Translate this page into:
Larvicidal potential of Thuja orientalis leaves and fruits extracts against Culex pipiens (Diptera: Culicidae)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Botanical pesticides targeted to avoid the pesticide resistance to synthetic ones that conventionally affecting ecosystems diversity. The study designed to evaluate the larvicidal potentials of leaves and fruits extracts (methanol, acetone, hexane, and aqueous) from Thuja orientalis (Pinales: Cupressaceae) against Culex pipiens 3rd instar larvae and to identify the extracts compounds by gas chromatography-mass spectrometry (GC–MS) method of analysis. Leaves and fruits extracts used in concentrations, 25, 50, 100, 200, and 400 ppm against the 3rd larval instar of Cx. pipiens in five replicates and larval mortalities recorded after 24 and 48 h post exposure. The larvicidal potentials of extracts showed concentration dependent and varied between leaves and fruits extracts. At 400 ppm concentration, leaves extracts showed 100 % larval mortality except in hexane extract exhibited 98 %. Acetone, methanol, aqueous and hexane leaves extracts recorded LC50 values, 58.04, 70.20, 77.19 and 84.25 ppm, respectively. Fruits extracts by hexane and methanol exhibited 100 % larval mortality and LC50, 68.26 and 83.21 ppm, respectively, while, acetone and aqueous fruits extracts showed 98 % and 96 % larval mortality and LC50, 92.81 and 102.97 ppm, respectively. Terpenoids and sesquiterpenoids, fatty acid esters mainly identified in both extracts.
The present study showed that extracts from Thuja orientalis leaves and fruits acquired promising larvicidal potential for the control of Cx. pipiens larvae with the role of their chemical constituents.
Keywords
Thuja orientalis
Methanol
Acetone
Hexane
Aqueous
Culex pipiens
Larvicidal
GC–MS
1 Introduction
Mosquitoes considered as a burden to human health by transmitting diseases including malaria, filariasis, dengue, and leishmaniasis (Wilson et al., 2020). Culex pipiens mosquitoes is common house mosquito and one of the most widely distributed mosquitoes worldwide due to its adaptation to human environments and its mode of feeding on birds and mammals reflected by its role in transmission of the West Nile virus and other pathogens (Farajollahi et al., 2011) that urged researches of its control.
Vector control is the main way to reduce public concerns about mosquito-borne diseases including filariasis, dengue, malaria, and leishmaniasis (Wilson et al., 2020). Control of mosquito larvae in aquatic phases is an effective method for reducing mosquito-spread (WHO, 2013). Excessive use of synthetic insecticides, with a complete lack of awareness of the strategy of changing the pesticides, led to resistance to pesticides along with environmental pollution and health risks to humans and non-target biota. Therefore, the search for environmentally friendly alternatives as plants or oils rich in secondary metabolites is a recent trend since they are more efficient, less toxic, biodegradable, and capable of insect decrease plant resistance to these natural compounds (Mouden et al., 2017; Ahmed et al., 2021) besides serving as larvicides, adult pesticides, insect repellents and deterrents (Govindarajan et al., 2016) they destroy only the insects they are meant to kill, leaving no residue on food or in the environment.
Thuja orientalis is a dense, evergreen and coniferous tree belongs to the family Cupressaceae growing in Saudi Arabia (Elsharkawy et al., 2017; Elsharkawy and Ali, 2019). In folk medicine T. orientalis used as herbal medicine for treatment of psoriasis, amenorrhea, enuresis, rheumatism, cystitis, bronchial catarrh and uterine carcinomas (Srivastava et al., 2012). Studies evaluated different activities of T. orientalis like, antimicrobial (Choi et al., 2021), antifungal activity (Caruntu et al., 2020), antioxidant (Nizam and Mushfiq, 2007), anticancer (Elsharkawy et al., 2017), anti-inflammatory (Darwish et al., 2021; Shin et al., 2015), hair growth promotion (Zhang et al., 2013).
The present study designed to investigate the larvicidal activities of methanol, hexane and acetone and aqueous extracts from leaves and fruits of Thuja orientalis plant against Cx. pipiens third instar larvae after exposure to extracts for 24 and 48 h. As well, identification of the chemical composition of the tested extracts by the aid of gas chromatography-mass spectrometry (GC–MS) method of analysis.
2 Materials and methods
2.1 Plants materials
Thuja orientalis L. (Pinales: Cupressaceae) leaves and fruits were obtained from Arar region, at Northern Region of Saudi Arabia, where growing wild in March 2022 as previously recorded (Elsharkawy and Ali, 2019). The plant was identified by a taxonomist, prof. Dr. Yahya Masrahi, from the Department of Biology, Faculty of Science, Jazan University, Saudi Arabia.
2.2 Culex pipiens colony
Mosquitoes (Cx. pipiens) were obtained from the Center for Environmental Research and Studies at Jazan University. Rearing was performed under controlled conditions (27 ± 2 °C, relative humidity at 70 % ± 10 %, and 12:12 h light:dark regime). Mosquito larvae were reared in round enamel plates (25 × 20 × 10 cm) filled with 2 L de-chlorinated water and fed with fish food daily. The third instar Cx. pipiens larvae were used for the larvicidal examination.
2.3 Plant extracts.
Leaves and fruits were dried in shade for 7 days at laboratory temperatures (27–29 °C). The dried leaves (40 g) and fruits (25 g) were powdered using a commercial electrical stainless-steel blender and extracted for each solvent including methanol, acetone, hexane, and aqueous using Soxhlet apparatus for 6–8 h according to the solvent type. The leaves and fruits extracts were filtered with Whatman number 1 filter paper through a Buchner funnel. The filtrates then dried using a rotary evaporator under vacuum at 40 °C. The plant leaves extract yields 3.7, 3.2, 2.8, and 1.7 g for methanol, acetone, hexane, and aqueous solvents and yields 2.1, 1.5, 2.3 and 1.1 g for plant fruits, respectively.
2.4 Larvicidal assay
The larvicidal activity was determined for the extracts in concentrations that prepared as 25, 50, 100, 200, and 400 ppm based on 1 g/1L (1000 ppm) from each extract stock solution against the 3rd larval instar of Cx. pipiens (WHO, 2005). Twenty Cx. pipiens third instar larvae of were subjected to each extract concentration in 250 mL glass beakers containing 150 mL de-chlorinated water (aqueous suspension) at 27 ± 2 °C, 70 ± 10 % RH, and a 12:12 h (L/D) photoperiod. Five replicates per concentration per extract and control were conducted. Larval mortalities were recorded after 24 and 48 h of exposure.
2.5 Identification of chemical compounds in extracts by gas chromatography-mass spectrometer.
Extracts from promising plant, Thuja orientalis L. were analyzed to investigate their chemical constituents by GC–MS using the Trace GC-TSQ mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) through TG–5MS direct capillary column (30 m × 0.25 mm × 0.25 m thickness of the film) where the column oven temperature initially maintained at 50 °C, then rate was increased 5 °C/min up to 250 °C, for 2 min, and then increased 30 °C/min to 300 °C. The lines for the injector and MS transfer were suspended at 280 °C and 260 °C, respectively, helium was the carrier gas at the rate of 1 mL/min. The solvent delay was 3 min and 2 µl samples were injected automatically using Autosampler AS1310 coupled with GC in the splitless mode. In full scanning mode, electrospray ionization (EI) mass spectra were obtained covering the range 50–650 m/s at an ionization voltage of 70 V. The ion source temperature was fixed at 200 °C. The chemical constituents were identified from the Total Ion Chromatogram (TIC), where the chemical compounds were identified by comparison of their retention times and mass spectra with those of WILEY 09 and NIST 11 mass spectral databases.
