Translate this page into:
Larvicidal activity of Artemisia absinthium extracts with special reference to inhibition of detoxifying enzymes in larvae of Aedes aegypti L
⁎Corresponding author. animanandabiomed@gmail.com (Anima Nanda) animananda72@gmail.com (Anima Nanda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Insect-borne diseases continue to be a major source of sickness and death worldwide. Resistance to chemical pesticides and their risks have been regarded as a setback in mosquito vector control. Due to the presence of various phytocompounds in plant species, botanicals can manage and prevent vector (insect) transmitted illnesses by killing insect eggs and larvae. The purpose of this study was to determine the toxicity of Artemisia absinthium extracts (methanolic and ethanolic) against Aedes aegypti larvae (vector of arboviruses). Larval mortality was recorded after 24 h of exposure. Our findings showed that the ethanolic leaf extract of Artemisia absinthium has higher larvicidal activity (92 ± 1.99 percent at 1000 ppm) than the methanolic extract (68 ± 1.99 percent at 1000 ppm). Furthermore, the ethanolic extract inhibited the activity of detoxifying enzymes acetylcholinesterase, α and β-carboxylesterases activity significantly. Besides, the protein level was also lowered upon exposure of ethanolic extract for 24 h against 4th instar larvae at an LC50 of 694.3 ppm. These findings clearly show that the Artemisia absinthium active extract (ethanolic) can be utilized as a pest and vector control option, helping to prevent the spread of infectious illnesses.
Keywords
Medicinal plants
Artemisia absinthium
Larvicidal activity
Aedes aegypti
Biochemical marker enzymes
GC–MS
- CBT
-
Centre for biodiversity and taxonomy
- BSA
-
Bovine serum albumin
- AChE
-
Acetylcholinesterase
- OD
-
Optical density
- RT
-
Room temperature
- DTNB
-
5-5-dithiobis 2-nitro benzoic acid
- ACh
-
acetylcholine
- TSM
-
Thermo Scientific Multiskan
- LC
-
Lethal concentration
- CoA
-
Coenzyme A
Abbreviations
1 Introduction
Mosquitoes, the major arthropods, are lethal disease carriers and a severe menace to millions of people worldwide. They serve as vectors for fatal parasites and diseases such as filariasis, dengue fever, malaria, West Nile Virus, yellow fever, and Chikungunya (Ramos et al., 2019; WHO, 2020; WHO, 2014). Mosquito-borne illnesses damage human and animal life and have a significant economic effect, particularly in nations with tropical or subtropical climates that are hotspots for these types of epidemics or pandemics (Liu et al., 2021). Aedes mosquitoes, in particular, are worrying in terms of public health since they are the primary vectors of arboviruses such as malaria, filariasis, dengue, chikungunya, and Zika, which cause fatal viral illnesses (Matthews et al., 2018). Synthetic chemical pesticides have long been used to control mosquitoes. Still, their indiscriminate use has resulted in well-known and significant issues, such as genetic resistance, rising application costs, handling risks, and environmental contamination (Bharathithasan et al., 2021). This has prompted the need to study and develop ecologically safe, biodegradable, and low-cost indigenous vector control methods that individuals and communities may employ with little care in certain situations.
Plant products are emerging as a viable alternative to synthetic pest management/control chemicals. Phytochemicals from different chemical classes, including steroids, alkaloids, terpenes, and phenolic components, have previously been studied for pest control potential and have shown to be promising agents (Senthil-Nathan, 2020). Artemisia absinthium, commonly known as wormwood is an important medicinal plant of ethnopharmacological interest. It is indigenous to temperate regions of Europe, South Africa, North America, and Asia. This aromatic has been extensively studied for its antifungal, antimicrobial, and antiprotozoal properties. Traditionally, its leaf powder is used to treat gastrointestinal disorders and intestinal worms (Liu et al., 2019; Rizvi et al., 2018). The capacity to control insects varies with plant age, species, extracted component, collecting place, and extraction solvent. Thus the present study is aimed at investigating the larvicidal activity of Artemisia absinthium leaf extracts against the 4th instar larvae of Aedes aegypti and its detoxifying enzymes under laboratory conditions.
