaweeds, seabream, barramundi and bivalve molluscs (e.g., clams, mussels, oysters, and scallops) (Ahmed and Thompson, 2019). Mariculture production is dominated by algae (46.2%) followed by bivalve 42.9% and marine fishes (3.7%) and crustaceans (1.8%) (FAO, 2018). Further, awareness of functional foods and therapeutic properties on marine natural products have been growing in recent times. According to the latest report, marine aquaculture products reach the global market at $226.2 billion by 2022 (Newswire, 2019a). These type of foods are mainly utilized for pharmacological and medicinal properties and extraction of metabolites such as fatty acids, protein, peptides and other carotenoid derivatives.
Therefore, this review focuses on wide variety of marine invertebrate proteins and peptides aiming for food application in future. In addition, different fraction of proteins and peptides from marine invertebrates with a detailed comparison of molecular weight for upscaling their uses for human wellness were discussed and evaluated with recent literature.
2 Protein fractions and its functional property
2.1 Biological action of protein sequence based on molecular weight
Bioactive peptides are discharged during enzymatic dehydration or solvent extraction; their biological activity might affect the type of protein fractions, hydrolytic compounds, catalyst substrate proportion, temperature and time of response (Xu et al., 2013). These conditions would influence the sub-atomic weight and peptides fractions and, this influence the functional property of proteins. As per recent report jellyfish had highest amount of bioactive peptides due to high protein content, mainly collagen from 40 to 60% of DW. Besides, the peptides PIIVYWK (Pro-Iso-Iso-Val-Tyr-Try-Lys) (1004.57 Da), and FSVVPSPK (Phe-Ser-Val-Val-Pro-Ser-Pro-Lys) (860.09 Da) from mytilus edulis exhibit hepatoprotective activity through upregulation of heme oxygenase-1 (HO-1) on hepatocytes against H2O2-induced hepatic damage (Park et al., 2016). The hydrolysis of ark shell Scapharca subcrenata by pepsin yielded two peptides MCLDSCLL(P1)(Met-Cys-Leu-Asp-Ser-Cys-Leu-Leu) and HPLDSLCL(P2) (His-Pro-Leu-Asp-Ser-Leu-Cys-Leu) with MW of 897.5 Da showed potent free radical scavenging activity to (2,2-diphenyl-1-picrylhydrazyl) DPPH, ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) and (oxygen radical absorbance capacity) ORAC (Jin et al., 2018). However, ark shell protein hydrolysate with less than 1 kDa fractions stimulate the production of bone morphogenetic protein-2 (BMP-2), p-Smad1/5, Runx2, Dlx5, osterix, and mitogen-activated protein kinase (MAPKs) in mouse mesenchymal stem cells (MSC) and also up-regulated alkaline phosphatase (ALP) activity, mineralization, type I collagen and osteocalcin seen in MSC (Hyung et al., 2017). Similarly, Hyung et al. (2018) reported that blue mussel M. edulis protein hydrolysates with less than 1 kDa stimulate the osteoblast differentiation in mouse MSC through enhance the ALP initiation, osteocalcin and type I collagen activity along with calcium deposition. In addition, the peptic hydrolysate of ark shell with the average MW of 235.17–897.52 Da showed inhibition of adipogenesis through down-regulating adipocyte-specific protein expression together with peroxisome proliferator-activated receptor γ, CCAAT/enhancer-binding protein α, with sterol regulatory element-binding protein 1c, but this action down-regulated fatty acid synthase expression and lipoprotein lipase (Hyung et al. 2017). This confirms that based on the amino acid sequences, peptides derived from different marine invertebrates had varied biological property (Fig. 1).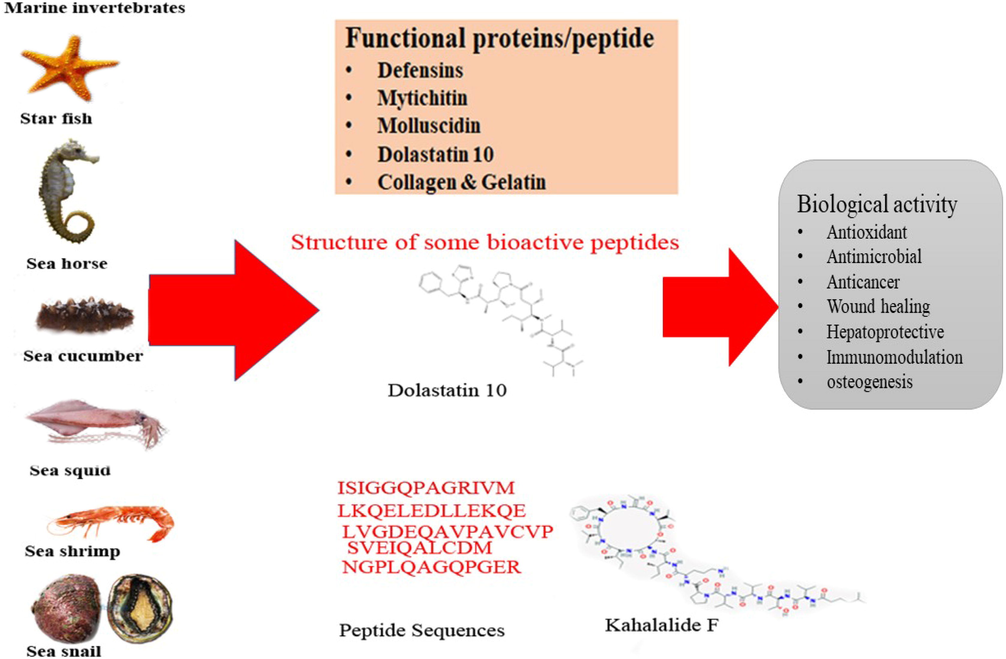
Functional protein and peptides from marine invertebrate.
2.2 Anticancer and antioxidant activity of protein and peptide of marine invertebrates
According to Hu et al. (2012) reported that polypeptide fraction from Arca subcrenata showed antitumor activity in vitro and in vivo HeLa and HT-29 (colon cancer cell line) cell lines (IC50 of 11.43 μg/mL for HeLa (cervical cancer cell line) and 13.00 μg/mL for HT-29, similarly the same species with purified polypeptide (H3) shows MW of 20,491.0 Da exhibited antioxidant action ranges from 56.8% and 47.5% against (DPPH). However, higher MW from protein-enriched fraction of M. edulis (50 kDa) displayed 90%, 89%, 85% and 81% mortality rate against PC3 (prostate cancer cell), A549 (type II pulmonary epithelial cell), HCT15 (colon carcinoma cell) and BT549 (breast carcinoma cell) cell lines, respectively at the concentration of 44 µg/mL (Beaulieu et al., 2013). The polypeptide fraction of A. subcrenata mediates anticancer activity through apoptosis which arrests the G2/M phase via ROS-Mediated MAPKs Pathways (Hu et al., 2015). The M. edulis α-chymotrypsin hydrolysate protect the human umbilical vein endothelial cells (HUVECs) against H2O2-Induced cytotoxicity and increased HUVEC viability up to 85.35% at the concentration of 0.5 mg/mL. The M. edulis α-chymotrypsin hydrolysate increased HUVEC cell viability of toxicity induced by H2O2 was mediated by inhibition of apoptotic pathway via downregulating apoptotic gene p53, caspase-3, and the bax and upregulating bcl-2. Besides, M. edulis α-chymotrypsin hydrolysate increase the intracellular antioxidant status, such as glutathione (GSH) , superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) (Oh et al., 2019).