2.6 Data analysis
The percentage mortalities were determined according to Abbott, (1925). The larval control results did not need correction, as the mortality was less than 5 %, according to the WHO guidelines (WHO, 2005) (no larval control mortality recorded throughout the study). Mortality data from all the replicates were performed using one-way analysis of variance (ANOVA) to find the differences among the activity between each plant extract concentrations using the least significant difference (LSD) test. Also, data from all the replicates were subjected to analysis to determine the larval LC50, LC90, and LC95 as well as chi-square values within confidence limits at 95 % (lower confidence limit (LCL) and upper confidence limit (UCL) by using probit analysis and regression between log extract concentration and probit values. Data analysis was performed using SPSS software (IBM SPSS Statistics v22 – 64 bit), and p < 0.05 was considered significant.
3 Results
3.1 Larvicidal activity
The data about the larvicidal activities of the tested Thuja orientalis leaves extracts against the third instar larvae of Cx. pipiens after 24 h are summarized in Table 1, and after 48 h are summarized in Table 2. The analysis of responses of the leaves extracts revealed that the larvicidal activities after 24 h showed 100 % mortalities at 400 ppm of methanol, acetone and aqueous extracts. While hexane leaves extract at 400 ppm showed 98 % larval mortality. Acetone leaves extract showed the highest efficacy by inducing 100 % mortality (LC50, 58.04, LC90, 169.27, and LC95, 229.27 ppm), followed by methanol extract by 100 % mortality (LC50, 70.20, LC90, 221.66, and LC95, 307.07 ppm), followed by aqueous extract by 100 % mortality (LC50, 77.19, LC90, 244.26, and LC95, 338.95 ppm). While, hexane extract showed the least efficacy as compared to others, inducing 98.00 % mortality (LC50, 84.25, LC90, 283.47, and LC95, 399.84 ppm) (Table 1). The larvicidal activities of the leaves extracts after 48 h showed 100 % mortalities at 400 ppm for all tested extracts. Acetone leaves extract showed the highest efficacy (LC50, 43.40, LC90, 102.62, and LC95, 130.98 ppm), followed by methanol extract (LC50, 54.75, LC90, 156.00, and LC95, 209.92 ppm), followed by the aqueous extract (LC50, 63.76, LC90, 198.50, and LC95, 273.89 ppm). Then, hexane showed (LC50, 69.02, LC90, 225.14, and LC95, 314.78 ppm) (Table 2). Significance at 0.05 level between different superscripts. (a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.05. Significance at 0.05 level between different superscripts. (a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.05.
Leaves extract
Conc. ppm
Mortality%
(Mean ± SE)LC50
(LCL–UCL.)LC90
(LCL–UCL.)LC95
(LCL–UCL.)Chi
(Sig)Regression equation
R2
Methanol
0.0
0.00 ± 0.0a
70.20
(61.92–79.19)221.66
(185.53–278.05)307.07
(248.31–404.83)4.417
(0.220a)Y = −4.33 + 2.33*x
0.997
25
15.00 ± 2.24b
50
35.00 ± 3.16c
100
61.00 ± 4.30d
200
86.00 ± 2.45e
400
100.00 ± 0.00f
Acetone
0.0
0.00 ± 0.0a
58.04
(51.25–65.24)169.27
(143.49–208.94)229.27
(188.22–296.63)1.884
(0.597a)Y = −4.68 + 2.65*x
0.997
25
18.00 ± 2.55b
50
41.00 ± 2.92c
100
72.00 ± 2.55d
200
93.00 ± 2.55e
400
100.00 ± 0.00f
Hexane
0.0
0.00 ± 0.0a
84.25
(74.21–95.43)283.47
(234.18–361.87)399.84
(318.35–537.98)3.348
(0.341a)Y = −4.95 + 2.6*x
0.976
25
12.00 ± 2.00b
50
29.00 ± 3.32c
100
54.00 ± 2.45d
200
79.00 ± 2.45e
400
98.00 ± 1.22f
Aqueous
0.0
0.00 ± 0.0a
77.19
(68.22–87.06)244.26
(204.00–307.18)338.95
(273.22–447.30)5.630
(0.131a)Y = −4.32 + 2.27*x
0.999
25
13.00 ± 2.00b
50
31.00 ± 4.00c
100
58.00 ± 2.55d
200
82.00 ± 1.22e
400
100.00 ± 0.00f
Leaves extract
Conc. ppm
Mortality %
(Mean ± SE)LC50
(LCL–UCL.)LC90
(LCL–UCL.)LC95
(LCL–UCL.)Chi
(Sig)Regression equation
R2
Methanol
0.0
0.00 ± 0.0a
54.75
(48.63–61.48)156.00
(132.59–192.02)209.92
(172.87–270.73)2.260
(0.520a)Y = −4.8 + 2.77*x
0.992
25
20.00 ± 1.58b
50
42.00 ± 4.90c
100
75.00 ± 4.18d
200
95.00 ± 3.16e
400
100.00 ± 0.00e
Acetone
0.0
0.00 ± 0.0a
43.40
(38.72–48.22)102.62
(88.81–123.99)130.98
(110.22–165.22)3.112
(0.375a)Y = −5.26 + 3.21*x
0.988
25
24.00 ± 2.45b
50
53.00 ± 4.06c
100
89.00 ± 1.87d
200
100.00 ± 0.00e
400
100.00 ± 0.00e
Hexane
0.0
0.00 ± 0.0a
69.02
(60.68–78.06)225.14
(187.68–284.10)314.78
(253.15–418.36)4.622
(0.202a)Y = −4.15 + 2.24*x
0.999
25
16.00 ± 1.87b
50
36.00 ± 1.87c
100
62.00 ± 2.55d
200
85.00 ± 2.24e
400
100.00 ± 0.00f
Aqueous
0.0
0.00 ± 0.0a
63.76
(56.17–71.92)198.50
(166.68–248.05)273.89
(222.26–359.70)4.041
(0.257a)Y = −4.38 + 2.43*x
0.991
25
18.00 ± 2.55b
50
37.00 ± 2.00c
100
65.00 ± 4.18d
200
90.00 ± 4.18e
400
100.00 ± 0.00f
The results about the larvicidal activities of the tested T. orientalis fruits extracts against the third instar larvae of Cx. pipiens after 24 h are summarized in Table 3 and after 48 h are summarized in Table 4. The analysis of fruits extracts data revealed that the larvicidal activities after 24 h showed 100 % mortalities at 400 ppm of methanol and hexane extracts. While acetone and aqueous fruits extract at 400 ppm showed 98 % and 96 % larval mortalities, respectively. Hexane fruits extract showed the highest efficacy by inducing 100 % mortality (LC50, 68.26, LC90, 201.48, and LC95, 273.84 ppm), followed by methanol extract by 100 % mortality (LC50, 83.21, LC90, 253.85, and LC95, 348.25 ppm). The acetone extract induced 98 % mortality (LC50, 92.81, LC90, 296.34, and LC95, 411.83 ppm). While, the aqueous extract showed the least efficacy as compared to others, inducing 96 % mortality (LC50, 102.97, LC90, 333.60, and LC95, 465.54 ppm) (Table 3). The larvicidal activities of the fruits extracts after 48 h showed 100 % mortalities at 400 ppm for all tested extracts. Hexane extract showed the highest efficacy (LC50, 50.60, LC90, 119.30, and LC95, 152.14 ppm), followed by methanol extract (LC50, 68.62, LC90, 205.88, and LC95, 281.12 ppm), followed by the acetone extract (LC50, 76.43, LC90, 236.40, and LC95 (), 325.58 ppm). While, aqueous showed (LC50, 81.85, LC90, 253.48, and LC95, 349.23 ppm) (Table 4). Significance at 0.05 level between different superscripts. (a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.05. Significance at 0.05 level between different superscripts. (a) In Chi-Square Tests, no heterogeneity factor was used in the calculation of confidence limits because the significance level was greater than 0.05.
