Lycopene augments and enhances anti-oxidant/antibacterial efficiency of ethanolic leaf extract of Helianthus annuus over multidrug-resistant bacterial isolates
⁎Corresponding author at: Research Center for Advanced Materials Science (RCAMS), King Khalid University, P.O. Box 9004, Abha 61413, Saudi Arabia. mona.kilany@yahoo.com (Mona Kilany)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Lycopene, the potential antioxidant naturally occurring in red carotenoid pigment is found in many fruits and vegetables. In this work, antioxidant power and antimicrobial potentials of lycopene against the multidrug-resistant (MDR) Streptococcus agalactiae (S. agalactiae) and Streptococcus pyogenes (S. pyogenes) were studied when added to ethanolic leaf extracts of Helianthus annuus (H. annuus). Supplementation of the lycopene along with ethanolic leaf extract of H. annuus indicates a 30 % enhancement in the antioxidant activity by 2, 2-Diphenyl-1-Picrylhydrazyl (DPPH) assay and reduction of 24 % reactive oxygen species (ROS) in human fibroblast cells under calcium stress compared to leaf extract alone. The antibacterial activity of the leaf extract + lycopene showed improved bacterial inhibition as low as 40 and 70 μg of the leaf extract compared with extract alone against S. pyogenes and S. agalactiae respectively. Taken together, the observations show that the natural anti-oxidant, lycopene, when added to the extract enhanced the antibacterial activity at lower concentrations which could possibly reduce the larger dose as observed in alternative or complementary medical practices.
Keywords
Leaf extract of Helianthus annuus
Multidrug resistance
Lycopene
Streptococcus. Pyogenes
Streptococcus agalactiae
1 Introduction
Traditional and folklore medicinal literatures (Laurieri and Delgoda, 2017; Shahat et al., 2017) have a huge reference of herbal extracts and formulations which needs to be scientifically evaluated to suit modern medical practices. In recent times antibiotic drug resistance (Medina and Pieper, 2016) has emerged as a huge global problem with increasing risk of multidrug-resistant forms evolving due to antibiotic misuse (Llor and Bjerrum, 2014), immunodeficiency (Denegre et al., 2019) and environmental mutations (Cantón, 2009). A large chuck of investment has been made in these two decades to screen small molecule libraries (Zulauf and Kirby, 2020), synthetic and semi-synthetic derivatives (Jeya et al., 2011), natural products (Leisner, 2020) and secondary metabolites (Gorlenko et al., 2020) and finally crude extract (Mohamed et al., 2020) as original traditional formulations.
The bioactivity of H. annuus flower and seeds is well documented in the literature (Amirul, 2020). There are studies which shows the ozonated sunflower seed oil has a wide range of antibacterial and antifungal activity (Sechi et al., 2001). There are few other studies that describe the H. annuus leaf extracts exhibiting antibacterial activities (Akpor et al., 2019). A notable point is sunflower plant extracts have shown antibacterial activity against both gram +ve and gram −ve organisms (Mutlu-Ingok et al., 2020). Drug resistance especially in streptococcus species has been well studied (Alves-Barroco et al., 2020). There are significant infections caused by S. agalactiae including meningitis, urinary tract infections and pneumonia while S. pyogenes is known to cause scarlet fever, streptococcus septic shock etc are every day threat in pediatric and geriatric populations (Al-Bayati et al., 2020). Drug resistance has become a common problem especially with these streptococcus species and the high demand for new and effective drugs (Nayak et al., 2019). Further, extracts or natural products, small molecules and secondary metabolites are known to exhibit t toxicity to the host tissues poses a bigger hurdle in the drug screening studies (Şeremet et al., 2016). Though H. annuus products are known to be antibacterial and their mechanism of action does not involve a pathway to describe, not much toxicity to the normal cells of the host is reported (Fatrcová-Šramková et al., 2015).