The peptide obtained from pepsin hydrolysate of Octopus aegina exhibited DPPH scavenging activity of 44.39% at the concentration of 1.5 mg/mL and hydroxyl activity of 38.84% at a concentration of 0.25 mg/mL (Sudhakar and Nazeer, 2017). The different type of peptide was obtained by hydrolysis of M. edulis with eight different types of protease like alcalase, α-chymotrypsin, flavourzyme, neutrase, papain, pepsin, protamex, and trypsin. Amongst α-chymotrypsin mediated hydrolysate had highest ABTS+ radical scavenging (117 μM TE/mg sample), ORAC (199.62 μM TE/mg sample) and DPPH radical scavenging activity (IC50 of 0.35 mg/mL). The report from Yang et al. (2019), purified six antioxidant peptides EPLSD (Glu-Pro-Leu-Ser-Asp), WLDPDG (Trp-Ile-Asp-Pro-Asp-Gly), MDLFTE (Met-Asp-Leu-Phe-Thr-Glu), WPPD (Trp-Pro-Pro-Asp), EPVV (Glu-Pro-Val-Val), and CYIE (Cys-Tyr-Ile-Glu) from marine bivalve mollusk tergillarca granosa. These peptides could be applied as functional food ingredients to enhance the nutraceutical value of regular diet (Fig. 2).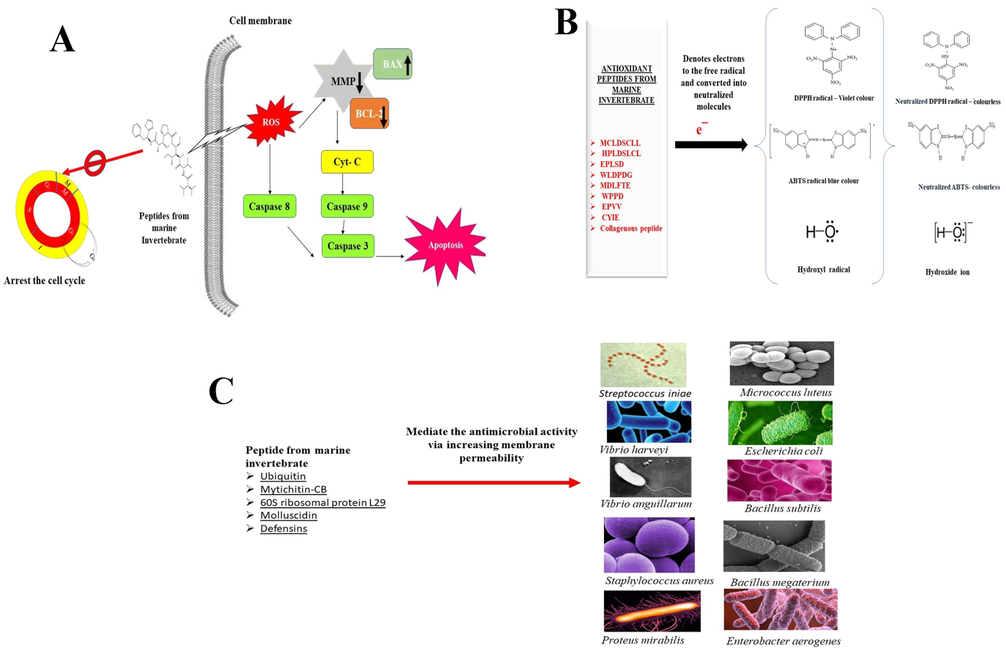
Biological activity of peptides and proteins from marine invertebrate; A – Anticancer activity; B – Antioxidant activity; C – antimicrobial activity.
2.3 Antimicrobial activity of protein sequence from edible marine invertebrates
Seo et al. (2013) reported that polypeptide ubiquitin (74 amino acid residues) with MW of 8.47 kDa exhibited strong antimicrobial activity against gram-negative, positive bacteria including Streptococcus iniae and Vibrio parahaemolyticus with the minimal inhibitory concentrations (MIC) ranging from 7.8 and 9.8 (μg/mL) without hemolytic activity, respectively. The cysteine-rich polypeptide myricetin-CB from M. coruscus showed better antimicrobial activity against gram-positive strains like Bacillus subtilis, Staphylococcus aureus, S. luteus and B. megaterium with MIC of less than 5 μM and showed moderate antifungal active against Candida albicans and Monilia albican (MIC > 5 μM) (Qin et al., 2014). Similarly, pacific oyster Crassostrea gigas protein with MW 6.4 kDa consists of 60S ribosomal protein L29 displayed potent antimicrobial activity (Seo et al., 2017). Molluscidin, a polypeptide from Haliotis discus has MW of 4.76 kDa with 46 amino acids and showed broad and potent antimicrobial spectrum without hemolysis (Seo et al., 2016), besides, molluscidin (5.6 kDa) purified from Atrina pectinata had 59 amino acid residues with repeats of Lys-Lys and Lys-Gly di-basic amino acid reported antimicrobial activity against B. subtilis at MIC of 2.1 μg/mL and Escherichia coli of MIC = 0.5 μg/mL, without hemolytic activity (Hong et al., 2018). Antimicrobial activity of polypeptide defensins from Venerupis phlippinarum and reported that defensins increased the cell membrane permeability of bacteria (Yang et al., 2018), antiviral activity of proline-rich peptides from marine snail rapanavenosa and reported that its isoforms against Epstein-Barr virus (Dolashka et al., 2011; 2014). Further, defensins had broad spectrum of antimicrobial action against S. aureus, Micrococcus luteus, V. anguillarum, Enterobacter cloacae, V. harveyi, Proteus mirabilis, Enterobacter aerogenes, V. parahaemolyticus, V. splendidus and E. coli with the MIC ranging from 0.5 μM to 8 μM. The antimicrobial peptide defensin from V. philippinarum demonstrated potential inhibitory activity against M. luteus and destroyed this bacteria through membrane permeability (Zhang et al., 2015). The antimicrobial property of this peptide from marine invertebrate could act as a nutraceutical molecule with food preserving entity (Fig. 2).