Fruit extract
Conc. ppm
Mortality%
(Mean ± SE)LC50
(LCL–UCL.)LC90
(LCL–UCL.)LC95
(LCL–UCL.)Chi
(Sig)Regression equation
R2
Methanol
0.0
0.00 ± 0.0a
83.21
(73.84–93.61)253.85
(212.80–317.49)348.25
(282.48–456.44)5.980
(0.113a)Y = −4.55 + 2.34*x
1.000
25
10.00 ± 1.58b
50
29.00 ± 2.92c
100
55.00 ± 4.74d
200
80.00 ± 3.16e
400
100.00 ± 0.00f
Acetone
0.0
0.00 ± 0.0a
92.81
(82.18–104.78)296.34
(246.13–375.30)411.83
(330.40–547.84)3.894
(0.273a)Y = −5.27 + 2.71*x
0.974
25
9.00 ± 1.00b
50
25.00 ± 1.58c
100
51.00 ± 3.32d
200
76.00 ± 2.92e
400
98.00 ± 1.22f
Hexane
0.0
0.00 ± 0.0a
68.26
(60.49–76.66)201.48
(170.32–249.29)273.84
(224.24–354.73)2.659
(0.447a)Y = −4.68 + 2.54*x
1.000
25
13.00 ± 2.55b
50
36.00 ± 1.00c
100
65.00 ± 4.18d
200
88.00 ± 3.00e
400
100.00 ± 0.00f
Aqueous
0.0
0.00 ± 0.0a
102.97
(91.14–116.45)333.60
(275.53–425.82)465.54
(371.08–624.83)2.596
(0.458a)Y = −5.18 + 2.59*x
0.987
25
7.00 ± 1.22b
50
23.00 ± 2.00c
100
46.00 ± 4.30d
200
73.00 ± 2.00e
400
96.00 ± 1.87f
Fruits extract
Conc. ppm
Mortality%
(Mean ± SE)LC50
(LCL–UCL.)LC90
(LCL–UCL.)LC95
(LCL–UCL.)Chi
(Sig)Regression equation
R2
Methanol
0.0
0.00 ± 0.0a
68.62
(60.74–77.16)205.88
(173.67–255.45)281.12
(229.59–365.45)3.189
(0.363a)Y = −4.56 + 2.47*x
0.999
25
14.00 ± 2.92b
50
34.00 ± 3.67c
100
66.00 ± 4.30d
200
87.00 ± 5.83e
400
100.00 ± 0.00e
Acetone
0.0
0.00 ± 0.0a
76.43
(67.66–86.07)236.40
(198.09–295.92)325.58
(263.83–427.56)5.274
(0.153a)Y = −4.44 + 2.34*x
0.997
25
13.00 ± 3.00b
50
31.00 ± 4.85c
100
57.00 ± 4.66d
200
84.00 ± 4.00e
400
100.00 ± 0.00e
Hexane
0.0
0.00 ± 0.0a
50.60
(45.43–56.07)119.30
(103.29–143.64)152.14
(128.27–190.61)4.305
(0.230a)Y = −5.21 + 3.04*x
0.994
25
18.00 ± 3.39b
50
45.00 ± 3.54c
100
82.00 ± 6.04d
200
100.00 ± 0.00e
400
100.00 ± 0.00e
Aqueous
0.0
0.00 ± 0.0a
81.85
(72.54–92.17)253.48
(212.04–317.98)349.23
(282.50–459.55)6.258
(0.100a)Y = −4.46 + 2.3*x
0.998
25
11.00 ± 1.87b
50
30.00 ± 2.24c
100
54.00 ± 2.92d
200
81.00 ± 3.32e
400
100.00 ± 0.00f
3.2 Chemical analysis
The GC–MS analysis revealed that the main constituents of the T. orientalis leaves extracts were the sesquiterpenoids and terpenoids (Table 5) and (Fig. 1). The main % area for sesquiterpenoids compounds commonly detected in all extracts, were, cedrol (33.03, 35.77, 33.99 and 38.25 %), caryophyllene (18.49, 25.31, 21.68 and 20.67 %) and humulene (10.94, 15.22, 12.28 and 12.35 %) for methanol, hexane, acetone and aqueous leaves extracts, respectively. Followed by, germacrene D (2.80, 4.00, 3.60 and 3.35 %), allocedrol (2.26, 2.22, 2.16 and 2.43 %), α-Eudesmol (1.95, 1.92, 2.91 and 2.58 %), cadina-1(10),4-diene (1.29, 1.67, 1.30 and 1.58 %), selinene (1.27, 1.18, 1.32 and 1.59 %), γ-muurolene (1.17, 1.47, 1.08 and 1.26 %), caryophyllene oxide (1.16, 1.25, 1.39 and 1.29 %), cedrene (0.39, 2.66, 0.60 and 2.33 %) and γ-elemene (0.28, 1.54, 0.62 and 1.52 %) for methanol, hexane, acetone and aqueous leaves extracts, respectively. Also, fatty acid methyl ester, cis-4,7,10,13,16,19-docosahexaenoic acid, methyl ester (1.22, 1.31, 0.29 and 0.97 %) for the same aforementioned extracts, respectively. other compounds detected in three extracts only, pimara-7,15-dien-3-ol (6.04, 1.53 and 0.54 %), α-muurolene (0.29, 1.80 and 0.31 %), alloaromadendrene (1.11, 2.11 and 0.64 %) and butyl 4,7,10,13,16,19-docosahexaenoate (3.52, 1.43 and 3.00 %) where, were detected in methanol, acetone and aqueous leaves extracts, respectively. The retinoid compound 4,14-retro-retinol represented by 1.44, 1.25 and 1.25 area % in hexan, acetone and aqueous leaves extracts, respectively. The compounds, Bornyl acetate (0.39 and 0.44 %), α-terpinyl acetate (0.48 and 0.36 %), β-elemene (0.52 and 1.73 %), 1,7-Di-epi-β-cedrene (2.47 and 2.06 %), valencen (1.05 and 1.44 %), cedarn-8-ol (0.19 and 0.14 %) and α-acorenol (1.20 and 1.64 %) were recorded in methanol and acetone leaves extracts, respectively. Meanwhile, the terpenoid compound, ehydro-4-epiabietol represented by 0.73 and 1.03 area % and the sesquiterpenoid, γ-gurjunene represented by 0.33 and 1.10 area % in acetone and aqueous leaves extracts, respectively. There are compounds were detected only in the methanol leaves extract as 4-hydroxy-3,3′,4-trimethoxystilbene (3.95 %), sugiol (1.49 %), totarol (0.52 %) and copalol (0.51 %). β-Chamigrene and linolenic acid, methyl ester were detected in hexane leaves extract and represented by 1.81 and 1.26 area %, respectively.
