Translate this page into:
Magnesium correlation with boys’ height and other anthropometric measurements
⁎Address: College of Science and Health Professions, King Saud bin Abdulaziz University for Health Sciences, Jeddah, Saudi Arabia. Sufyania@ksau-hs.edu.sa (Amal A AlSufyani)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The primary objective of this investigation is to explore the relationship between serum magnesium (Mg) concentrations and the height and various anthropometric measurements of boys aged 9–13. Additionally, the correlations were examined between magnesium levels and other key macrominerals, namely Chloride (Cl), calcium (Ca), sodium (Na), potassium (K), and phosphorus (P). A group of 45 children free from chronic and systematic diseases and who do not take any medication or supplements was selected. The height and weight were measured, and blood samples were collected for serum analysis of Cl, Mg, P, Ca, Na, and K. Spearman’s rho and Pearson’s correlation (r) analysis were used to study the correlations between these six minerals and anthropometric analysis controlling for age. They were also used to study the correlations between Mg and the other five minerals. The Mg concentration mean for the children in this study was 0.8510 mmol/L with variables ranging between 0.71 and 0.95 mmol/L, which falls between the normal ranges for Mg concentration for the study’s gender and age group. However, Mg/Ca ratio mean SD is 0.3587 0.0244, which is considered low, with 40 % of the sample’s ratio below 0.36. Mg concentration was negatively correlated (R = −0.321) with the children’s height. This correlation was statistically significant p = 0.032 < 0.05. Mg concentration was insignificantly correlated (P > 0.05) with the concentration of Na (0.146), percent of body fat (0.169), muscle mass (−0.211), and basal metabolic rate (−0.212). This study is the first to unveil the negative correlation between serum magnesium concentrations and the height of children. Additionally, it explores the potential links between magnesium levels and weight, muscle mass, basal metabolic rate, visceral fat levels, and percent body fat. These intriguing correlations underscore the need for further research to investigate the safety and efficacy of magnesium supplementation in the prevention of childhood obesity without compromising normal growth patterns.
Keywords
Magnesium
Height
Anthropometry
Macrominerals
Weight
1 Introduction
Minerals are essential for the human body to function regularly. Approximately twenty minerals are required by the body’s biochemical process for good health and proper physiological regulation. Regardless of the minerals’ significance or physiological role, they are classified into macrominerals and microminerals, respectively, based on whether the required daily intake is higher or lower than 100 mg/day (Farag et al., 2023). In the human body, macrominerals include Calcium (Ca), Magnesium (Mg), Sodium (Na), Potassium (K), Sulfur (S), Phosphorus (P), and Chloride (Cl). Mg is the earth's eighth most abundant mineral, the fourth most prevalent essential mineral in the body, and the second most common divalent cation intracellularly (Gröber et al., 2015; Volpe, 2013).
The high availability of magnesium does not prevent its insufficient consumption. This is due to food processing methods that remove magnesium from the food during preparation. Additionally, food choices have changed to favor fast and processed foods instead of natural foods with high magnesium levels, such as whole grains, green leafy vegetables, legumes, seeds, nuts, bananas, almonds, and broccoli (Razzaque, 2018). The daily recommended intake (RDI) for magnesium ranges from 130 mg/day for children 4–8 years old to 240 mg/day for children 9–13 years old (Gröber et al., 2015).
Magnesium’s functions in the body range from being critical in DNA replication, stability, repair, and RNA transcription, to playing roles in the cardiovascular system, bone proliferation, and brain. Magnesium is an enzymatic cofactor for about two hundred enzymes and an extra two hundred activators, therefore affecting many biochemical processes (Glasdam et al., 2016; Fiorentini et al., 2021). With this wide range of functions of magnesium, its deficiency is linked to a variety of severe conditions such as mental disorders, atherosclerosis, blood lipids, sugar alteration, type 2 diabetes, myocardial infarction, hypertension, kidney stones, premenstrual syndrome, electrolyte abnormalities, and renal disease (Gröber et al., 2015). The bone is the storing site for over 50 % of the body’s Mg, where one-third of it is used to maintain extracellular Mg levels. Over 40 % is distributed between muscles and other tissues. Only two percent is found in erythrocytes and serum. About 55 % of serum magnesium is found as free ionic Mg2+ (Glasdam et al., 2016).