2 Materials & methods
2.1 Chemicals
Methanol, ethanol Fast blue –B salt and ACh (Iodide) were procured from Hi-media (Mumbai). α- and β-naphthol and DTNB were purchased from Sisco Research Laboratories (Mumbai, India). All other chemicals and glassware used were of analytical grade.
The Artemisia absinthium plant was collected from the Daksum area of Anantnag, Jammu and Kashmir, India at an altitude of 2438 m above sea level (Fig. 1 and Fig. 2). The identification and authenticity process of the plant was completed in the Kashmir University (CBT-botany) vide voucher specimen number 3070-(KASH) Herbarium. The leaves of the plant were shade dried in hygienic conditions for at least fifteen days. Thereafter the leaves were crushed into a coarse powder in an electrical grinder machine and packaged carefully for further processing.
Artemisia absinthium plant.
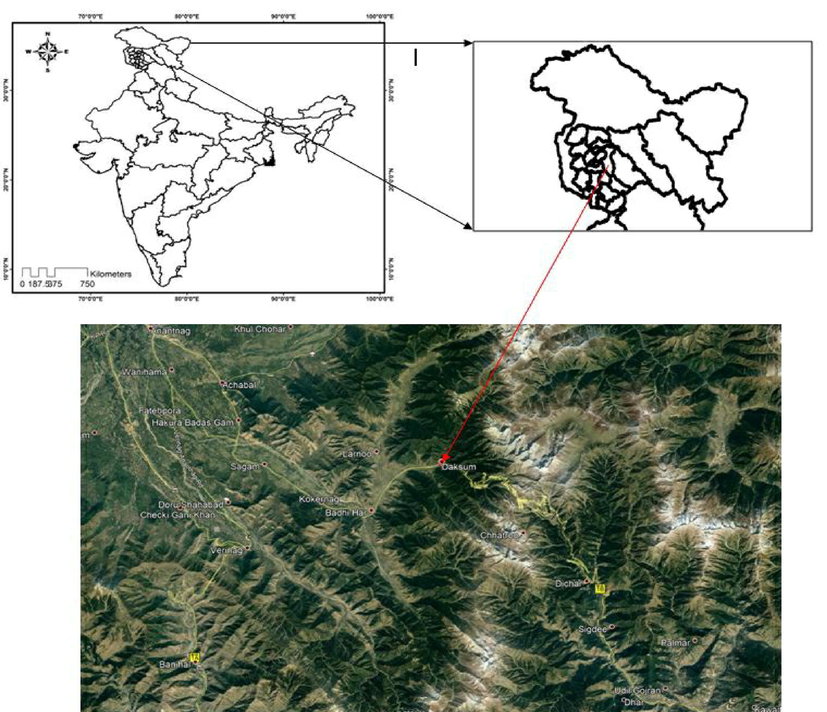
Collection site Daksum Anantnag Jammu and Kashmir, India. Latitude 33°36′43″N & longitude 75°26′6″E.
2.2 Extraction process
A simple maceration process was employed for the extraction of the plant material. 10 g of coarse powder was mixed with 100 ml of methanol and ethanol separately in a 250 ml flask and kept the mixture on a shaker for 24 h. This was followed by filtration of the reaction mixture using Whatman filter paper No1. The filtrate so collected was left for evaporation of the solvent to obtain a concentrated mass. The procedure was repeated three times to get the desired quantity and quality of the sample for further analysis. The total extraction yield percentage was calculated by the following formula.
2.3 GC–MS analysis
The phytochemical profiling was done was done according to (Vasantharaja et al., 2019) using GC–MS Shimadzu-QP2010 Ultra analyzer, with ion source temperature and interface temperature as 240 °C and 250 °C, respectively. The column oven temperature was initially programmed from 80 °C to 200 °C at a rate of 3 °C/minute, then to 260 °C at a rate of 10 °C/minute, maintained for 5 min. The sample was injected in a split mode into the capillary column, which contains helium as a carrier gas, with a column flow rate of 1 ml/minute, and run time was 54 min.