Many of the screened natural products including herbal extracts have less efficacy and bioavailability thus need a larger dose for longer periods (Bhattaram et al., 2002; Kesarwani and Gupta, 2013). This was overcome in the last few years with the new concept of combination or added naturally occurring compounds which is known to have profound bioactivity or antioxidant itself (McCarrell et al., 2008). Adding an antioxidant enhances the extracts bioactivity at lower concentrations while increasing the bioavailability and acts as a drug delivery system (Kanellos et al., 2013). Lycopene, vitamin C and other pigmented natural products are usually supplemented with drugs and food products or given as such to enhance antioxidant levels as a disease management strategy (Yonar et al., 2019). However, the idea of these antioxidants along with herbal extracts are unusual in drug screening studies.
Therefore, in the current study, evaluation of ethanolic extracts of leaves of H. annuus was tested for their antioxidant property and supplemented with known antioxidant lycopene to enhance oxidant scavenging as well as antibacterial activity at very low concentrations. This will pave the way for the use of safe drugs which will be only active against bacterial cells without harming the host tissues.
2 Materials and methods
2.1 H. annuus leaf extract preparation
Fresh leaves of H. annuus (Asteraceae), commonly known as the sunflower plant were gathered from Abha, Saudi Arabia, cleaned and shade dried. The dry leaves were pounded and kept in airtight dark containers. The ethanolic extract was prepared as per standard protocol adopted in the laboratory (Ibrahim et al., 2021). Briefly, 10 g of the grinded leaf were blended with 200 mL of absolute ethanol and agitated for 50 h. The mixture was centrifuged at 5000 rpm two times to get rid of solid materials and dried at 55 °C. A stock solution (1 %) was prepared by dissolving the dried dimethyl formamide (DMFO, Sigma-Aldrich) and sterilized utilizing 0.45 µm syringe filter.
2.2 Culture of microorganisms
Culture, maintenance, source of bacterial strains (MDR isolates; Streptococcus pyogenes and Streptococcus agalactiae) and antibiotic sensitivity assays were done as previously described by Alshahrani et al. (2022).
2.3 Disc diffusion method
A 100 μL (108 cfu/mL) of the logarithmic phase of test bacteria were spread on the surface of MHA plates. Ethanolic H. annuus extract discs were placed at a 2 cm space between discs in the MHA plates. All plates were kept at 37 °C for 48 h. The preparation showing least zone of clearance was considered and compared with the positive antibiotic controls (Bshabshe et al., 2020).
2.4 Determination of minimum inhibitory concentration
Alamar blue (AB) microplate adaptation was used to determine the minimum inhibitory concentration (MIC). Briefly, the bacterial cultures of S. pyogenes and S. agalactiae were adjusted to 4.2X 105 CFU/ml and 5.3 X105 CFU/mL equivalent to 0.002 at OD600 respectively. Penicillin and erythromycin served as positive controls while media served as a negative control. For MIC, one concentration before and after the complete zone of clearance was selected. Therefore, for the current experiment extract (100, 125 and 150 μg for S. pyogenes & 150, 175 and 200 μg for S. agalactiae) and extract + lycopene (40, 50 and 60 μg for S. pyogenes & 70, 80 and 90 μg for S. agalactiae) were used. The extract and test organisms were incubated along with alamar blue for 12 h in 10 replicates. The fluorescence was measured at 530 nm and 590 nm respectively at 0, 6, and 12 h. MIC was noted as the concentration at which the purple color of alamar blue is not reduced to bright red fluorescence.
2.5 DPPH assay
Antioxidant activity of the ethanolic leaf extract of H. annuus was determined by DPPH assay (Akar et al., 2017). Briefly, different concentrations of the leaf extract alone or with lycopene were added to 0.3 mM methanolic solution of DPPH in a ratio of 1:1. Graded doses of 5, 25, 50, 75, 100, 125, 150,175 and 200 μg/mL of extract alone or with 1 μM lycopene were used to screen the scavenging activity. The mixture was incubated for 30 min at room temperature in dark. The change in color was measured at 517 nm. DPPH solution and methanol served as positive and negative controls respectively. Graded doses (10–100 μg/ml) of l-ascorbic acid were taken as the standard reference. Percentage-free radical scavenging of the sample was calculated as follows.