Due to the high importance of magnesium for many functions of the body, the body's systems works to maintain its homeostasis so that it is readily available even when the rate of food consumption decreases. For example, in response to the low intake of Mg, the rate of its absorption from food increases, and the rate of excretion with urine declines (Nielsen, 2010). The effect of Mg concentration on Mg absorbance is the main factor in Mg homeostasis, and no hormone controls the serum level of Mg (Ghosh and Joshi, 2008). Razzaque (2018) reminds us that the ordinary serum level of magnesium does not necessarily signify the absence of moderate to severe deficiency in magnesium since it is drawn from the cells to compensate for its insufficient levels in the blood. Therefore, magnesium levels appear within the normal ranges in blood analyses even when an individual is magnesium deficient (Razzaque, 2018). It is suggested to use Mg/Ca ratio as a better indicator of Mg level, where a Mg/Ca ratio of 0.4 is considered optimal while a ratio value below 0.36 is too low (Rosanoff and Wolf, 2016).
Despite its abundance, the lack of magnesium consumption is prevalent. In 2021, Kutbi reported on 424 Saudi children between the ages of 6 and 12 years old, and found that most of the studied children did not consume a sufficient amount of Mg (Kutbi, 2021). This low essential nutrient consumption was also reported by Allam et al. (2012) who found out that medical students were consuming only 24 % of the RDA (Allam et al., 2012). Several reports indicated low magnesium intake in many areas of the world including the US (Nielsen, 2010), China (Huang et al., 2022), and several European countries (Mensink et al., 2013).
Children in the prepubertal period require a sufficient amount of all nutrients necessary for proper development. An important aspect of children’s growth is bone elongation and remodeling which is orchestrated by many hormones, enzymes, and nutrients. Ibrahim et al. (2002) found that stunted Egyptian children have significantly lower magnesium levels when compared to healthy controls (Ibrahim et al., 2002). Magnesium plays a role in the function of many of these hormones, enzymes, and nutrients. Mg aids in bone crystals' size and formation by increasing the solubility of minerals like Pi and Ca2+, which compose the hydroxyapatite crystals (Fiorentini et al., 2021). Bone develops from osteoblasts, whereas cartilage grows from chondrocytes (Aghajanian and Mohan, 2018) Children's increased height results from their bones growing via endochondral ossification (Satoh and Hasegawa, 2022). Endochondral ossification is the process that starts with cartilage formation and continues to cartilage replacement by bone (Boyce et al., 2009). Bone remodeling is a process that may happen under normal and pathological conditions via osteoclasts (Boyce et al., 2009). Osteoclasts' secretions make microscopic trenches on the bones, which recruit osteoblasts to put and mineralize a new matrix (Boyle et al., 2016). Hormones, vitamins, and minerals affect these processes.
Parathyroid hormone (PTH), which regulates calcium absorption and conservation, is affected by magnesium levels. Slightly low levels of Mg induce PTH, while an extreme drop in Mg concentration inhibits PTH secretion. At low calcium concentrations, Mg inhibits PTH secretion (Fiorentini et al., 2021). Thus, Mg influences PTH regulation of Ca absorbtion which affects bones.
Magnesium salts enhance osteogenesis in two ways. First, it induces the Notch signaling pathway, which is exceedingly involved in bone formation. Second, Mg stimulates mesenchymal stem cell (MSC) proliferation and differentiation into osteoblasts (Díaz-Tocados et al., 2017).
Extracellular Ca enhances osteoblasts and chondrocytes (Aghajanian and Mohan, 2018), where a high extracellular level of Mg promotes the differentiation of osteoclasts and inhibits osteoblast activity and osteogenesis (Mammoli et al., 2019). Limited data are available on osteoclasts' and osteoblasts' responses to high levels of Mg, and none are in vitro. Mammoli et al. (2019) hypothesized that increased extracellular Mg causes an imbalance between osteoblasts and osteoclasts (Mammoli et al., 2019).
Investigators have studied children’s growth spurts, including height, weight, and body composition in relation to age and puberty. The relationships between children’s height and serum magnesium concentration in relation to other macroelement concentrations have not been examined in healthy children. This study aims to investigate the correlation between the level of serum Mg and height and nutritional status defined according to BMI in healthy children aged 9–13 living in Jeddah, Saudi Arabia.
2 Materials and methods
2.1 Study design
This is an observational cross-sectional study. The included volunteer children were boys living in Jeddah, Saudi Arabia. For concision and better differentiation between late childhood and late pubertal and post-puberal stages, this study selected prepubertal individuals (Rico et al., 1993) from boys’ Tanner’s stages 1 and 2 (aged 9–13 years) (Emmanuel and Bokor, 2022), which are the transitional stage between childhood, and puberty (Tanner’s stages 3 and 4).