2.4 Biological action of collagen and gelatin
Collagen and properase E hydrolysate from jellyfish had the ability to increase the moisture in skin of UV-induced mice and also restore the endogenous collagen and elastin fibers, through type I to III collagen (Fan et al., 2013). The collagenous peptide extracted from Chrysaora sp. by the action of enzyme trypsin displayed highest DPPH radical scavenging activities (94% at 2 mg/mL) which possess angiotensin-I-converting enzyme (ACE) inhibitory activity of 89% as compared to other protein hydrolysate extracted using enzymes alcalase and Protamex. The high biological activity of collagenous peptide from trypsin hydrolysate may due to high proposition of hydrophobic amino acids with unique amino acid arrangements (Barzideh et al., 2014). Similarly, jellyfish Nemopilema nomurai stimulate the immune system in mouse by activating bone marrow-derived dendritic cells and produces inflammatory cytokines tumor necrosis factor (TNF)-α, Interleukin 6 (IL-6), IL-1β and IL-12. This collagen peptide underwent macrophage J774.1 cells and stimulated cytokine production via activation of the (nuclear factor kappa-light-chain-enhancer of activated B cells) NF-κB and JNK (c-Jun N-terminal kinase) signalling cascades through TLR4 (Toll-like receptor 4) (Putra et al., 2015). The collagen from jellyfish Rhopilema esculentum acts like collagen type I which enhanced the hemostatic process. This mechanism of action was due to improved physical absorption of collagen on the system (Cheng et al., 2017) similar to other marine based biopolymer like carrageenan (Ganesan et al., 2018a, 2018b; Mohan et al., 2018). The collagen peptide fractions from R. esculentum with less than 25 kDa MW presented significant effects on scratch closure at a concentration of 6.25 μg/mL for 48 h and also showed that increased the production of β-fibroblast growth factor (β-FGF) and transforming growth factor-β1 (TGF-β1) expression (Felician et al., 2019). This confirms collagen found to be suitable candidate for wound dressing applications. Table 1 depicts the protein from the source of edible marine invertebrates and its biological action.
Invertebrate type
Phylum
Species
Protein/peptide fraction
Biological activity
Molecular weight (MW)/Extraction method/Techniques used for characterization
Reference
Squid
Mollusca
Loligoduvauceli
Hexapeptide Trp-Cys-Thr-Ser-Val-Ser,
Antioxidant activity (inhibited lipid peroxidation)
MW 682.5 Da/ion exchange chromatography and gel filtration chromatography using fast protein liquid chromatography (FPLC)
Sudhakar et al. (2015)
Squid
Mollusca
Todarodespacificus
Protein hydrolysate
anti-inflammatory tumour necrosis factor (TNF) and antioxidant (DPPH)
high hydrostatic pressure (HHP at 200, 400 and 600 MPa)
Zhang et al. (2016)
Sea snail
Mollusca
Cumia reticulata
Polypeptides, three vWFA1 domains and named vWFA48, vWFA59 and vWFA105
Anti-homeostatic compounds
Molecular docking
Modica et al. (2018)
Snail
Mollusca
Helix lucorum
RvH2-g hemocyanins
Anticancer activity (CAL-29 bladder cancer cell lines)
Gene expression
Antonova et al. (2015)
Sea snail
Mollusca
Rapanavenosa
RvH1 and RvH2 Hemocyanin
Antiviral action against (Epstein-Barr virus) in vitro
Pyridylethylation and Enzymatic Digestions
Dolashka et al. (2014)
–
Mollusca
Mollusc species
Hemocyanin,haliotisin peptides
Antimicrobial (B. subtilis and E. carotovara)
HPLC
Zhuang et al. (2015)
Sea snail
Mollusca
Haliotis rubra
Hemocyanin
antiviral activity (herpes simplex virus type-1)
700–800 kDa/Ultrafiltration
Zanjani et al. (2014)
Sea snail
Mollusca
Haliotis rubra
Hemocyanin
antiviral activity (vero cells vUL37-GFP HSV-1)
Gel filtration chromatography
Talaei Zanjani et al. (2016)
Clam
Mollusca
Arcasubcrenata
Polypeptide
Anticancer activity (HeLa (human cervical cancer cell) and RAW264.7 cells)
ion-exchange chromatography
Wu et al. (2014)
Snail
Mollusca
Helix pomatia
Hemocyanin
Immunomodulation action
anti-TT IgG (tetanus toxoid (TT)
Gesheva et al. (2015)
Oyster
Mollusca
Crassostrea madrasensis
Protein