2.6 Reactive oxygen species detection
Reactive oxygen species (ROS) detection Assay Kit (BioVision, Catalog # K936, CA 95,035 USA) was used as per manufacturer instructions adapted to microplate reader protocol. Briefly 2.5 × 104 human fibroblast cells were seeded in a 96-well cell culture plate, kept at 37 °C and 5 % CO2 for 18 h to get ∼ 75–85 % confluency. At the test day, cells were treated with calcium solution (100 μM in culture medium) for 30 min to create calcium stress, washed once with phosphate-buffered saline (PBS) and incubated with 100 μL/well 1X ROS Label (diluted in ROS Assay Buffer) for 45 min at 37 °C in the dark. Post incubation, the ROS label was washed and then 100 μL of blank (reagent), positive and negative control along with extract or extract + lycopene were added in triplicates for 10 min. The cells were once again washed and loaded with PBS (100 μL) and fluorescence was measured instantly at Ex/Em = 495/529 nm in end point mode. The ROS was determined as the change in the fluorescence of treated over untreated after the background subtraction as instructed in the kit instructions.
3 Result
The antibiotic sensitivity results (Table 1) show the susceptibility pattern of S. agalactiae and S. pyogenes clinical isolates. Typically, S. agalactiae was intermediately susceptible to Clindamycin and showed resistance to Erythromycin and Vancomycin compared to S. pyogenes. Hence Erythromycin and Penicillin were selected as a positive control for S. pyogenes and S. agalactiae respectively.
| Clinical Isolates Antibiotics | S. agalactiae | S. pyogenes |
|---|---|---|
| Ampicillin | R | R |
| Amoxiclav | R | R |
| Amikacin | NA | NA |
| Ceftazidime | NA | NA |
| Cefotaxime | S | S |
| Ciprofloxacin | NA | NA |
| Cefuroxime | S | S |
| Cefazolin | S | S |
| Gentamicin | S | S |
| Imipenem | NA | NA |
| Nalidixic acid | NA | NA |
| Nitrofurantoin | NA | NA |
| Norfloxacin | NA | NA |
| Erythromycin | R | S |
| Clindamycin | I | S |
| Penicillin | S | S |
| Rifampicin | NA | NA |
| Vancomycin | R | NA |
NA: Not Applicable; S: Sensitive; R: Resistant; I: Intermediate.
The disc diffusion method outcomes (Table 2) demonstrated that ethanolic extracts of H. annuus were able to inhibit S. agalactiae at 175 μg compared to a positive control (Penicillin) (Table 3). Similarly, the extract, at 125 μg concentration, showed complete clearance zone parallel with the positive control (Erythromycin) for S. pyogenes (Tables 2 & 3).
| Herb | Nature of extract | Clinical Isolates | Concentration (μg/ml) of the extract +/- Lycopene versus Zone of Clearance (mm)* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 20 | 30 | 40 | 50 | 60 | 70 | 80 | 90 | 100 | 125 | 150 | 175 | |||
| H. annuus | Ethanolic | S. agalactiae | -/- | -/- | -/- | -/- | -/- | -/- | -/- | 02 | 05 | 08 | 14 | 17.5 | 19 |
| S.pyogenes | -/- | -/- | -/- | -/- | -/- | 4 | 8.4 | 11 | 14 | 15.5 | 19 | 20 | 20 | ||
| Ethanolic + 1 μM Lycopene |
S. agalactiae | -/- | -/- | -/- | 07 | 07 | 13 | 17 | 19 | 20 | – | – | – | – | |
| S.pyogenes | -/- | 02 | 08 | 16 | 19 | 19 | 20 | – | – | – | – | – | – | ||
*The results are demonstrated as the average inhibition zone (mm) obtained from four independent tests.
-/-: No inhibition zone.
-: Compete clearance.