2.4 Mosquito larvicidal bioassay
Mosquito larvae were collected from residential areas of Chennai, Tamil Nadu (Fig. 3). The early fourth instar larvae were chosen for the larvicidal bioassay, which was carried out according to the (WHO standard with slight modifications, 1996). In brief, batches of ten early fourth instar larvae were placed in 100 ml of tap water containing each solvent extract at final concentrations of 200, 400, 600, 800, and 1000 ppm. As a control, ordinary tap water with 1 % DMSO was utilized. The larvae were fed a 3:1 combination of dog biscuits and yeast and kept in the laboratory at a temperature of 27 2 °C and an ideal photoperiod (12 h light:12 h dark). The larvicidal bioassays were replicated five times, and the percentage of larvae death was calculated after 24 h of exposure.
Aedes aegypti larvae.
2.5 Preparation of larval whole body homogenates
The control and Artemisia absinthium extract-treated (LC50) larvae were taken separately and washed with double distilled water. The water content from the body surface was removed by blotting with tissue paper. The larvae of ten individuals were homogenized in eppendorf tubes (held in crushed ice) using a Teflon hand homogenizer containing 0.5 ml of ice-cold 20 mM phosphate buffer (pH 7.2). The whole body homogenates were centrifuged at 10,000×g in 4 °C for 15 min, and the clear supernatants were used for the quantitative analysis. The homogenates were stored at −20 °C until use.
2.6 Total protein
The total protein in larval homogenate (treated with plant extract) and control was calculated by following the method described by (Lowry et al., 1951), using BSA as a standard.
2.7 Acetylcholinesterase assay
The activity of AChE in the larval homogenate was determined using acetylcholine iodide as a substrate, as described by (Ellman et al., 1961), with a minor modification by (Parthiban et al., 2019). After mixing 50uL of larval homogenate into 800 µL of 100 mM Na3PO4 buffer (pH 7.5), the experimental mixture was mixed with 50 µL of 10 mM DTNB and 50 µL of 12.5 mM cholinergic iodide and incubated at room temperature for 5 min. The OD of the prepared sample was measured against a blank at a wavelength of 405 nm.
2.8 Carboxylesterase assays
The determination of α and β-carboxylesterase activity from larval homogenate was performed using (Van Asperen's, 1962) method. 50 µL of the larval homogenate was put into 1 ml of 100 mM Na3PO4 buffer (pH 7.0) containing 250 µM of α and β-naphthyl acetate for the appropriate tests and incubated for 30 min at room temperature. To stop the enzymatic activity, 400 µL fast blue B salt (0.3 %) in SDS (3.3 %) was added to each reaction mixture and left for 15 min at room temperature for color development. The OD of the sample was measured against a blank (with TSM. EX-200–240 V) at 430 nm (for α-carboxylesterase) and 588 nm (for β-carboxylesterase). The carboxylesterase activity was determined by a standard curve formed by using α- and β-naphthol standard.
3 Results
Analysis of the mass Spectra of the obtained phytocompounds through gas chromatographic separation by comparing with the references in the Wiley and NIST led to the identification of 11 different bioactive phytocompounds (as shown in Fig. 4 and Table 1). Out of these, 3 compounds were dominant with a total percentage of 77.66 %. Concentration of Tris(2,4-di-tert-butylphenyl) phosphate (43.86 %) was highest, followed by Cycloisolongifolene,8,9-dehydro-9-formyl- (25.50 %), and 9,12,15-Octadecatrienoic acid, methyl ester, (8.30 %).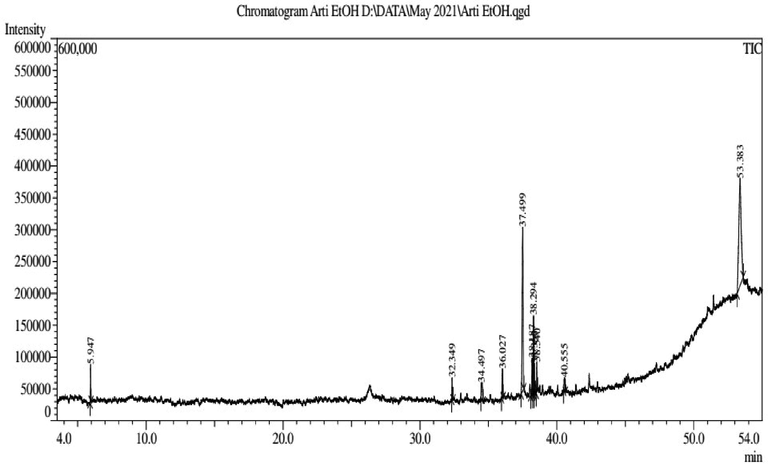
Chromatogram of ethanolic leaf extract of Artemisia absinthium.