Antibacterial activity (V. parahaemolyticus, Salmonella sp, Shigella sp, Streptococcus sp and Staphylococcus sp)
Dialysis
Muthezhilan et al. (2014)
Saltwater clam
Mollusca
Venerupisphilippinarum
Defensins (Protein)
Antimicrobial activity (Staphyloccocus aureus and M. luteus) and eight Gram-negative bacteria (V. anguillarum, Entherobacter cloacae, Pseudomonas putida, Proteus mirabilis, Enterobacter aerogenes, V. parahaemolyticus, V. splendidus and V. harveyi)
Recombinant expression
Zhang et al. (2015)
Sea snail
Mollusca
Rapanavenosa
Proline rich pepdite
Antiviral Activity (Staphylococcus aureus) and a Gram-negative (Klebsiella pneumoniae))
3000 and 9500Da/ ultrafiltration and reverse-phase high-performance liquid chromatography (RP-HPLC)
Dolashka et al. (2011)
Mussel
Mollusca
Mytilus coruscus
Pepdite (mytichitin-CB)
Antimicrobial activity (B. subtilis, S. aureus, S. luteus; and B. megaterium)
RP-HPLC
Qin et al. (2014)
Mussel
Mollusca
Mytilus coruscus
Cysteine-rich myticin, mytilin and mytimycin
Antimicrobial activity (Sarcina luteus and Escherichia coli)
11,269.37 Da/HPLC purification
Liao et al. (2013)
Mussel
Mollusca
Mytilus coruscus
Myticusin
Antimicrobial activity (gram-positive, gram-negative bacteria)
6202 Da/C18 reversed-phase high-performance liquid chromatography (HPLC)
Oh et al. (2018)
Sea snail
Mollusca
Haliotis discus
HdMolluscidin
Antimicrobial activity (Bacillus subtilis and Staphylococcus aureus (minimal effective concentrations [MECs]; 0.8–19.0 μg/mL) and Gram-negative bacteria including Aeromonas hydrophila, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Shigella flexneri, and Vibrio parahemolyticus)
4.7 kDa/C18 reversed-phase high-performance liquid chromatography (HPLC)
Seo et al. (2016)
Oyster
Mollusca
Crassostrea gigas
Ubiquitin with terminal Gly–Gly doublet
Antimicrobial activity (Streptococcus iniae and Vibrio parahemolyticus)
8471 Da/C18 reversed-phase HPLC
Seo et al. (2013a)
Oyster
Mollusca
Crassostrea gigas
60S ribosomal protein L29
Antimicrobial activity (B. subtilis and E. coli)
∼6.4-kDa/C18 reversed-phase HPLC
Seo et al. (2017)
Oyster
Mollusca
Crassostrea gigas
cgMolluscidin
Antimicrobial activity (Bacillus subtilis, Micrococcus luteus, and Staphylococcus aureus)
5.5 kDa/C18 reversed-phase HPLC
Seo et al. (2013b)
Pen shell
Mollusca
Atrinapectinata
cgMolluscidin
Antimicrobial activity (Candida albicans, Bacillus subtilis)
5.6 kDa/cation exchange and C18 reversed-phase HPLC
Hong et al. (2018)
Sea horse
Chordata
Hippocampus trimaculatus
Peptide (Oligomeric Aβ42) Gly-Thr-Glu-Asp-Glu-Leu-Asp-Lys
neuroprotective effects against Aβ42-induced neuronal death in PC12 cells
906.4 Da/Enzymatic degradation
Pangestuti et al. (2013)
Clam
Mollusca
Sinonovaculaconstricta
Peptide
antihypertensive activity (ACE-inhibitory activity)
3 kDa/Ion exchange
Li et al. (2016)
Jellyfish
Cnidaria
Rhopilema esculentum
Peptide Ser-Tyr
Antioxidant (DPPH, super oxygen anion scavenging activities) and antihypertensive activity (ACE inhibitory activity)
268.1 Da/ultrafiltration, gel filtration chromatography, and RP-HPLC
Q. Zhang et al. (2018)
2.5 Collagen and gelatin prospective application
The commercially useful compounds from edible marine invertebrates are collagen and gelatin. Collagen is present in all animals in skins and bones around 30% of total content (Silva et al., 2014). Marine organism-based collagen is a promising candidate which replaces mammal-derived collagen and widely used for much biomedical application (Silva et al., 2014; Silvipriya et al., 2015). In food application, collagen is a primary raw material for the production of gelatin and acts as a functional protein for its gelling property. Further, the rheological properties of collagen found to be desirable such as improved elasticity, shear thinning and apparent viscosity which is directly used in food application as a texture enchancer (Ganesan et al., 2019). Gelatin is water soluble protein with MW of 80–250 k Da and it exhibits 88% protein, 10% moisture, and 1–2% salts. In gelatin and collagen, some of the amino acid derivatives are glycine, proline and