Positive activity was calculated as per zone of clearance compared to the zone of clearance obtained with susceptible antibiotics from antibiotic susceptibility testing.
| Antibiotics | Zone of Clearance (mm)* | |
|---|---|---|
| S. agalactiae | S.pyogenes | |
| Penicillin | 21 | 19 |
| Erythromycin | 09 | 19 |
Next the additive effect of 1 μM Lycopene with extracts showed (Table 2) 80 μg was able to inhibit S. agalactiae while 50 μg was able to inhibit S. pyogenes compared with their respective positive antibiotic controls (Table 3). The antibacterial effect was observed at a minimum concentration of 20 μg signifying the additive effect of Lycopene. However, the varied concentrations of the extracts and lycopene showing antibacterial activity indicated that the nature of the organism plays a major role in the susceptibility pattern to natural extracts.
Further, the ethanolic H. annuus extract exhibited well-marked antioxidant activity compared to Lycopene (a well-established anti-oxidant). When the combination of various concentrations of extract and 1 μM Lycopene was tested for oxidant scavenging activity, the results showed a dose-dependent incremental oxidant scavenging (Fig. 1). 100 μg showed maximum scavenging activity compared with standard Ascorbic acid. In order to validate the antioxidant activity of the combined extract and Lycopene, specific ROS clearance was assessed (Fig. 2). The results well corroborated with the oxidant scavenging activity (Fig. 1) showing an accelerated ROS clearance with extract and Lycopene combination.
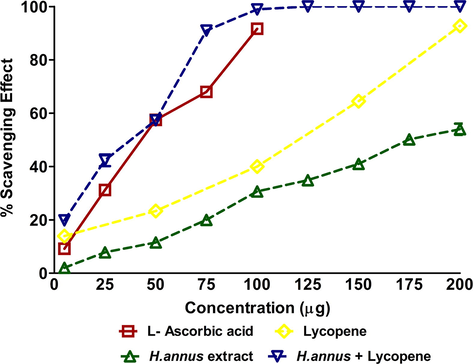
- The DPPH assay showing oxidant scavenging of H. annuus extract and H. annuus extract + 1 μM Lycopene.
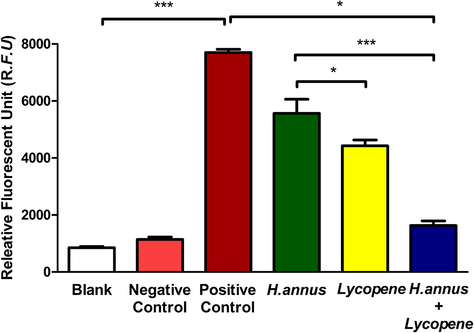
- ROS clearance activity of H. annuus extract and H. annuus extract + 1 μM Lycopene.
Finally, the MIC was determined based on the concentrations of the extracts or extracts + Lycopene obtained with both the organisms by the disc diffusion method and confirmed by alamar blue method (Table 2 & Fig. 3). Accordingly, the MIC was confirmed as 175 μg and 80 μg for S. agalactiae by ethanoic extract of H. annuus and ethanolic extract of H. annuus + 1 μM Lycopene respectively (Fig. 3a). Similarly, a concentration of 125 μg and 50 μg exhibited complete inhibition of S. pyogenes by extract and extract + Lycopene respectively.
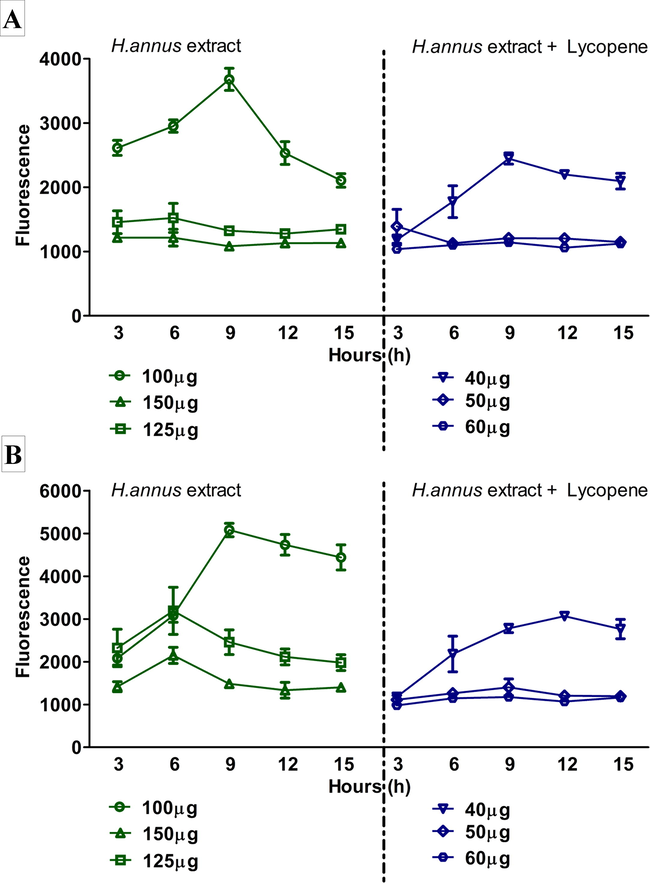
- A: MIC determination of H. annuus extract and H. annuus extract + 1 μM Lycopene against S. agalactiae. B: MIC determination of H. annuus extract and H. annuus extract + 1 μM Lycopene against S. pyogenes.