The study participants were devoid of any systemic or local diseases. Children who were severely ill and participants who had a history of current or recent (within the last month) antibiotic use were excluded.
2.2 Anthropometry
A shoeless standing height was measured. Basal metabolic rate, and all body composition measurements including weight, body mass index, percent body fat, and muscle masswere conducted using a bioelectrical impedance analysis (InBody 120).
2.3 Biochemical measurements
A certified technician collected blood from the children's veins after 8 h of fasting and left it to coagulate. After the coagulation, samples were centrifugated for seven minutes, at 6000 rpm (4,427 g) to separate the serum. The serum was taken to clinical biochemistry laboratory at King Abdulaziz Medical City, King Khalid Hospital for National Guard, Jeddah, Saudi Arabia, that is associated with KSAU-HS for the analysis of the elements. The serum concentrations of Chloride (Cl), Magnesium (Mg), Phosphate (P), Calcium (Ca), Sodium (Na), and Potassium (K) was measured using an automated clinical chemistry analyzer, Architect c8000 (Abbott, Abbott Park, IL, USA). Determination of the concentrations was conducted using reagents for each mineral from Abbott Company according to instructions for the kit insert to ensure the reliability of the results.
The following protocols were used for minerals detection: Abbott ion selective electrode for chloride, potassium and Sodium, Abbott ARCHITECT enzymatic assay for magnesium (3P68), Abbott phosphomolybdate (7D71) for phosphate, and Abbott Architect Arsenazo III for calcium (3L79).
Prior to running the test on the ARCHITECT, the presence of adequate sample volume was ensured, and all samples were initially tested using the ARCHITECT STANDARD Dilution Protocol. If a sample result was greater than the upper value of the analytical measurement range (AMR), this sample was retested using the dilution protocol. If the result obtained was within the AMR, the sample was run as neat. Studies were conducted on the ARCHITECTc System based on guidance, using the ARCHITECT Reagents. Quality control samples were run daily, and they were within acceptable limits. The Architect minerals assays were investigated for within and between run precision (percentage of coefficient of variation (%CV)). The precision, linearity, maximum observed Limit of Blank (LoB), Limit of Detection (LoD), and Limit of Quantitation (LoQ) studies were verified based on the reagents insert kit protocol and the guidance for clinical laboratory standards.
2.4 Statistical analysis
All measurements including minerals and Anthropometric data were collected using an excel entry data sheet. The results were statistically analyzed using the two softwares SPSS v 25 and rstudio v 4.2.1. The mean value, standard deviation (SD) and minimum and maximum values range were computed for the anthropometric measurements and the Cl, Mg, P, Ca, Na, and K levels. The Shapiro-Wilk test was used to test the distribution of the data. The Spearman’s rho and Pearson’s correlation (r) analyses were done to study the strength of correlation that trace elements demonstrate with anthropometric parameters controlling for age as a cofounder. Pearson stipulates that both variables follow a normal distribution, otherwise, Spearman’s was used. The statistical correlation was considered significant when p ≤ 0.05.
3 Results
The research population was comprised of 45 boys aged 9–13. The mean height was 143 cm with a minimum value of 129 cm and a maximum value of 155 cm. Table 1 illustrates the summary of the statistics for a general description of the subjects including anthropometric measurements. The serum Mg concentration mean was 0.8510 mmol/L with variables ranging between 0.71 and 0.95 mmol/L, which falls between the normal ranges for Mg concentration for the study’s gender and age group. However, Mg/Ca ratio mean
SD was 0.3587
0.0244, which is considered low, with 40 % of the sample’s ratio below 0.36.