Peak#
R.Time
Area
Area%
Name
1
5.947
118,274
2.59
Silicic acid (h4sio4), tetraethyl ester
2
32.349
99,418
2.18
Neophytadiene
3
34.497
133,906
2.93
1,3b,6,6-tetramethyldecahydro-1 h-cyclopropa[7,8]azulen
4
36.027
147,362
3.23
3-Phenanthrenol,tetradecahydro-4b,8,8-trimethyl-,[3R-(3.alpha.,4a.alpha.,4b.beZ)-
5
37.499
1,165,188
25.50
Cycloisolongifolene,8,9-dehydro-9-formyl-
6
38.187
163,657
3.58
Methyl 10-trans,12-cis-octadecadienoate
7
38.294
379,205
8.30
9,12,15-Octadecatrienoic acid, methyl ester, (
8
38.360
136,951
3.00
1,4-benzenediol,2,5-bis(1,1-dimethylethyl)-
9
38.540
139,767
3.06
2-hexadecen-1-ol,3,7,11,15-tetramethyl-,[r-[r*,r*-(e)]]-
10
40.555
81,306
1.78
1-[2-(2,2,6-trimethylbicyclo[4.1.0]hept-1-yl)ethyl]vinylacet
11
53.383
2,003,662
43.86
Tris(2,4-di-tert-butylphenyl) phosphate
4,568,696
100.00
3.1 Larvicidal activity of Artemisia absinthium extracts against Aedes aegypti.
Plant extract yields (%) using methanol and ethanol solvents were determined and the result are mentioned in the (Table 2). The larvicidal activity of crude extracts was determined by using varying concentrations (200, 400, 600, 800 and 1000 ppm). The larval mortality percentage was scored after 24 h, and the results are presented in (Table 3). Ethanolic extract of Artemisia absinthium showed (92 ± 1.99 %) mortality at 1000 ppm, while methanolic extract showed (68 ± 1.99 %) mortality at 1000 ppm after 24 h. The lethal concentrations LC50 and LC90 values and other related parameters as the fiducial limit were calculated and presented in (Table 3) In the case of ethanolic extract tested on larvae, LC50 and LC90 were found to be 694.3 ppm and 1045.4 ppm, respectively. While, for methanolic extract, these values came out to be 888.6 ppm and 1159.7 ppm. Mean of five replication ± SE. AM: Artemisia absinthium Methanolic extract. AE: Artemisia absinthium ethanolic extract. LC50: Lethal concentration that kills 50% of the exposed larvae; LC90: Lethal concentration that kills 90% of the exposed larvae; LCL: Lower confidence limit; UCL: Upper confidence limit.