4 Discussion
The antibacterial effect of ethanolic leaf extract of H. annuus was well documented from the results. Though, antibacterial (Al-Shukaili and Hossain, 2019), antifungal (Lawson et al., 2019), antioxidant and antidiabetic (Saini and Sharma, 2013) activity of the flower and essential oil of H. annuus are known, traditional and flock lore uses leaves for treatment of wounds in humans and animals for long (Gai et al., 2020). Though, many plant-based formulations have shown antibacterial activities including H. annuus products, additive profound effects when mixed with standard natural plant based pure products are currently trending (Cowan, 1999; Safi and Al-Mariri, 2014). This is owing to extracts added with pure natural products complexity in mechanism of action that may overcome drug resistance a common phenomenon observed in recent times (Gonelimali et al., 2018). The additive effects of Lycopene along with ethanolic extract were well in agreement with other studies done similarly with different herbs and nanoparticles (Ozen et al., 2011; Zhang et al., 2017).
It is evident from literature and current results; that the herbal extracts and natural products from plant origin are effective in antibacterial activity and it has been noted that several parts of H. annuus and extracted with different solvents show varied of bio-activity. For instance, aqueous extracts from flowers and seeds have been implicated to be active against gram-ve organisms (Liu et al., 2020) while leaf extracts on both gram +ve and gram −ve organisms (Amirul, 2020; Sechi et al., 2001). H. annuus extract and Lycopene are known for its antioxidant activity, many studies have suggested its antibacterial activity to be directly killing the cells. The difference between the activities exhibited for gram +ve or gram −ve are due to its affinities towards membrane proteins on the organism itself (Mutlu-Ingok et al., 2020). However, the results of the current study showed dose-dependent clearance which might explain the inhibition of bacterial cells by direct killing.
The enhanced MIC of the extracts with the addition of Lycopene at a lower concentration is synonymous with other studies where other antioxidants like vitamin C which is naturally present or added (38). This offers a new set of natural product formulations that could very well overcome the problem of drug resistance and the question of the in-vivo toxicity observed with chemical substitutes, and small molecules (Khameneh et al., 2019; Silver, 2011). Therefore, it may be assumed that the traditional formulations like ethanolic extracts of herbal leaves along with additives like naturally occurring antioxidants may boost antibacterial activities or other bioactivities.
5 Conclusion
The ethanolic extracts of H. annuus along with 1 μM Lycopene a known antioxidant over its profound antibacterial activity at lower concentrations interestingly seems to be a new formulation. Further, the antioxidant nature of the sunflower and its essential oils are known, however, added Lycopene to the leaf extract not only elevates its antioxidant potential but also antibacterial effects. This will in turn reduce the large dosage of herbal formulation usually observed in traditional medical practices.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this work through the small research group program number 42-7.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A new colorimetric DPPH• scavenging activity method with no need for a spectrophotometer applied on synthetic and natural antioxidants and medicinal herbs. J. Enzyme Inhib. Med. Chem.. 2017;32(1):640-647.
- [Google Scholar]
- Antibacterial and antioxidant potentials of leave extracts of Helianthus annuus. Potravin. Slovak J. Food Sci.. 2019;13:1026-1033.