Range
Mean
SD
Variable
9–13
11.19
0.917
Age
129–155
142
6.652
Height (cm)
24.1–61.5
35.7262
9.3251
Weight (kg)
9.4–20.1
13.7476
2.3052
Muscle Mass (kg)
0.03–0.506
0.2279
0.1104
Percent Body Fat (%)
12.7–31.8
17.65
4.2288
Body Mass Index (kg/m2)
793–1179
947
82.559
Basal Metabolic Rate (Kcal/24 h)
1–10
3.24
2.314
Visceral Fat Level
0.71–0.95
0.8510
0.0495
MAG (in mmol/L)
0.30–0.42
0.3587
0.0244
Mg/Ca (in mmol/L)
2.37–3.3
2.8
0.1869
Ca/Mg (in mmol/L)
Serum magnesium concentration was negatively correlated with the children’s height controlling for age. The negative correlation between Mg and height was weak (R = -0.321) but significant (p = 0.032 < 0.05). Fig. 1 shows the distribution of Mg and height data in a scatter plot to visualize the trend line and the inverse relationship between height and Mg.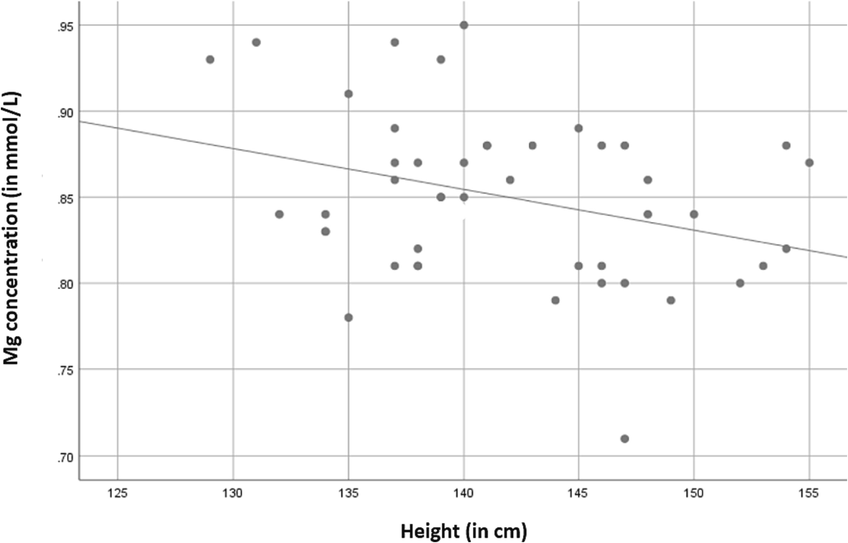
The negative correlation between height and serum Mg concentration in the study population.
As seen from the data in Table 2, there were no significant correlations between height and serum concentration of Cl, Ca, Na, K, and P controlling for age. Table 3 shows the relationship results between Mg and other tested trace elements. There is no significant correlation between the trace elements serum concentration and Mg serum concentration in the study group. S, significant (P < 0.05). NS, not significant (P > 0.05). NS, not significant (P > 0.05).
Correlation
Variable
Correlation Coefficient
r
Significant (2-tailed)
p
Spearman's rho
K
−0.060
0.701 NS
Cl
−0.055
0.721 NS
Pearson’s correlation
Mg
−0.308
0.042 S
Ca
−0.029
0.853NS
Na
0.052
0.738 NS
P
−0.047
0.762NS
Ca/Mg
0.241
0.111NS
Mg/Ca
−0.249
0.099NS
Correlation
Variable
Correlation Coefficient
Significant
Pearson’s correlation
R
p (2-tailed)
Ca
−0.067
0.662NS
Na
0.146
0.338NS
P
−0.014
0.926NS
Spearman's rho
rs
p (2-tailed)
K
0.087
0.571NS
Cl
0.034
0.825NS
There was no significant correlation between the child’s age, weight, BMI, muscle mass, basal metabolic rate, percent body fat, nor visceral fat level and Mg serum concentration in the study population (p > 0.05, see Table 4). S, significant (P < 0.05). NS, not significant (P > 0.05).
Correlation Coefficient r
Significant (2-tailed)
Pearson’s correlation
Height
−0.308
0.042 S
Muscle Mass
−0.211
0.164 NS
Basal Metabolic Rate
−0.212
0.163 NS
Spearman's rho
Age
−0.132
0.406
Weight
−0.044
0.776
Percent Body Fat
0.169
0.266
BMI
0.093
0.544NS
Visceral Fat Level
0.070
0.648NS
4 Discussion
Previously, the role of macrominerals in the height and growth of children has had more focus on Zn and Ca, and less attention has been given to Mg, the forgotten electrolyte (Castiglioni et al., 2013; Ahmed and Mohammed, 2019). This research is the first to explore correlations between serum macrominerals concentrations and to investigate whether Mg is related to height and body composition in boys between the ages of 9–13 years old living in Saudi Arabia and free from other conditions. The selected subjects' serum concentrations of the measured electrolytes Mg, Ca, Cl, K, Na, and P are normal. However, the mean value of the Mg/Ca ratio is low, and individual values showed several children with less than the optimal ratio of 0.36 (Rosanoff and Wolf, 2016). The results of the study (Table 2) show a significant (P = 0.032) inverse relationship between the serum concentration of Mg in children and their height. Fig. 1 represents the scatter diagram of serum magnesium levels with body height in the study sample (r = -0.321, p < 0.032). The weak correlation indicated by the value of r = -0.321 suggests a collective effect of Mg with other trace elements that allow this relationship between Mg and height. When considering the effect of age, it was determined as a confounding factor in the correlation between height and minerals. Furthermore, the correlation between age and Mg concentration was calculated, and no significant correlation was found. Thus, the inverse relationship between Mg and height is more likely to be true regardless of any developmental factor associated with age.