No
Molecular formula
Chemical compound
Methanol
(%)Hexan
(%)Acetone
(%)Aqueous (%)
Nature of compound
1
C12H20O2
Bornyl acetate
0.39
–
0.44
–
Bicyclic monoterpenoid
2
C12H20O2
α-Terpinyl acetate
0.48
–
0.36
–
Monoterpene ester monoterpenoid
3
C17H28O2
Elemyl acetate
–
–
0.75
–
Monocyclic monoterpenoid
4
C20H32O
Pimara-7,15-dien-3-ol
6.04
–
1.53
0.54
Terpenoid
5
C20H34O
Copalol
0.51
–
–
–
Terpenoid
6
C20H30O
Sugiol
1.49
–
–
–
Terpenoid
7
C20H30O
Totarol
0.52
–
–
–
Terpenoid
8
C20H30O
Dehydro-4-epiabietol
–
–
0.73
1.03
Terpenoid
9
C15H24
β-Elemene
0.52
–
1.73
–
Sesquiterpenoid
10
C15H24
1,7-Di-epi-β-cedrene
2.47
–
2.06
–
Sesquiterpenoid
11
C15H24
Caryophyllene
18.49
25.31
21.68
20.67
Sesquiterpenoid
12
C15H24
α-Muurolene
0.29
–
1.80
0.31
Sesquiterpenoid
13
C15H24
γ-Elemene
0.28
1.54
0.62
1.52
Sesquiterpenoid
14
C15H24
Humulene
10.94
15.22
12.28
12.35
Sesquiterpenoid
15
C15H24
Cedrene
0.39
2.66
0.60
2.33
Sesquiterpenoid
16
C15H24
Selinene
1.27
1.18
1.32
1.59
Sesquiterpenoid
17
C15H24
Germacrene D
2.80
4.00
3.60
3.35
Sesquiterpenoid
18
C15H24
Valencen
1.05
–
1.44
–
Sesquiterpenoid
19
C15H24
γ-Muurolene
1.17
1.47
1.08
1.26
Sesquiterpenoid
20
C15H24
Cadina-1(10),4-diene
1.29
1.67
1.30
1.58
Sesquiterpenoid
21
C15H24
Alloaromadendrene
1.11
–
2.11
0.64
Sesquiterpenoid
22
C15H24
Cedrenol
–
–
–
0.20
Sesquiterpenoid
23
C15H24
Aromandendrene
–
–
–
1.65
Sesquiterpenoid
24
C15H24O
Caryophyllene oxide
1.16
1.25
1.39
1.29
Sesquiterpenoid
25
C15H26O
Allocedrol
2.26
2.22
2.16
2.43
Sesquiterpenoid
26
C15H26O
Cedrol
33.03
35.77
33.99
38.25
Sesquiterpenoid alcohol
27
C15H26O
Cedran-8-ol
0.19
–
0.14
–
Sesquiterpenoid
28
C15H26O
α -acorenol
1.20
–
1.64
–
Sesquiterpenoid
29
C15H26O
α-Eudesmol
1.95
1.92
2.91
2.58
Cycloeudesmane sesquiterpene
30
C15H24
β-Chamigrene
–
1.81
–
–
Sesquiterpenoid
31
C15H24
γ-Gurjunene
–
–
0.33
1.10
Sesquiterpenoid
32
C19H32O2
Linolenic acid, methyl ester
–
1.26
–
–
Fatty acid methyl ester
33
C23H34O2
cis-4,7,10,13,16,19-Docosahexaenoic acid, methyl ester
1.22
1.31
0.29
0.97
very long-chain fatty acid methyl ester
34
C26H40O2
Butyl 4,7,10,13,16,19-docosahexaenoate
3.52
–
1.43
3.00
very long-chain fatty acid
35
C17H18O4
4-Hydroxy-3,3′,4-trimethoxystilbene
3.95
Stilbene polyphenol
36
C20H30O
4,14-retro-retinol
–
1.44
1.25
1.25
Retinoid
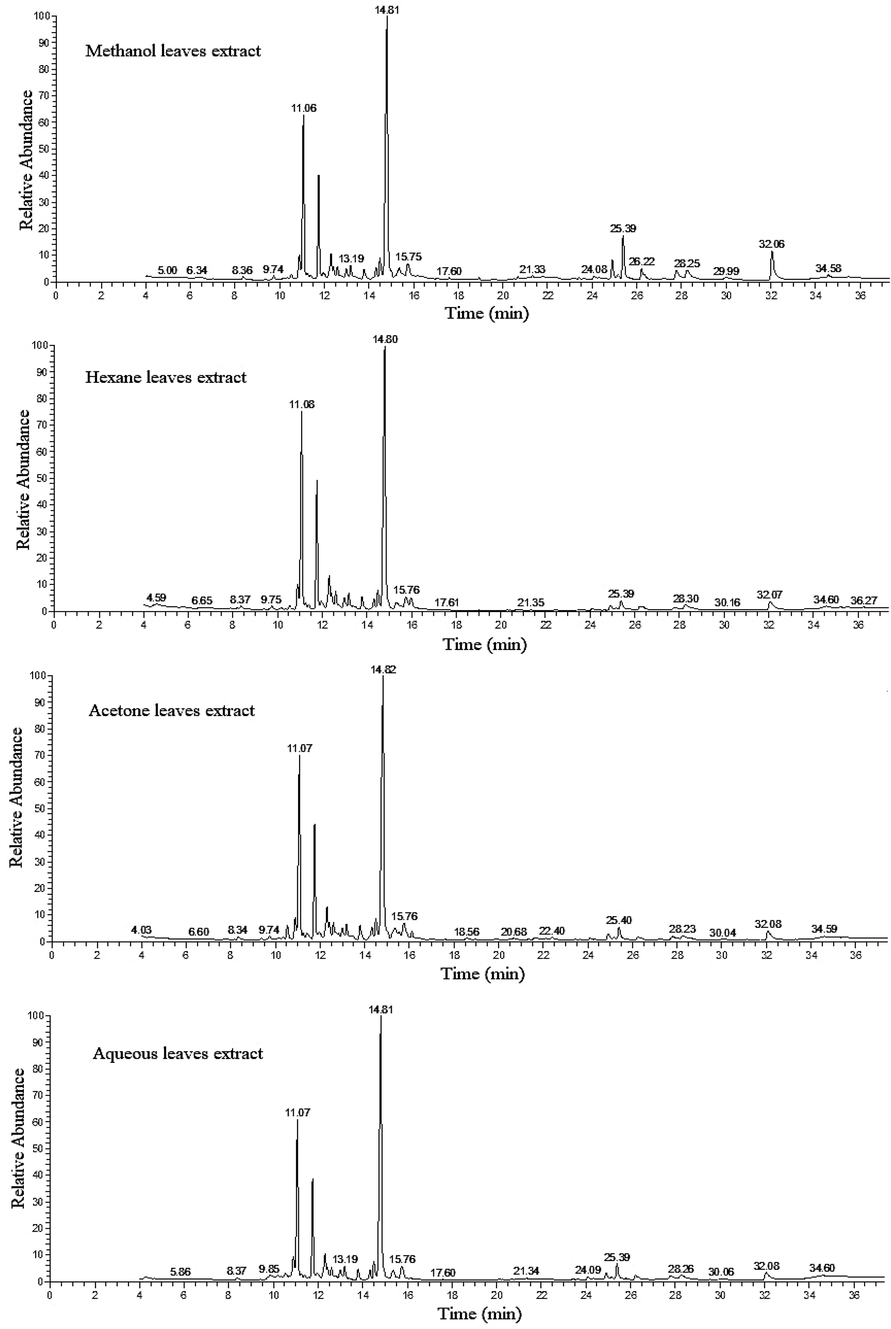
The TIC chromatograms of leaves, methanol, hexane, acetone and aqueous extracts from Thuja orientalis showing chemical constituents separation detected by GC–MS.