hyroxyproline which play a role towards thermal stability and improve rheological properties. Moreover, using mammalian tissue causes serious health risk for human i.e hoof and mouth disease, bovine spongiform encephalopathy which is infectious disease spreading via food. Subsequently, marine source is the best alternative to prevent these kinds of disease widespread and also topped with functional properties (Silvipriya et al., 2015). Furthermore, the complex structure of gelatin is poly-ampholyte cross-linking and protein structure constituents biocompatible properties, because of this gelatin does not come under E-number which is classified as a food product (Ofokansi et al., 2010). In bioengineering, gelatin plays a crucial role as encapsulating material or coated gelatin shows efficient loading and drug release properties. There are many advantages of using gelatin in nanoencapsulation. This will improve the product deprivation, free from oxidation, and therefore extend core product shelf-life before its final (Nikkhah et al., 2016; Oh et al., 2019).
3 Commercial value of protein from edible marine invertebrate’s origin
The global market value of functional proteins expected to reach $7.98 billion by 2026 with an annual growth rate of 6.93% from 2019 to 2026 (Newswire, 2019a). Collagen is one of the important macromolecule from marine invertebrates. These molecules have vast application on food, pharmaceuticals and healthcare industry. Therefore, the demand for collagen and its hydrolyzed gelatin were found increased utilization in tissue engineering, bone grafting, drug delivery system, wound healing and cosmetic surgeries. According to recent data, the growth rate of global collagen market increased by 9.4% over the period from 2015 to 2023 and collagen market value expected to attain a value of $9.37 billion by 2023 from a value of $4.13 billion in 2014 which mainly derived from marine sources. Gelatin has global market value of 7.14% annual growth rate between 2019 and 2024 (Newswire, 2019b,c). The commercial market value is expected to reach around $22 billion by 2016 with the annual growth rate of 20.2% over the estimated time frame from 2019 to 2026 (Newswire, 2019a).
4 Conclusion
Thus, marine peptides and proteins have vast biomedical applications like antioxidant, antimicrobial, anticancer, hepatoprotective, bone marrow regeneration and tissue regeneration properties. The market value of these proteins and peptides is increasing every year, and this shows the value in nutraceutical and biomedical industries. In future, these proteins and peptides from marine invertebrates could be a valuable ingredient in the food, feed and pharmaceutical industries.
Acknowledgements
The necessary facilities provided at the Fiji National University, Far Eastern Federal University, Sejong University-South Korea and Periyar University were much appreciated. The authors BB and IHK would like to extend their sincere appreciation to the National Research Foundation of South Korea for support through the Basic Research Project (Grant number: 2018R1C1B5086232) by Ministry of Education, Science, and Technology.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The blue dimensions of aquaculture: a global synthesis. Sci. Total Environ.. 2019;652:851-861.
- [Google Scholar]
- Changes in the gene expression profile of the bladder cancer cell lines after treatment with Helix lucorum and Rapana venosa hemocyanin. J. Buon. 2015;20(1):180-187.
- [Google Scholar]
- ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.) Food Technol. Biotechnol.. 2014;52(4):495-504.
- [Google Scholar]
- Evidence of anti-proliferative activities in blue mussel (Mytilus edulis) by-products. Mar. Drugs. 2013;11(4):975-990.
- [Google Scholar]
- Isolation, Characterization and evaluation of collagen from jellyfish Rhopilema esculentum Kishinouye for use in hemostatic applications. PloS one. 2017;12(1):e0169731
- [Google Scholar]
- Antimicrobial proline-rich peptides from the hemolymph of marine snail Rapana venosa. Peptides. 2011;32(7):1477-1483.