Species
Mass of the Extract (g) yield %
Mass of the dried leaf (g)
Methanol
Ethanol
Artemisia absinthium
10
0. 9793 (9.793 %)
0.7753 (7.753 %)
Sample ID
Concentration (ppm)
Larval toxicity (%) 24 h
LC50 ppm (LCL - UCL)
LC90 ppm (LCL - UCL)
P-level
AM
200
0.0 ± 0.0
888.6 (841.4–947.4)
1159.7 (1075.8–1296.3)
1.000
400
0.0 ± 0.0
600
10 ± 3.15
800
36 ± 2.45
1000
68 ± 1.99
AE
200
6 ± 2.45
694.3 (643.1–749.8)
1045.4 (961.5–1166.9)
0.992
400
16 ± 2.45
600
28 ± 1.99
800
62 ± 1.99
1000
92 ± 1.99
3.2 Quantitative analysis of biochemical components
Protein concentrations were determined in both control and extract-exposed fourth instar larval whole-body homogenates (Table 4). The protein concentration in control larval homogenates was 1.56 ± 0.01 µg/µL, whereas the protein concentration in the extract exposed larval homogenates was 1.16 ± 0.06 µg/µL. The drop in assessed larval protein levels was statistically significant (p ≤ 0.05) compared to the control.
3.2.1 Effect of Artemisia absinthium ethanol extract on acetylcholinesterase activity in larvae of Aedes aegypti
The acetylcholine esterase level in extract-treated larvae for 24 h at their lethal threshold concentration (LC50) was significantly lower, 70.33 ± 2.56**, than in control larval homogenates 99.3 ± 1.91 as shown in (Table 4). The decrease in acetylcholinesterase enzyme activity in the treated larvae was statistically significant (p ≤ 0.05) compared to the control. Acetylcholinesterase is an esterase that degrades the neurotransmitter acetylcholine to stop nerve impulses. Esterase is one of the enzymes that are most sensitive to environmental influences. Insects can employ the enzyme to lower pesticide sensitivity at the target location. When acetylcholinesterase activity is decreased to a certain level, insects become paralyzed and die. Mean of three replication ± SE. Quantitative analysis of biochemical components in the whole-body homogenates of fourth instar larvae Aaedes aegypti upon the 24 h exposure of ethanolic leaf extract of Artemisia absinthium * Significant at P ≤ 0.05 level.
Biochemical Studies
Control
Treated
Total Protein (µg/µL of homogenate)
1.56 ± 0.01
1.16 ± 0.06
Acetylcholinesterase activity (µM of AcT Hydrolyzed/min/mg of protein)
99.3 ± 1.91
70.33 ± 2.56**
Alpha-carboxylesterase activity (µM of α-Naphthol released/min/mg of protein)
134.78 ± 0.61
71.73 ± 1.4**
Beta-carboxylesterase activity (µM of β-Naphthol released/min/mg of protein)
100.17 ± 0.99
77.41 ± 1.51**
3.2.2 Effect of Artemisia absinthium ethanol extract on carboxylesterase activity in larvae Aedes aegypti
α-carboxylesterase activity: The impact of Artemisia absinthium ethanolic leaf extract on (24 h) exposure of larval homogenates for determination of α-carboxylesterase activity was observed (Table 4), with downregulation due to release of low-level α-naphthol 71.73 ± 1.4** (µM of α-Naphthol released/min/mg of protein) compared to control larval homogenate of around 134.78 ± 0.61 (µM of α-Naphthol released/min/mg of protein). The decreased values for α-carboxylesterase in the tested homogenate were statistically significant (p ≤ 0.05) compared to the control.
Activity of β-carboxylesterase: The β-carboxylesterase level was reduced by the production of low levels of α-naphthol, 77.41 ± 1.51** mM/min/mg of protein compared to the control the larval homogenate, which was around 100.17 ± 0.99 mM/min/mg of protein. The decrease in values for β-carboxylesterase in the tested homogenate was statistically significant (p ≤ 0.05) compared to the control. Similar effects were seen for α-carboxylesterase activity after 24 h exposure of extract to larval homogenates (Table 4).
4 Discussion
Since arthropod-borne illnesses are predominantly treated by insecticide-based vector activity, the development of resistance is a critical issue (Gan et al., 2021). Insecticide resistance is rising in Southeast Asia, matching the global trend of other kinds of resistance. Given their availability and safety, natural herbs may serve as possible insecticides in controlling disease vectors, minimizing the demand for synthetic pesticides (Lengai et al., 2020).