This negative relationship between body height and Mg concentration is in alignment with Rębacz-Maron (2016) results who reported a significantly strong negative correlation between hair Mg concentration versus body height (r = –0.63) and weight (r = –0.66) in the underweight secondary school students aged 16.60 ± 1.92 in Tanzania (Rębacz-Maron, 2016). While the children in this study and Rębacz-Maron (2016) study differ in age and ethnicity, the similarity in the nature of correlation in Mg and height gives a supporting reason to trust that this correlation is worth further investigation. These results show that Mg is related in an inverse fashion to the child’s height, which suggests an accelerated endochondral ossification. The mechanism by which Mg influences the height of the child or is influenced by it is yet to be investigated. Several hypotheses to be considered include: 1) the antagonism between Ca and Mg so that high Mg inhibits hydroxyapatite crystal formation (Navarro-González et al., 2009), 2) Mg impairs parathyroid function (Rodríguez-Ortiz et al., 2014), and 3) high Mg inhibits osteoblast activity (Leidi et al., 2011) as well as bone mesenchymal stem cell osteogenesis (Mammoli et al., 2019).
Interpreting the data using Mg concentration alone suggests that children’s height between the ages of 9–13 and Mg absorbance influence each other; taller children may absorb Mg more, resulting in lower serum concentrations, or that increased Mg serum levels result in shorter children. It also suggests that with children’s development, the need for Mg increases due to growing organs (like bones), which is supported by the low Mg/Ca ratio values obtained.
The limited research on Mg and children’s height suggests that this relationship is observed as the child grows during elementary school age. Other studies showed no relationship between height and Mg concentration in children aged 0–6 years old. Yin et al. (2017) measured serum concentrations of Mg in children 6–36 months and found no correlation with their height (Yin et al., 2017). Cao et al. (2016) conducted a study on 2141 children from 0 to 6 years old and found no correlation between Mg concentration and the height of the child (Cao, et al., 2016).
Other than Mg, none of the remaining measured electrolytes showed a significant correlation with height in this study population. The absence of a significant correlation between Ca and height is consistent with the findings of other studies on children’s growth and Ca concentrations (Suárez-Ortegón et al., 2011; Ye et al., 2015; Cao et al., 2016; Yin et al., 2017). Suárez-Ortegón et al. (2011) did not find a significant correlation between Ca and height in children of ages 2–5 years old (Suárez-Ortegón et al., 2011). Cao et al. did not find an effect of Ca on height in children younger than 6 years old (Cao et al., 2016). Other study found no effect of additional Ca intake on height (Lee et al., 1995). However, supplementation with Ca was positively associated with the mineral content of bones (Lee et al., 1995).
This study did not find a significant relationship between children’s weight and magnesium levels, as evidenced by correlation coefficient values of −0.044 and 0.093 for weight and BMI, respectively. These values are very close to 0, which indicates no relation. In the P values of 0.776 for weight and 0.544 for BMI, the absence of correlation is confirmed. The basal metabolic rate, and remaining composition elements of body muscle mass, percent body fat, and visceral fat level, were weakly and not significantly correlated to Mg concentrations. The absence of a correlation between Mg concentration and body composition suggests that Mg does not affect the body weight of children by influencing fat and muscle percentage.
While this study found an insignificant negative correlation between children’s weight and Mg concentrations, a study done by Lu et al. (2020) found a significant inverse relationship between Mg and body weight in obese adults (Lu et al., 2020). The absence of significance in this study could be due to variance in sample size.