- [CrossRef] [Google Scholar]
- Meningitis from invasive Streptococcus agalactiae in a healthy young adult. IDCases. 2020;21:e00907.
- [Google Scholar]
- Antimicrobial and cytotoxic potential of seeds and flowers crude extracts of sunflower. Grain {\&} Oil Sci. Technol.. 2019;2:103-108.
- [CrossRef] [Google Scholar]
- Alves-Barroco, C., Rivas-Garc\’\ia, L., Fernandes, A.R., Baptista, P.V., 2020. Tackling Multidrug Resistance in Streptococci {\textendash} From Novel Biotherapeutic Strategies to Nanomedicines. Front. Microbiol. 11. doi:10.3389/fmicb.2020.579916.
- Literature Review: Study of Antibacterial Activity of Sunflower (Helianthus Annuus L.) Extract and Its Phytochemical Profiles | Amirul | Journal of Nutraceuticals and Herbal Medicine. J. Nutraceuticals Herb. Med.. 2020;3:29-37.
- [Google Scholar]
- Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine. 2002;9:1-33.
- [CrossRef] [Google Scholar]
- Clinical Relevance and Antimicrobial Profiling of Methicillin-Resistant Staphylococcus aureus ({MRSA}) on Routine Antibiotics and Ethanol Extract of Mango Kernel (Mangifera indica L.) BioMed Res. Int.. 2020;2020:1-8.
- [CrossRef] [Google Scholar]
- Antibiotic resistance genes from the environment: a perspective through newly identified antibiotic resistance mechanisms in the clinical setting. Clin. Microbiol. Infect.. 2009;15(Suppl 1):20-25.
- [CrossRef] [Google Scholar]
- Plant products as antimicrobial agents. Clin. Microbiol. Rev.. 1999;12:564-582.
- [CrossRef] [Google Scholar]
- Emergence of antibiotic resistance in immunocompromised host populations: A case study of emerging antibiotic resistant tuberculosis in AIDS patients. PLoS One. 2019;14(2):e0212969.
- [Google Scholar]
- Biologically active antimicrobial and antioxidant substances in theHelianthus {annuusL}. bee pollen. J. Environ. Sci. Heal. Part B. 2015;51:176-181.
- [CrossRef] [Google Scholar]
- Sunflower (Helianthus annuus L.) Plants at Various Growth Stages Subjected to Extraction{ extemdash}Comparison of the Antioxidant Activity and Phenolic Profile. Antioxidants. 2020;9:535.
- [CrossRef] [Google Scholar]
- Antimicrobial Properties and Mechanism of Action of Some Plant Extracts Against Food Pathogens and Spoilage Microorganisms. Front. Microbiol.. 2018;9:1639.
- [CrossRef] [Google Scholar]
- Gorlenko, C.L., Kiselev, H.Y., Budanova, E. V, Zamyatnin, A.A.J., Ikryannikova, L.N., 2020. Plant Secondary Metabolites in the Battle of Drugs and Drug-Resistant Bacteria: New Heroes or Worse Clones of Antibiotics? Antibiot. (Basel, Switzerland) 9. doi:10.3390/antibiotics9040170.
- Rosemary Extract and Its Biogenic Silver Nanoparticles Induce Apoptosis and Arrest Cell Cycle in HT-29 Colon Cancer Cells. Sci. Adv. Mater.. 2021;13:36-49.
- [CrossRef] [Google Scholar]
- Glycopeptide antibiotics and their novel semi-synthetic derivatives. Curr. Pharm. Biotechnol.. 2011;12:1194-1204.
- [CrossRef] [Google Scholar]
- Absorption and bioavailability of antioxidant phytochemicals and increase of serum oxidation resistance in healthy subjects following supplementation with raisins. Plant Foods Hum. Nutr.. 2013;68:411-415.
- [CrossRef] [Google Scholar]
- Bioavailability enhancers of herbal origin: an overview. Asian Pac. J. Trop. Biomed.. 2013;3:253-266.