Our study evaluated the potential larvicidal action of Artemisia absinthium. Ethanolic and Methanolic leaf extracts against the 4th instar larvae of Aedes aegypti. After 24 h of exposure, larval mortality was found to be 92 ± 1.99 % in ethanolic extract and 68 ± 1.99 % in the methanolic extract at 1000 ppm. The overall activity of the crude plant extracts is attributed to a diverse mixture of active compounds.
Artemisia absinthium ethanolic leaf extract has strong larvicidal activity when compared to methanolic extract. As a result, Artemisia absinthium ethanolic leaf extract was subjected to GC–MS analysis to identify the chemical components of the extract. The GC–MS profile of the chemicals revealed some essential biologically active metabolites with very strong insecticidal properties. Neophytadiene, 9,12,15-Octadecatrienoic acid, methyl ester, have been reported to have larvicidal or insecticidal activity (Ravi et al., 2018; Evanjaline and Vr, 2018; Kannathasan et al., 2008; Hikal et al., 2017). Cycloisolongifolene, 8,9-dehydro-9-formyl is a bicyclic monoterpenoid, and such compounds have been seen to inhibit acetylcholinesterase (AChE) activity (Miyazawa and Yamafuji, 2005). The synergistic action of the identified compounds may also be responsible for the death of the larvae.
Knowing the specific mechanism of botanicals' interaction with the physiological system of target insects is critical for developing better insecticides. The systemic effect of Artemisia absinthium crude methanol and ethanol extract was studied at the threshold period (24 h) for its fatal effect utilizing specific lethal concentration (LC50) tests on 4th instar larvae with regard to alterations in Aedes aegypti biochemical components.
Proteins, such as chitin production and cuticle development, play a crucial role in insect metamorphosis. When insects are subjected to plant-based biopesticides, their whole-body protein levels may vary due to downregulation or upregulation (Parthiban et al., 2021; Parthiban et al., 2019). In our investigation, the extract-exposed fourth instar larval protein level was seen to be significantly lower than the control larvae. Similar effects were also observed by (Parthiban et al., 2020). Acetylcholinesterase is a neurotransmitter enzyme that plays an important role in hydrolyzing its substrate acetylcholine into acetate and choline. The acetylcholine is continuously released from the pre-synaptic neuron into the synaptic cleft, where it binds to the receptor /ligand-gated sodium channels, allowing the heavy movement of sodium ions to pass through this channel (Jankowska et al., 2018; Oni et al., 2019). Once the action has been completed, acetylcholinesterase, a special enzyme, is released to break acetylcholine into choline and acetate. The choline is reabsorbed and combined with acetyl CoA to take part in regular cycles to synthesize acetylcholine (Wilkinson, 1976). AChE has been reported to be carbamate and organophosphorus resistant, and it is generally known that AChE alteration is one of the primary resistance mechanisms in insect pests (Ghosh et al., 2012). In our investigation, the extract-exposed fourth instar larval protein level was seen to be significantly lower than the control larvae. Similar effects were also observed by (Koodalingam et al., 2011; Parthiban et al., 2020). AChE inhibitory properties of plant extracts have been reported (Silvério et al., 2020). The death of insects due to plant extract treatment suggested that the chemicals present in the extracts may interfere with the cholinergic synapse and disrupt the communication network from one exonic end to the other, thereby stopping nerve impulse transmission. Thus, the deadly impact may be due to a build up of acetylcholine (ACh), a neurotransmitter, at synaptic junctions, disrupting coordination between the nerve and muscle junctions (Jankowska et al., 2018; Oni et al., 2019).