- [Google Scholar]
- Antiviral activity of hemocyanin Rapana venosa and its isoforms against Epstein-Barr virus. Glob. J. Pharmacol.. 2014;8:206-212.
- [Google Scholar]
- Effects of collagen and collagen hydrolysate from jellyfish umbrella on histological and immunity changes of mice photoaging. Nutrients. 2013;5(1):223-233.
- [Google Scholar]
- The State of World Fisheries and Aquaculture 2018‐Meeting the Sustainable Development Goals. Rome, Italy: FAO; 2018.
- The wound healing potential of collagen peptides derived from the jellyfish Rhopilema esculentum. Chin. J. Traumatol.. 2019;22:12-20.
- [Google Scholar]
- Helix pomatia hemocyanin-A novel bio-adjuvant for viral and bacterial antigens. Int. Immunopharmacol.. 2015;26(1):162-168.
- [Google Scholar]
- Development of edible film from Acanthophora spicifera: Structural, rheological and functional properties. Food Biosci.. 2018;23:121-128.
- [Google Scholar]
- Effect of potassium hydroxide on rheological and thermo-mechanical properties of semi-refined carrageenan (SRC) films. Food Biosci.. 2018;26:104-112.
- [Google Scholar]
- Quality enhancement of chicken sausage by semi-refined carrageenan. J. Food Process. Preserv. 2019:e13988.
- [Google Scholar]
- Purification and cDNA cloning of the antimicrobial peptide apMolluscidin from the pen shell, Atrina pectinata. Fish Shellfish Immunol.. 2018;81:408-415.
- [Google Scholar]
- Antitumor effect of a polypeptide fraction from Arca subcrenata in vitro and in vivo. Mar. Drugs. 2012;10(12):2782-2794.
- [Google Scholar]
- Polypeptide fraction from Arca subcrenata induces apoptosis and G2/M phase arrest in HeLa cells via ROS-mediated MAPKs pathways. Evid. Based Complementary Altern. Med.. 2015;2015
- [Google Scholar]
- Ark shell protein hydrolysates inhibit adipogenesis in mouse mesenchymal stem cells through the down-regulation of transcriptional factors. RSC Adv.. 2017;7(11):6223-6228.
- [Google Scholar]
- Blue mussel (Mytilus edulis) protein hydrolysate promotes mouse mesenchymal stem cell differentiation into osteoblasts through up-regulation of bone morphogenetic protein. Food Chem.. 2018;242:156-161.
- [Google Scholar]
- Purification and characterization of antioxidant peptides from enzymatically hydrolyzed ark shell (Scapharca subcrenata) Process Biochem.. 2018;72:170-176.
- [Google Scholar]
- Identification of angiotensin I-converting enzyme inhibitory peptides derived from enzymatic hydrolysates of razor clam Sinonovacula constricta. Mar. Drugs. 2016;14(6):110.
- [Google Scholar]
- Molecular characterization of a novel antimicrobial peptide from Mytilus coruscus. Fish Shellfish Immunol.. 2013;34(2):610-616.
- [Google Scholar]
- Anti-haemostatic compounds from the vampire snail Cumia reticulata: molecular cloning and in-silico structure-function analysis. Comput. Biol. Chem.. 2018;75:168-177.
- [Google Scholar]
- Purification of protein from marine edible oyster Crassostrea madrasensis for bactericidal potency. Biosci. Biotechnol. Res. Asia. 2014;11(1):25-29.
- [Google Scholar]
- Application of marine-derived polysaccharides as immunostimulants in aquaculture: a review of current knowledge and further perspectives. Fish Shellfish Immunol.. 2018;86:1177-1193.
- [Google Scholar]
- NEWSWIRE, G. (2019a). Global Functional Proteins Market is Expected to Reach USD 7.98 Billion by 2026: Fior Markets.
- NEWSWIRE, G. (2019b). Collagen Market to Expand at a CAGR of 9.4% by 2023; Marine Collagen to Gain Maximum Prominence, Says TMR.
- NEWSWIRE, G. (2019c). Food Gelatin: Insights Into & Future of the Market (2019-2024).
- Nikkhah, M., Akbari, M., Paul, A., Memic, A., et al., 2016. Gelatin‐Based Biomaterials for Tissue Engineering and Stem Cell Bioengineering. Biomaterials from Nature for Advanced Devices and Therapies, 1st Ed. 37–62.