- [CrossRef] [Google Scholar]
- Review on plant antimicrobials: a mechanistic viewpoint. Antimicrob. Resist. {&} Infect. Control. 2019;8
- [CrossRef] [Google Scholar]
- Essential Oil Compositions and Antifungal Activity of Sunflower (Helianthus) Species Growing in North Alabama. Appl. Sci.. 2019;9:3179.
- [CrossRef] [Google Scholar]
- The Diverse Search for Synthetic, Semisynthetic and Natural Product Antibiotics From the 1940s and Up to 1960 Exemplified by a Small Pharmaceutical Player. Front. Microbiol.. 2020;11
- [CrossRef] [Google Scholar]
- Chemical Composition and Antimicrobial and Antioxidant Activities of Essential Oil of Sunflower (Helianthus annuus L.) Receptacle. Molecules. 2020;25:5244.
- [CrossRef] [Google Scholar]
- Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf.. 2014;5(6):229-241.
- [Google Scholar]
- Antimicrobial activities of pomegranate rind extracts: enhancement by addition of metal salts and vitamin C. BMC Complement. Altern. Med.. 2008;8
- [CrossRef] [Google Scholar]
- Tackling threats and future problems of multidrug-resistant bacteria. Curr. Top. Microbiol. Immunol.. 2016;398:3-33.
- [CrossRef] [Google Scholar]
- Antimicrobial activity, phytochemical screening of crude extracts, and essential oils constituents of two Pulicaria spp. growing in Sudan. Sci. Rep.. 2020;10
- [CrossRef] [Google Scholar]
- Antibacterial, Antifungal, Antimycotoxigenic, and Antioxidant Activities of Essential Oils: An Updated Review. Molecules. 2020;25:4711.
- [CrossRef] [Google Scholar]
- Computational screening of potential drug targets for pathogens causing bacterial pneumonia. Microb. Pathog.. 2019;130:271-282.
- [CrossRef] [Google Scholar]
- Screening of antioxidant, antimicrobial activities and chemical contents of edible mushrooms wildly grown in the Black Sea Region of Turkey. Comb. Chem. {&} High Throughput Screen.. 2011;14:72-84.
- [CrossRef] [Google Scholar]
- In vitro antibacterial activity of several plant extracts and essential oils against Brucella melitensis. Herba Pol.. 2014;60:29-38.
- [CrossRef] [Google Scholar]
- Antidiabetic effect of Helianthus annuus l., seeds ethanolic extract in streptozotocin-nicotinamide induced type 2 diabetes mellitus. Int. J. Pharm. Pharm. Sci.. 2013;5:382-387.
- [Google Scholar]
- Antibacterial activity of ozonized sunflower oil (Oleozon) J. Appl. Microbiol.. 2001;90:279-284.
- [CrossRef] [Google Scholar]
- Şeremet, O.C., Bărbuceanu, F., Ionică, F., M, M.D., GuŢu, C.M., Olaru, O.T., Ilie, M., Gonciar, V., Negreş, S., ChiriŢă, C., 2016. Oral toxicity study of certain plant extracts containing pyrrolizidine alkaloids. J Morphol Embryol. 57, 1017–1023.
- Antimicrobial activities of some Saudi Arabian herbal plants. African. J. Tradit. Complement. Altern. Med. AJTCAM. 2017;14:161-165.
- [CrossRef] [Google Scholar]
- Challenges of Antibacterial Discovery. Clin. Microbiol. Rev.. 2011;24:71-109.
- [CrossRef] [Google Scholar]
- Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish {&} Shellfish Immunol.. 2019;89:83-90.
- [Google Scholar]
- Novel biomolecule lycopene-reduced graphene oxide-silver nanoparticle enhances apoptotic potential of trichostatin A in human ovarian cancer cells ({SKOV}3) Int. J. Nanomed.. 2017;12:7551-7575.
- [CrossRef] [Google Scholar]
- Discovery of small-molecule inhibitors of multidrug-resistance plasmid maintenance using a high-throughput screening approach. Proc. Natl. Acad. Sci. U. S. A.. 2020;117:29839-29850.
- [CrossRef] [Google Scholar]