Elemyl acetate compound detected in acetone leaves extract only and represented by 0.75 area %. Cedrenol and aromandendrene compounds detected in aqueous leaves extract only and represented by 0.20 and 1.65 area %, respectively.
The GC–MS analysis revealed that the main constituents of the fruits extracts were represented in Table 6 and (Fig. 2). The main commonly sesquiterpenoids compounds in all extracts were, cedrol (21.26, 25.11, 18.55 and 36.66 %) and caryophyllene (17.06, 19.93, 15.21 and 17.04 %) for methanol, hexane, acetone and aqueous fruits extracts, respectively. Followed by the compounds, humuline (9.05, 10.93, 8.62 and 9.06 %), germacrene D (4.93, 5.76, 5.16 and 4.44 %), elemol (4.20, 4.57, 3.42 and 4.05 %), bornyl acetate (3.14, 3.08, 2.48 and 3.35 %), terpinolene (1.14, 2.75, 1.77 and 1.62 %), α-terpinyl acetate (1.10, 0.93, 1.10 and 1.22 %), cedrene (1.45, 1.36, 1.22 and 1.44 %), allocedrol (1.48, 1.18, 1.26 and 1.84 %), β-elemene (1.15, 1.28, 0.86 and 1.07 %), caryophyllene oxide (1.08, 1.14, 1.04 and 1.62 %), selinene (0.60, 0.59, 0.56 and 0.65 %) for methanol, hexane, acetone and aqueous fruits extracts, respectively. Two compounds were detected in three extracts only, labda-8(20),12,14-triene (4.64, 1.80 and 8.02 %) and trachyloban (0.81, 0.63 and 0.52 %), in methanol, hexan and acetone fruits extracts, respectively. Alloaromadendrene recorded in methanol and acetone fruits extracts by 0.48 and 0.53 area%, respectively. While, γ-elemene detected in methanol, hexane and aqueous extracts by 0.51, 0.53 and 0.42 area %, respectively. Isopimaral and olic acid detected in hexan and acetone extracts by 5.64, 5.64 and 2.02 and 0.76 area %, respectively. cis-5,8,11,14,17-eicosapentaenoic acid, methyl ester represented by 7.11 and 3.48 area %, i-Propyl 7,10,13,16,19-docosapentaenoate represented by 2.26 and 2.26 area %, arachidonic acid methyl ester represented by 0.58 and 1.13 area %, 6,9,12,15-docosatetraenoic acid, methyl ester represented by 0.63 and 1.89 area %, dodecanoic acid, 2,3-bis(acetyloxy)propyl ester represented by 0.76 and 0.42 area %, and in methanol and aqueous extracts, respectively. β-Eudesmol compound detected in hexane and aqueous fruits extracts and represented by by 0.80 and 0.70 area %, respectively.
No
Molecular formula
Chemical compound
Methanol (%)
Hexan (%)
Acetone (%)
Aqueous (%)
Nature of compound
1
C10H16
Terpinolene
1.14
2.75
1.77
1.62
Menthane monoterpenoid
2
C10H16
2-Carene
1.00
–
–
–
Bicyclic monoterpenoid
3
C20H32
Labda-8(20),12,14-triene
4.64
1.80
8.02
Terpenoid
4
C21H32O2
Labda-8(20) 12 14-triene-19-oic acid methyl ester (z)-
–
2.95
–
–
Terpenoid
5
C21H34O
Labda-8(20),14-dien-13-ol, (13S)-
–
–
–
1.62
Terpenoid
6
C20H32
(-)-Atisirene
0.42
–
–
–
Terpenoid
7
C20H32
Trachyloban
0.81
0.63
0.52
–
Terpenoid
8
C12H20O2
Bornyl acetate
3.14
3.08
2.48
3.35
Bicyclic monoterpenoid
9
C12H20O2
α-Terpinyl acetate
1.10
0.93
1.10
1.22
Monoterpene ester
10
C20H28O6
16-Hydroxyingenol
0.45
–
–
–
Terpenoid
11
C20H30O2
Communic Acid
0.93
–
–
–
Terpenoid
12
C20H32O
Pimara-7,15-dien-3-ol
–
–
1.46
–
Terpenoid
13
C23H38O2Si
Pimaric acid TMS derivative
–
–
8.43
–
Terpenoid
14
C23H38O2Si
Isopimaric acid TMS ester
–
–
–
–
Terpenoid
15
C20H30O
Isopimaral
–
5.64
5.64
–
Terpenoid
16
C20H34O
Copalol
–
1.05
–
–
Terpenoid
17
C20H30O
Totarol
–
–
–
–
Terpenoid
18
C20H30O
Dehydro-4-epiabietol
–
–
–
–
Terpenoid
19
C21H32O2
Isopimaric acid, methyl ester
–
1.00
–
–
Terpenoid
20
C15H24
β-Elemene
1.15
1.28
0.86
1.07
Sesquiterpenoid
21
C15H24
γ-Elemene
0.51
0.53
–
0.42
Sesquiterpenoid
22
C15H24
δ-EIemene
0.68
–
–
–
Sesquiterpenoid
23
C15H24
1,7-Di-epi-β-cedrene
–
–
–
Sesquiterpenoid
24
C15H24
Caryophyllene
17.06
19.93
15.21
17.04
Sesquiterpenoid
25
C15H24
Humulene
9.05
10.93
8.62
9.06
Sesquiterpenoid
26
C15H24
Cedrene
1.45
1.36
1.22
1.44
Sesquiterpenoid
27
C15H24
Selinene
0.60
0.59
0.56
0.65
Sesquiterpenoid
28
C15H24
Germacrene D
4.93
5.76
5.16
4.44
Sesquiterpenoid
29
C15H24
Isogermacrene D
0.31
–
–
–
Sesquiterpenoid
30
C15H24
Valencen
–
–
–
1.05
Sesquiterpenoid
31
C15H24
Cadina-1(10),4-diene
0.47
–
–
–
Sesquiterpenoid
32
C15H24