The key esterase enzymes in the insect physiological system, acetylcholinesterase, α and β-carboxylesterase, detoxify xenobiotics and other exogenous compounds that enter the insect body (Lushchak et al., 2018). Insecticide-resistant mosquitoes develop due to enhanced detoxifying enzyme activity brought on by increased gene amplification or upregulation after exposure to synthetic pesticides (Şengül Demirak and Canpolat, 2022). Plant extracts and derivatives have been shown to reduce carboxylesterase (α-β-carboxylesterase) levels in Aedes aegypti larvae (Koodalingam et al., 2014; Lija-Escaline et al., 2015). As a result, several researchers began to report diverse plant metabolites with esterase inhibitory activities tested on insect models, including mosquitoes. They then investigated how esterase inhibitors target enzyme binding sites in insects to develop effective pesticides (Al-Solami, 2021). According to our findings, when Aedes aegypti fourth instar larvae were exposed to plant extract for 24 h, the activity of and α and β-Carboxylesterase enzymes was lowered due to the downregulation of these enzymes. Similar results were observed for β-Carboxylesterase upon exposure of the aqueous extract of Sapindus emarginatus to the fourth instar larva Aedes aegypti (Koodalingam et al., 2011). This demonstrates that the ethanolic extract of Artemisia absinthium can suppress the tested organism's particular enzyme activity without acquiring resistance.
Using chemical pesticides in water sources endanger both humans and the environment. Natural pesticides derived from plants are thus potential agents, particularly for mosquito larvae management (Alois et al., 2022).
5 Conclusion
To summarize, the leaf extract of the plant Artemisia absinthium included in the research has significant larvicidal activity, with ethanol extract exhibiting the highest activity. Ethanol extract significantly lowered the activity of AChE, α and β-carboxylesterase enzymes in the larvae. The study also needs in-depth phytochemical analysis using different solvents and extraction methods that will reveal more bioactive compounds in the leaves having larvicidal properties and can assist us in devising novel larvicidal formulations with minimal toxicity risks to the environment and life. In the meantime, the crude extract of Artemisia absinthium or isolated phytocompounds could be employed in stagnant water bodies, which are known mosquito breeding grounds. Besides, more research on the active principles involved and their mode of action and field testing is typically required before recommending this plant as an anti-mosquito product in a mosquito control program.
Acknowledgements
The authors acknowledge and extend their appreciation to the Researchers Supporting Project Number (RSP-2021/124) King Saud University, Riyadh, Saudi Arabia. The authors are also thankful to the Department of Biomedical Engineering, Sathyabama Institute of Science & Technology, Chennai, Tamil Nadu, for providing laboratory facilities to carry out the experiments.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical constituents and larvicidal efficacy of leaf extracts of Aristolochia elegans (Aristolochiaceae) S. Afr. J. Bot.. 2022;146:383-394.
- [Google Scholar]
- Larvicidal activity of plant extracts by inhibition of detoxification enzymes in Culex pipiens. J. King Saud Univ. Sci.. 2021;33(3)
- [Google Scholar]
- Analysis of chemical compositions and larvicidal activity of nut extracts from Areca catechu Linn against Aedes (Diptera: Culicidae) PloS one. 2021;16(11)
- [Google Scholar]
- A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol.. 1961;7:88-95.
- [CrossRef] [Google Scholar]
- Determination of bioactive components of Caralluma umbellata haw. (apocynaceae) by gas chromatography and mass spectroscopy analysis. Asian J. Pharm. Clin. Res. 2018:194-199.
- [Google Scholar]
- Dengue fever and insecticide resistance in Aedes mosquitoes in Southeast Asia: a review. Parasites Vectors. 2021;14(1)
- [Google Scholar]
- Plant extracts as potential mosquito larvicides. Indian J. Med. Res.. 2012;135(5):581.
- [Google Scholar]
- Botanical insecticide as simple extractives for pest control. Cogent Biol.. 2017;3(1):1404274.
- [Google Scholar]
- Molecular targets for components of essential oils in the insect nervous system—a review. Molecules. 2018;23(1):34.
- [Google Scholar]
- Larvicidal activity of fatty acid methyl esters of Vitex species against Culex quinquefasciatus. Parasitol. Res.. 2008;103(4):999-1001.
- [Google Scholar]
- Effects of extract of soapnut Sapindus emarginatus on esterases and phosphatases of the vector mosquito, Aedes aegypti (Diptera: Culicidae) Acta Trop.. 2011;118(1):27-36.
- [Google Scholar]
- Effects of NeemAzal on marker enzymes and hemocyte phagocytic activity of larvae and pupae of the vector mosquito Aedes aegypti. J. Asia-Pac. Entomol.. 2014;17(2):175-181.