- Matrix-loaded biodegradable gelatin nanoparticles as new approach to improve drug loading and delivery. Eur. J. Pharm. Biopharm.. 2010;76(1):1-9.
- [Google Scholar]
- Purification and characterization of an antimicrobial peptide mytichitin-chitin binding domain from the hard-shelled mussel, Mytilus coruscus. Fish Shellfish Immunol.. 2018;83:425-435.
- [Google Scholar]
- Amino acid composition, antioxidant, and cytoprotective effect of blue mussel (Mytilus edulis) hydrolysate through the inhibition of caspase-3 activation in oxidative stress-mediated endothelial cell injury. Mar. Drugs. 2019;17(2):135.
- [Google Scholar]
- Optimization of hydrolysis conditions, isolation, and identification of neuroprotective peptides derived from seahorse Hippocampus trimaculatus. Amino Acids. 2013;45(2):369-381.
- [Google Scholar]
- Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J. Funct. Foods. 2016;20:88-95.
- [Google Scholar]
- Jellyfish collagen stimulates maturation of mouse bone marrow-derived dendritic cells. J. Funct. Foods. 2015;14:308-317.
- [Google Scholar]
- Characterization of a novel antimicrobial peptide with chiting-biding domain from Mytilus coruscus. Fish Shellfish Immunol.. 2014;41(2):362-370.
- [Google Scholar]
- Purification and antimicrobial function of ubiquitin isolated from the gill of Pacific oyster, Crassostrea gigas. Mol. Immunol.. 2013;53(1):88-98.
- [Google Scholar]
- Antimicrobial peptide, hdMolluscidin, purified from the gill of the abalone, Haliotis discus. Fish Shellfish Immunol.. 2016;52:289-297.
- [Google Scholar]
- Antimicrobial effect of the 60S ribosomal protein L29 (cgRPL29), purified from the gill of pacific oyster, Crassostrea gigas. Fish Shellfish Immunol.. 2017;67:675-683.
- [Google Scholar]
- Marine origin collagens and its potential applications. Mar. Drugs. 2014;12(12):5881-5901.
- [Google Scholar]
- Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci.. 2015;5(3):123-127.
- [Google Scholar]
- Structural characterization of an Indian squid antioxidant peptide and its protective effect against cellular reactive oxygen species. J. Funct. Foods. 2015;14:502-512.
- [Google Scholar]
- In vitro preparation and assessment of radical reducing peptide from Octopus aegina using digestive proteases. J. Biosci. Bioeng.. 2017;124(1):36-42.
- [Google Scholar]
- Abalone hemocyanin blocks the entry of herpes simplex virus 1 into cells: a potential new antiviral strategy. Antimicrob. Agents Chemother.. 2016;60(2):1003-1012.
- [Google Scholar]
- The inhibitory effect of a novel polypeptide fraction from Arca subcrenata on cancer-related inflammation in human cervical cancer HeLa cells. Sci. World J.. 2014;9(2014):768938
- [Google Scholar]
- A new in vitro anti-tumor polypeptide isolated from Arca inflata. Mar. Drugs. 2013;11(12):4773-4787.
- [Google Scholar]
- A defensin-like antimicrobial peptide from the manila clam Ruditapes philippinarum: investigation of the antibacterial activities and mode of action. Fish Shellfish Immunol.. 2018;80:274-280.
- [Google Scholar]
- Purification and characterization of antioxidant peptides derived from protein hydrolysate of the marine bivalve mollusk Tergillarca granosa. Mar. Drugs. 2019;17(5):251.
- [Google Scholar]
- Formulation of abalone hemocyanin with high antiviral activity and stability. Eur. J. Pharm. Sci.. 2014;53:77-85.
- [Google Scholar]
- A defensin from clam Venerupis philippinarum: Molecular characterization, localization, antibacterial activity, and mechanism of action. Dev. Comp. Immunol.. 2015;51(1):29-38.
- [Google Scholar]
- Separation and characterization of antioxidative and angiotensin converting enzyme inhibitory peptide from jellyfish gonad hydrolysate. Molecules. 2018;23(1):94.
- [Google Scholar]
- In vitro anti-inflammatory and antioxidant activities and protein quality of high hydrostatic pressure treated squids (Todarodes pacificus) Food Chem.. 2016;203:258-266.
- [Google Scholar]
- Identification of candidate antimicrobial peptides derived from abalone hemocyanin. Dev. Comp. Immunol.. 2015;49(1):96-102.
- [Google Scholar]