Alloaromadendrene
0.48
–
0.53
–
Sesquiterpenoid
33
C15H24
Chamigrene
–
0.61
–
–
Sesquiterpenoid
34
C20H32
Cubenol
–
–
–
1.48
Sesquiterpenoid
35
C15H24O
Caryophyllene oxide
1.08
1.14
1.04
1.62
Sesquiterpenoid
36
C15H26O
Allocedrol
1.48
1.18
1.26
1.84
Sesquiterpenoid
37
C15H26O
Cedrol
21.26
25.11
18.55
36.66
Sesquiterpenoid alcohol
38
C15H26O
α-Eudesmol
0.64
–
–
–
Cycloeudesmane sesquiterpene
39
C15H26O
β-Eudesmol
–
0.80
–
0.70
Cycloeudesmane sesquiterpene
40
C15H26O
Elemol
4.20
4.57
3.42
4.05
Sesquiterpenoid
41
C17H18O4
1-Heptatriacotanol
0.95
–
–
–
Fatty alcohol
42
C18H34O2
Oleic Acid
–
2.02
0.76
–
Fatty acid
43
C18H30O2
10-Heptadecen-8-ynoic acid, methyl ester,(E)-
–
–
–
0.49
Fatty acid methyl ester
44
C19H34O6
Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester
0.76
–
–
0.42
Lauric fatty acid ester with hydroxypropanediyl diacetate
45
C19H30O2
13,16-Octadecadiynoic acid, methyl ester
3.24
–
–
–
Fatty acid methyl ester
46
C19H32O2
6,9,12-Octadecatrienoic acid, methyl ester
–
–
0.62
Fatty acid methyl ester
47
C21H34O2
Arachidonic acid methyl ester
0.58
–
–
1.13
Fatty acid methyl ester
48
C21H32O2
cis-5,8,11,14,17-Eicosapentaenoic acid, methyl ester
7.11
–
–
3.48
Fatty acid methyl ester
49
C21H36O2
Linolenic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (Z,Z,Z)-
0.29
–
–
–
Fatty acid ethyl ester
50
C23H36O2
7,10,13,16,19-docosapentaenoic acid, methyl ester
–
–
–
0.81
Fatty acid ethyl ester
51
C22H32O2
Doconexent
1.42
–
–
–
very long-chain fatty acid
52
C23H34O2
cis-4,7,10,13,16,19-Docosahexaenoic acid
0.84
–
–
–
very long-chain fatty acid
53
C25H40O2
i-Propyl 7,10,13,16,19-docosapentaenoate
2.26
–
–
2.26
very long-chain fatty acid
54
C23H38O2
6,9,12,15-Docosatetraenoic acid, methyl ester
0.63
–
–
1.89
very long-chain fatty acid methyl ester
55
C21H36O4
9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z,Z,Z)-
0.59
–
–
1.24
1-monoglyceride derives from α-linolenic acid
56
C21H38O2Si
α-Linolenic acid, TMS derivative
–
–
12.23
–
Fatty acid trimethyl ester
57
C23H38O2Si
Eicosapentaenoic acid, TMS derivative
–
–
1.81
–
Fatty acid trimethyl ester
58
C21H34
Androst-5-ene, 4,4-dimethyl-, (13à)-
1.85
–
–
–
Steroid
59
C21H34O2
Androstan-17-one, 3-ethyl-3-hydroxy-, (5à)-
–
0.60
–
–
Steroid
60
C27H42O3
Pseduosarsasapogenin-5,20-dien
0.54
Steroidal saponin
61
C17H18O4
4-Hydroxy-3,3′,4-trimethoxystilbene
Stilbene polyphenol
62
C20H30O
4,14-retro-retinol
1.45
1.71
Retinoid
63
C20H28O6
Dotriacontane
0.45
Alkane
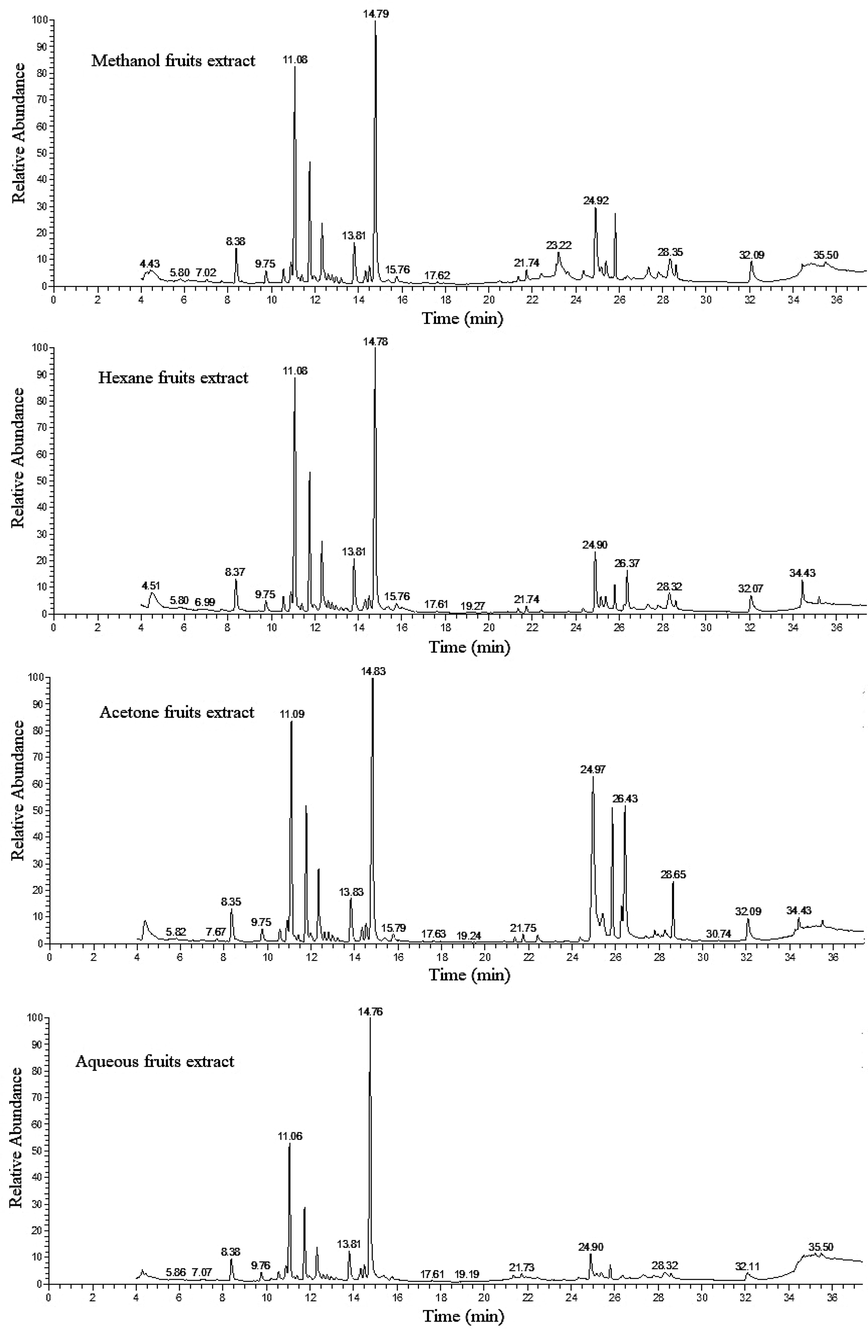
The TIC chromatograms of fruits, methanol, hexane, acetone and aqueous extracts from Thuja orientalis showing chemical constituents separation detected by GC–MS.
There are 15 compounds detected only in methanol fruits extract only which were, 13,16-octadecadiynoic acid, methyl ester (3.24 %), Androst-5-ene, 4,4-dimethyl-, (13à)- (1.85), doconexent (1.42 %), 2-Carene (1.00 %), 1-heptatriacotanol (0.95 %), communic acid (0.93 %), cis-4,7,10,13,16,19-docosahexaenoic acid (0.84 %), δ-eIemene (0.68 %), α-eudesmol (0.64 %), Pseduosarsasapogenin-5,20-dien (0.54 %), Cadina-1(10),4-diene (0.47 %), 16-hydroxyingenol (0.45), (-)-Atisirene (0.42 %), isogermacrene D (0.31 %), linolenic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester (Z,Z,Z)-(0.29 %). Six compounds detected only in hexane fruits extract which were, labda-8(20) 12 14-triene-19-oic acid methyl ester (z)-(2.95 %), copalol (1.05 %), isopimaric acid, methyl ester (1.00 %), chamigrene (0.61 %), Androstan-17-one, 3-ethyl-3-hydroxy-, (5à)-(0.60 %) and Dotriacontane (0.45 %). Four compounds detected only in acetone fruits extract were, α-linolenic acid, TMS derivative (12.23 %), pimaric acid TMS derivative (8.43 %), eicosapentaenoic acid, TMS derivative (1.81 %) and pimara-7,15-dien-3-ol (1.46 %). While, in aqueous fruits extract there are six compounds detected which were, labda-8(20),14-dien-13-ol, (13S)- (1.62 %), valencen (1.05 %), cubenol (1.48 %), 7,10,13,16,19-docosapentaenoic acid, methyl ester (0.81 %), 6,9,12-octadecatrienoic acid, methyl ester (0.62 %) and 10-heptadecen-8-ynoic acid, methyl ester,(E)- (0.49 %) (Table 6) and (Fig. 2).