- [Google Scholar]
- Phytochemical activity and role of botanical pesticides in pest management for sustainable agricultural crop production. Sci. Afr.. 2020;7:e00239.
- [Google Scholar]
- Physiological and biochemical effects of botanical extract from Piper nigrum Linn (Piperaceae) against the dengue vector Aedes aegypti Liston (Diptera: Culicidae) Parasitol. Res.. 2015;114(11):4239-4249.
- [Google Scholar]
- A bibliometric analysis on dengue outbreaks in tropical and sub-tropical climates worldwide since 1950. Int. J. Environ. Res. Public Health. 2021;18(6):3197.
- [Google Scholar]
- Wormwood (Artemisia absinthium L.) as a promising nematicidal and antifungal agent: Chemical composition, comparison of extraction techniques and bioassay-guided isolation. Ind. Crops Prod.. 2019;133:295-303.
- [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193(1):265-275.
- [Google Scholar]
- Improved reference genome of Aedes aegypti informs arbovirus vector control. Nature. 2018;563(7732):501-507.
- [Google Scholar]
- Inhibitory effects of oil extract of green Acalypha (Acalypha wilkesiana) on antioxidant and neurotransmitter enzymes in Callosobruchus maculatus. J. Basic Appl. Zool.. 2019;80(1):1-13.
- [Google Scholar]
- Biocompatible green synthesized silver nanoparticles impact on insecticides resistant developing enzymes of dengue transmitted mosquito vector. SN Appl. Sci.. 2019;1(10):1-9.
- [Google Scholar]
- Annona muricata: An alternate mosquito control agent with special reference to inhibition of detoxifying enzymes in Aedes aegypti. Ecotoxicol. Environ. Saf.. 2020;189:110050.
- [Google Scholar]
- Mosquito larvicidal activity of Annona reticulata extract and its lethal impacts on allelochemicals detoxifying enzymes in wild population dengue vector, Aedes aegypti. Int. J. Pest Manage. 2021:1-16.
- [Google Scholar]
- Identification of potential inhibitors from pyriproxyfen with insecticidal activity by virtual screening. Pharmaceuticals. 2019;12(1):20.
- [Google Scholar]
- Chemical composition and larvicidal activities of Azolla pinnata extracts against Aedes (Diptera: Culicidae) PloS ONE. 2018;13(11):e0206982.
- [Google Scholar]
- Toxicity and enzyme inhibition activities of the essential oil and dominant constituents derived from Artemisia absinthium L. against adult Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae) Ind. Crops Prod.. 2018;121:468-475.
- [Google Scholar]
- Plant-based bioinsecticides for mosquito control: impact on insecticide resistance and disease transmission. Insects. 2022;13(2):162.
- [Google Scholar]
- A review of resistance mechanisms of synthetic insecticides and botanicals, phytochemicals, and essential oils as alternative larvicidal agents against mosquitoes. Front. Physiol.. 2020;10:1591.
- [Google Scholar]
- Plant natural products for the control of Aedes aegypti: The main vector of important arboviruses. Molecules. 2020;25(15):3484.
- [Google Scholar]
- A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol.. 1962;8:401-416.
- [CrossRef] [Google Scholar]
- Influence of seaweed extracts on growth, phytochemical contents and antioxidant capacity of cowpea (Vigna unguiculata L. Walp) Biocatal. Agric. Biotechnol.. 2019;17:589-594.
- [Google Scholar]
- WHO, 2 March 2020, Vector-borne diseases.
- WHO, 2014, A Global Brief on Vector-Borne Diseases, Document number: WHO/DCO/WHD/2014.1.
- Wilkinson C.F. 1976. Insecticide Biochemistry and Physiology. Plenum Press, New York, pp 61–114.
- World Health Organization. 1996. Report of the WHO Informal Consultation on the Evaluation on the Testing of Insecticides, CTD/WHO PES/IC/96.1. Geneva: WHO. p. 69.