Hassan et al. (2017) reported a decrease in the mean serum Mg level in obese kids versus kids of normal BMI aged 2–14 years in Pakistan, which is consistent with this research’s finding of a negative correlation between Mg and weight (Hassan et al., 2017). The sample size may account for the degree of correlation and absence of significance in the current study. Both studies (Lu et al., 2020; Hassan et al., 2017), supported by this study’s finding of inverse correlation, indicate the relationship between Mg and body weight and compositions. The decrease in Mg levels in obese individuals may be due to the significant role of Mg in many metabolic pathways. It also suggests a hypothetical role of increasing Mg intake in obesity prevention. However, we support their call for more studies to evaluate the effect of Mg supplementation on weight control in kids, especially when considering this study’s findings of potential adverse effects of increased Mg concentration on bone elongation. While Mg supplementation may aid in weight control, it may also negatively affect children’s growth in other areas such as their height. Furthermore, mineral deficiency in children with developmental abnormalities like stunting (Ibrahim et al., 2002) results in the trend of micronutrient supplementation in children. The parental trend is to over supplement a nutrient to a child once its deficiency is a associated with developmental retardation. Thus, parents should be educated on the important effect of precisely obeying RDA, particularly for parents to avoid under and over providing.
The correlation between the five macrominerals of interest in this study was investigated in studies on the serum of cows that reported a significant negative correlation between K and Ca (Fadlalla et al., 2020) and a significant positive correlation between Mg and P (Mordak et al., 2021). Girardin and Paunier (1985) found a highly significant correlation between human erythrocytes concentrations of Mg and K (Girardin and Paunier, 1985). Girardin and Pauniers' results agree with Alaly et al. who reported a moderate correlation between Mg and K (Alaly et al., 2012). Thus, more studies on correlations between these minerals in the serum of healthy humans validate these findings. In the current study, Mg is correlated positively with the three electrolytes Na, K, and Cl but negatively with P and Ca. However, none of these correlations are statistically significant. Electrolytes interact with other proteins and molecules in the body. These metals may bind the same protein, use the same channel in an antiport or symport fashion, have metabolic and absorbance antagonism, and may positively or negatively affect each other’s concentrations. However, the detailed mechanism by which these electrolytes correlate to each other requires further investigation.
The small sample size is a limitation in this study. Additionally, it would be advisable to measure markers of endochondral ossification and levels of osteocalcin to be assessed. Despite these limitations, this study successfully exposed the significant correlation between Mg and height, which requires more attention. The significance of the correlation between the height and Mg concentration, despite the small size, is a good indicator of the existence of a relationship that requires further research.
5 Conclusions
This study shows that Mg serum concentrations have a significant negative correlation with children’s height in boys with normal Mg levels. This correlation suggests the role of Mg intake on height in children and gives a potential hypothesis that Mg may influence height through one of three mechanisms: 1) inhibiting hydroxyapatite crystal formation due to antagonism between Ca and Mg, 2) impairing parathyroid function, and 3) inhibiting osteoblast activity and osteogenesis. This role calls for more research investigating the safety and effect of Mg supplementation to maintain healthy levels of Mg to prevent children’s obesity without impairing their height. Moreover, studies on larger populations, cross-genders, and ethnic groups are needed to elucidate the correlations and understand more mechanisms behind these correlations.
6 Author Contributions
Single-author manuscript.
7 Funding
This research received no external funding.
8 Institutional Review Board Statement
The volunteers in this study were invited based on an approved ongoing research number NRJ22J/266/10 - Serum Biochemical Parameters Correlation with Body Weight and Composition, Food Consumption, and Medical Status; by Institutional Review Board (IRB) for the Clinical Research Committee of King Abdullah International Medical Research Center (KMARC), Jeddah, Saudi Arabia (IRB/0247/23).
9 Informed Consent Statement
Informed consent was obtained from all parents of the subjects involved in the study.
Acknowledgments
The author wishes to extend sincere gratitude to Ms. Massa M. Bitar, the Director of Nutrition & Food Experience at the International Medical Center, for graciously providing the InBody instrument. Additionally, gratitude is expressed to Dr. Jeanette Maier for her generous cooperation during the discussion of the results, and Ruth Weiss for her careful editing of the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The art of building bone: emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res.. 2018;6(1):1-9.
- [Google Scholar]
- Ahmed, F., Mohammed, A., 2019. Magnesium: The Forgotten Electrolyte—A Review on Hypomagnesemia. Med. Sci. 7(4), 56. Retrieved from /pmc/articles/PMC6524065/.
- The Relation of Serum Sodium, Potassium and Magnesium with Rheumatoid Arthritis Symptoms. Med. J. Babylon. 2012;9(4)
- [Google Scholar]
- Allam, A., Taha, I., Al-Nozha, O., Sultan, I., 2012. Nutritional and health status of medical students at a university in Northwestern Saudi Arabia. Retrieved from www.smj.org.sa.