4 Discussion
Generally the study reviled larvicidal activities of T. orientalis leaves and fruits extracts in a concentration dependent manner whatever the activities varied between leaves and fruits tested extracts. Leaves extracts ordered according to larval toxicity as acetone > methanol > aqueous > hexane. While, fruits extracts ordered as hexane > methanol > acetone > aqueous. Collectively acetone T. orientalis leaves extract showed throughout the tested extracts, the most effective larvicidal activity against C. pipiens larvae (100 % mortality) and (LC50, 58.04 ppm LC90, 169.27 ppm LC50, 229.27 ppm) at concentration 400 ppm.
Different Plant extracts previously tested for their larvicidal activities against mosquitoes, El-Sheikh et al. (2012) tested the larval toxicity of ethanol, acetone and petroleum ether extracts of Tribulus terrestris leaves, against the third instar larvae Ae. aegypti and predicted dose dependent larvicidal activities for the tested extracts in addition to variation of larvicidal activities related to solvent used. Where, crude plant extracts showed previously more efficiency in controlling mosquitoes over the purified compounds toxicity, which in line with the present study results (Ghosh et al., 2012). Another study estimated larval toxicity of leaves aqueous extracts in three tested concentrations from Ricinus communis L. (0.06, 0.12 and 0.2 g/l), Daphne gnidium L (0.09, 0.18 and 0.3 g/l) and Thymus vulgaris L. (0.0225, 0.045 and 0.09 g/l), against early instar larvae of Cx. pipiens and Cs. Longiareolata and showed in line with the present study results that the larval mortality increased with exposure times (24, 48 and 72 h), and the LC50 recorded values decreased in the same manner (Dahchar et al., 2016).
The chemical compounds detected in the extracts in line with that detected in previous studies, however, there were differences in the amount or number of the main components (Elsharkawy et al., 2017; Guleria et al., 2008; Nickavar et al., 2003; Ololade et al., 2014; Sanei-Dehkordi et al., 2018).
The common sesquiterpenoids highly represented as % areas in the chromatograms of all tested leaves and fruits extracts were, Cedrol, Caryophyllene, Humulene, Germacrene D and Elemol (common only in fruits extracts). The medium common ones were, γ-Elemene, Cedrene, Selinene, γ-Muurolene, Cadina-1(10),4-diene, Caryophyllene oxide, Allocedrol, α-Eudesmol and the fatty acid methyl ester, cis-4,7,10,13,16,19-Docosahexaenoic acid, methyl ester in leaves extracts and bornyl acetate, α-Terpinyl acetate, Cedrene, β-Elemene, Selinene and Caryophyllene oxide were medium common in fruits extracts. The observed larvicidal activities of the tested extracts may related to their chemical constituents synergistic actions, either the major or minor ones that may impact on the predicted larvicidal activities (Huong et al., 2020).
The essential oil P. orientalis L. (Family Cupressaceae) oil showed insecticide and molluscicidal activity (Hashemi and Safavi 2012; Ju-Hyun et al., 2005; Lei, et al., 2010). Thuja orientalis previously acquired cytotoxic principles and contained terpenoids including pimaric and isopimaric acids, fatty acids, aliphatic compounds like alkanes and bioflavonoids (Mehta et al., 1999). The essential oil extracted from T. orientalis leaves predicted larvicidal activity against Anopheles stephensi and Culex pipiens late third or young 4th instar larvae, where carene and cedrol were from the main constituents of the extract (Sanei-Dehkordi et al., 2018). Also extracts predicted diterpenes and labdane-type diterpenes in line with the previously recorded (Kim et al 2012; Kim et al., 2013). Cedrol the main constituent in the tested extracts was postulated as alternative to conventional synthetic acaricides for the black-legged ticks, Ixodes scapularis Say which is a human vector causing disease (Eller et al., 2014). Caryophyllene oxide and germacrene D individual compounds previously showed potent larvicidal activities against A. anthropophagus (Zhu and Tian, 2013). Also β-elemene and α-humulene showed larvicidal activities against A. subpictus, Ae albopictus, and Cx. tritaeniorhynchus (Govindarajan and Benelli, 2016). Jeon et al. (2005) showed that essential oils extracted from P. orientalis had strong activities on mosquito larvae Cx. pipiens and Ae. aegypti recording 100 % mortality at 400 ppm. Furthermore, Sanei-Dehkordi et al. (2018) verified the high efficiency of P. orientalis oil against A. stephensi (LC50, 11.67 ppm) and Cx. pipiens (LC50, 18.60 ppm). Ethanol and acetone leaves extracts of T. orientalis previously evaluated larvicidal activities against mosquitoes recording LC50, 13.10 and 200.87 ppm after 24 h and 9.02 and 127.53 ppm after 48 h, against A. stephensi third instar larvae, respectively. Besides recording against C. quinquefasciatus third instar larvae, LC50, 22.74 and 69.03 ppm after 24 h and 16.72 and 51.14 ppm after 48 h, respectively (Sharma et al., 2005).
In addition and in accordance with the present results behavior, hexane plant parts and seeds extracts of Physalis angulate, Peganum harmala, Tecrium polium and Thymus vulgaris were evaluated for their larvicidal potentials against the fourth instar larvae of Culex pipiens molestus, and showed that mortality increased with exposure times (24 and 48 h) and the LC50 recorded values decreased with exposure times (Mekhlif and Muhammad, 2021). A study tested larvicidal activities leaves oils extracts against A. aegypti and recorded that the main components in leaves of G. blepharophylla was the caryophyllene oxide, while, in G. friesiana were α-,β- and γ-eudesmols and in G. hispida, was (E)-caryophyllene. According to the predicted results oil extracted from G. friesiana recorded the best larvicidal effect against A. aegypti and hypothesized that sesquiterpenes in oils can reflect more controlling activity as compared to monoterpenes (Aciole et al., 2011). The aforementioned study results may declare the predicted advantage of acetone leaves extract results where the extract acquired highest content of eudesmol as compared to other extracts.
5 Conclusion
The leaves and fruits methanol, hexane, acetone and aqueous extracts of T. orientalis reviled high larvicidal potential at 400 ppm concentration against the third instar larvae of Cx. pipiens with the advantage of leaves acetone extract that predicted the lowest effective larval toxicity concentration. Further studies about the mode of the larvicidal action of the extracts should be under investigation.
Authorship contribution
Author declare her acknowledgment to the Center for Environmental Research and Studies at Jazan University for the technical support.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
Ethics approval and consent to participate
Not applicable.
Acknowledgments
Author declare her acknowledgment to the Center for Environmental Research and Studies at Jazan University for the technical support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A method of computing the effectiveness of an insecticide. J. Econ. Entomol.. 1925;18(2):265-267.
- [CrossRef] [Google Scholar]
- Insecticidal activity of three species of Guatteria (Annonaceae) against Aedes aegypti (Diptera: Culicidae) Rev. Colomb. Entomol.. 2011;37:262-268.