- Osteoclasts have Multiple Roles in Bone in Addition to Bone Resorption. Crit. Rev. Eukaryot. Gene Expr.. 2009;19(3):171. Retrieved from /pmc/articles/PMC2856465/
- [Google Scholar]
- Boyle, N., Lawton, C., Dye, L., 2016. The effects of magnesium supplementation on subjective anxiety. Retrieved from https://pubmed.ncbi.nlm.nih.gov/27869100/.
- Cao, J., Gao, Z., Yan, J., Li, M., Su, J., Xu, J., & Yan, C., 2016. Evaluation of Trace Elements and Their Relationship with Growth and Development of Young Children. Biol. Trace Element Res. 171(2), 270-274. Retrieved from https://link.springer.com/article/10.1007/s12011-015-0537-7.
- Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients. 2013;5(8):3022. Retrieved from /pmc/articles/PMC3775240/
- [Google Scholar]
- Díaz-Tocados, J., Herencia, C., Martínez-Moreno, J., Montes de Oca, A., Rodríguez-Ortiz, M., Vergara, N., Muñoz-Castañeda, J., 2017. Magnesium Chloride promotes Osteogenesis through Notch signaling activation and expansion of Mesenchymal Stem Cells. Sci. Reports 7(1), 1-12. Retrieved from https://www.nature.com/articles/s41598-017-08379-y.
- Tanner stages. In: The SAGE Encyclopedia of Lifespan Human Development. Vol 2017. 2022. p. :12.
- [Google Scholar]
- Fadlalla, I., Omer, S., Atta, M., 2020. Determination of some serum macroelement minerals levels at different lactation stages of dairy cows and their correlations. Sci. African 8, e00351.
- Farag, M., Abib, B., Qin, Z., Ze, X., Ali, S., 2023. Dietary macrominerals: Updated review of their role and orchestration in human nutrition throughout the life cycle with sex differences. Retrieved from https://linkinghub.elsevier.com/retrieve/pii/S2665927123000187.
- Fiorentini, D., Cappadone, C., Farruggia, G., Prata, C., 2021. Magnesium: Biochemistry, Nutrition, Detection, and Social Impact of Diseases Linked to Its Deficiency. Retrieved from https://www.mdpi.com/2072-6643/13/4/1136/htm.
- Ghosh, A., Joshi, S., 2008. Disorders of calcium, phosphorus and magnesium metabolism. J. Assoc. Physicians India 56(AUG), 613-621. Retrieved from https://pubmed.ncbi.nlm.nih.gov/19051708/.
- Relationship between magnesium, potassium and sodium concentrations in lymphocytes and erythrocytes from normal subjects. Magnesium. 1985;4(4):188-192. Retrieved from
- [Google Scholar]
- The Importance of Magnesium in the Human Body: A Systematic Literature Review. Adv. Clin. Chem.. 2016;73:169-193. Retrieved from
- [Google Scholar]
- Gröber, U., Schmidt, J., Kisters, K., 2015. Magnesium in Prevention and Therapy. Retrieved from https://www.mdpi.com/2072-6643/7/9/5388/htm.
- Hassan, S., Ahmed, I., Nasrullah, A., Haq, S., Ghazanfar, H., Sheikh, A., Khan, A., 2017. Comparison of Serum Magnesium Levels in Overweight and Obese Children and Normal Weight Children. Cureus, 9(8). Retrieved from /pmc/articles/PMC5654973/.
- Huang, K., Fang, H., Yu, D., Guo, Q., Xu, X., Ju, L., Zhao, L., 2022. Usual Intake of Micronutrients and Prevalence of Inadequate Intake among Chinese Adults: Data from CNHS 2015-2017. Retrieved from https://pubmed.ncbi.nlm.nih.gov/36432400/.
- Ibrahim, S., Abd El Maksoud, A., Nassar, M., 2002. Nutritional stunting in Egypt: which nutrient is responsible? Eastern Mediterranean Health J. = La revue de sante de la Mediterranee orientale = al-Majallah al-sihhiyah li-sharq al-mutawassit, 8(2-3), 272-280. Retrieved from https://pubmed.ncbi.nlm.nih.gov/15339114/.
- Kutbi, H., 2021. Nutrient intake and gender differences among Saudi children. Retrieved from https://pubmed.ncbi.nlm.nih.gov/34888037/.
- Lee, W., Leung, S., Leung, D., Tsang, H., Lau, J., Cheng, J., 1995. A randomized double-blind controlled calcium supplementation trial, and bone and height acquisition in children. Br. J. Nutrit. 74(1), 125–139. Retrieved from https://pubmed.ncbi.nlm.nih.gov/7547823/.
- Leidi, M., Dellera, F., Mariotti, M., Maier, J., 2011. High magnesium inhibits human osteoblast differentiation in vitro. Magn. Res., 24(1), 1–6. Retrieved from https://pubmed.ncbi.nlm.nih.gov/21421455/.
- Lu, L., Chen, C., Yang, K., Zhu, J., Xun, P., Shikany, J., He, K., 2020. Magnesium intake is inversely associated with risk of obesity in a 30-year prospective follow-up study among American young adults. Eur. J. Nutrit. 59(8), 3745. Retrieved from /pmc/articles/PMC7483156/.
- Mammoli, F., Castiglioni, S., Parenti, S., Cappadone, C., Farruggia, G., Iotti, S., Frassineti, C., 2019. Magnesium Is a Key Regulator of the Balance between Osteoclast and Osteoblast Differentiation in the Presence of Vitamin D3. Int. J. Mol. Sci. 20(2). Retrieved from https://pubmed.ncbi.nlm.nih.gov/30658432/.
- Mensink, G., Fletcher, R., Gurinovic, M., Huybrechts, I., Lafay, L., Serra-Majem, L., Stephen, A., 2013. Mapping low intake of micronutrients across Europe. Retrieved from /pmc/articles/PMC3785176/.
- 7). Relationships among Macro-Minerals, Other Selected Serum Markers of Bone Profile and Milk Components of Dairy Cows during Late Lactation. Annals of. Anim. Sci.. 2021;21(3):887-898.
- [Google Scholar]
- Reviews: Clinical Implications of Disordered Magnesium Homeostasis in Chronic Renal Failure and Dialysis. Seminars Dial.. 2009;22(1):37-44.
- [CrossRef] [Google Scholar]
- Nielsen, F., 2010. Magnesium, inflammation, and obesity in chronic disease. Retrieved from https://pubmed.ncbi.nlm.nih.gov/20536778/.
- Razzaque, M., 2018. Magnesium: Are We Consuming Enough? Retrieved from https://www.mdpi.com/2072-6643/10/12/1863/htm.
- Body Mass Index (nutritional status) and concentrations of Ca, Cu, Fe, Mg, Zn, K, Na in the hair of young men from Tanzania. Acta Biologica. 2016;23:75-86.
- [Google Scholar]
- Body composition in children and Tanner's stages: a study with dual-energy x-ray absorptiometry. Metabolism. 1993;42(8):967-970.
- [Google Scholar]
- Rodríguez-Ortiz, M., Canalejo, A., Herencia, C., Martínez-Moreno, J., Peralta-Ramírez, A., Perez-Martinez, P., Almaden, Y., 2014. Magnesium modulates parathyroid hormone secretion and upregulates parathyroid receptor expression at moderately low calcium concentrations. Nephrol., Dial., Transplant.: Off. Publ. Eur. Dial. Transplant Assoc. – Eur. Renal Assoc. 29(2), 282–289. Retrieved from https://pubmed.ncbi.nlm.nih.gov/24103811/.
- Rosanoff, A., Wolf, F., 2016. A guided tour of presentations at the XIV International Magnesium Symposium. Retrieved from https://pubmed.ncbi.nlm.nih.gov/27829571/.
- Factors affecting prepubertal and pubertal bone age progression. Front. Endocrinol.. 2022;13 Retrieved from /pmc/articles/PMC9441639/
- [Google Scholar]
- Inverse correlation between serum calcium and copper levels in male urban Colombian preschool children: relationships with anthropometry and age. Biol. Trace Element Res.. 2011;144(1–3):445-453.
- [Google Scholar]
- Volpe, S., 2013. Magnesium in Disease Prevention and Overall Health.
- Relationship of blood levels of Pb with Cu, Zn, Ca, Mg, Fe, and Hb in children aged 0 ∼ 6 years from Wuhan, China. Biol. Trace Element Res.. 2015;164(1):18-24.
- [Google Scholar]
- Evaluation of the Relationship Between Height and Zinc, Copper, Iron, Calcium, and Magnesium Levels in Healthy Young Children in Beijing, China. Biol. Trace Element Res.. 2017;176(2):244-250.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2024.103102.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