- [Google Scholar]
- Ahmed, N., Alam, M., Saeed, M., Ullah, H., Iqbal, T., Al-Mutairi, K.A., Shahjeer, K., Ullah, R., Ahmed, S., Ahmed, N.A.A.H., Khater, H.F., Salman, M., 2021. Botanical Insecticides Are a Non-Toxic Alternative to Conventional Pesticides in the Control of Insects and Pests, in: Global Decline of Insects. IntechOpen. https://doi.org/10.5772/intechopen.100416.
- Thuja occidentalis L. (Cupressaceae): Ethnobotany, phytochemistry and biological activity. Molecules. 2020;25(22):5416.
- [CrossRef] [Google Scholar]
- Growth suppression of a gingivitis and skin pathogen Cutibacterium (Propionibacterium) acnes by medicinal plant extracts. Antibiotics (Basel, Switzerland). 2021;10(9):1092.
- [CrossRef] [Google Scholar]
- Larvicidal activity of some plant extracts against two mosquito species Culex pipiens and Culiseta longiareolata. J. Entomol. Zool. Stud.. 2016;4:346-350.
- [Google Scholar]
- Chemical profiling and identification of anti-inflammatory biomarkers of oriental Thuja (Platycladus orientalis) using UPLC/MS/MS and network pharmacology-based analyses. Nat. Prod. Res.. 2021;1–5
- [CrossRef] [Google Scholar]
- Bioactivity of cedarwood oil and cedrol against arthropod pests. Environ. Entomol.. 2014;43(3):762-766.
- [CrossRef] [Google Scholar]
- Effect of drought condition of North Region of Saudi Arabia on accumulation of chemical compounds, antimicrobial and larvicidal activities of Thuja Orientalis. Orient. J. Chem.. 2019;35(2):738-743.
- [Google Scholar]
- Comparative study of antioxidant and anticancer activity of Thuja orientalis growing in Egypt and Saudi Arabia. Br. J. Pharm. Res. 2017;15:1-9.
- [CrossRef] [Google Scholar]
- Insecticidal and repellent activities of methanolic extract of Tribulus terrestris L. (Zygophyllaceae) against the malarial vector Anopheles arabiensis (Diptera: Culicidae). Egypt. Acad. J. Biol. Sci. A, Entomol.. 2012;5:13-22.
- [Google Scholar]
- “Bird biting” mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infect. Genet. Evol.. 2011;11:1577-1585.
- [CrossRef] [Google Scholar]
- Plant extracts as potential mosquito larvicides. Indian J. Med. Res.. 2012;135(5):581-598. PMID: 22771587
- [Google Scholar]
- α-Humulene and β-elemene from Syzygium zeylanicum (Myrtaceae) essential oil: highly effective and eco-friendly larvicides against Anopheles subpictus, Aedes albopictus, and Culex tritaeniorhynchus (Diptera: Culicidae) Parasitol. Res.. 2016;115:2771-2778.
- [CrossRef] [Google Scholar]
- Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res.. 2016;115(2):807-815.
- [CrossRef] [Google Scholar]
- Chemical composition and fungitoxic activity of essential oil of Thuja orientalis L. grown in the north-western Himalaya. Zeitschrift für Naturforsch. C. 2008;63:211-214.
- [CrossRef] [Google Scholar]
- Chemical constituents and toxicity of essential oils of oriental arborvitae, Platycladus orientalis (L.) Franco, against three stored-product beetles. Chil. J. Agr. Res.. 2012;72(2):188-194.
- [CrossRef] [Google Scholar]
- Mosquito larvicidal activity of the essential oil of Zingiber collinsii against Aedes albopictus and Culex quinquefasciatus. J. Oleo Sci.. 2020;69(2):153-160.
- [CrossRef] [Google Scholar]
- Larvicidal activity of Chamaecyparis obtusa and Thuja orientalis leaf oils against two mosquito species. J. Appl. Biol. Chem.. 2005;48(1):26-28.
- [Google Scholar]
- Larvicidal activity of Chamaecyparis obusta and Thuja orientalis leaf oils against two mosquito species. Agric. Chem. Biotech.. 2005;48(1):26-28.
- [Google Scholar]
- Three new diterpenoids from the leaves of Thuja orientalis. Planta Med.. 2012;78:485-487.
- [CrossRef] [Google Scholar]
- Kim, T.-H., Li, H., Wu, Q., Lee, H.J., Ryu, J.-H., 2013. A new labdane diterpenoid with anti-inflammatory activity from Thuja orientalis. J. Ethnopharmacol. 146, 760–767. https://doi.org/https://doi.org/10.1016/j.jep.2013.02.001.
- Composition and variability of essential oils of Platycladus orientalis growing in China. Biochem. Syst. Ecol.. 2010;38(5):1000-1006.
- [CrossRef] [Google Scholar]
- Identification of novel compounds from Thuja orientalis (leaves) Indian J. Chem. - B Org Med. Chem.. 1999;38(8):1005-1008.
- [Google Scholar]
- Larvicidal potentials of four medicinal plant extracts on mosquito vector, Culex pipiens molestus (Diptera: Culicidae) Int. J. Mosq. Res.. 2021;8(4):01-05.
- [Google Scholar]
- Towards eco-friendly crop protection: natural deep eutectic solvents and defensive secondary metabolites. Phytochem. Rev.. 2017;16:935-951.
- [CrossRef] [Google Scholar]
- Volatile constituents of the fruit and leaf oils of Thuja orientalis L. grown in Iran. Zeitschrift für Naturforsch.. 2003;C 58(3–4):171-172.
- [CrossRef] [Google Scholar]
- Antioxidant activity of water and alcohol extracts of Thuja orientalis leaves. Adv. Tradit. Med.. 2007;7:65-73.
- [CrossRef] [Google Scholar]
- Phytochemical and Therapeutic Studies of the Fruit essential oil of Thuja orientalis from Nigeria. Glob. J. Sci. Front. Res. B Chem. 2014;14(7):14-20.
- [Google Scholar]
- Essential oil composition and larvicidal evaluation of Platycladus orientalis against two mosquito vectors, Anopheles stephensi and Culex pipiens. J. Arthropod. Borne. Dis.. 2018;12(2):101-107.
- [Google Scholar]
- Larvicidal potential of Nerium indicum and Thuja oriertelis extracts against malaria and Japanese encephalitis vector. J. Environ. Biol.. 2005;26(4):657-660.
- [Google Scholar]
- Thuja orientalis reduces airway inflammation in ovalbumin-induced allergic asthma. Mol. Med. Rep.. 2015;12:4640-4646.
- [CrossRef] [Google Scholar]
- Biological properties of Thuja orientalis Linn. Adv. Life Sci.. 2012;2:17-20.
- [CrossRef] [Google Scholar]
- Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization 2005:1-41. Retrieved from
- [Google Scholar]
- WHO. (2013). Larval source management: a supplementary malaria vector control measure, 1–2. https://apps.who.int/iris/bitstream/handle/10665/85379/9789241505604_eng.pdf.
- The importance of vector control for the control and elimination of vector-borne diseases. PLoS Negl. Trop. Dis.. 2020;14:e0007831.
- [Google Scholar]
- Hair growth-promoting activity of hot water extract of Thuja orientalis. BMC Complement. Altern. Med.. 2013;13:1-12.
- [CrossRef] [Google Scholar]
- Chemical composition and larvicidal activity of essential oil of Artemisia gilvescens against Anopheles anthropophagus. Parasitol. Res.. 2013;112(3):1137-1142.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102396.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







