Translate this page into:
Larvicidal activity and chemical compositions of Aloe ferox mill, and Commipora abyssinica (O.Berg) combination against the mosquito vectors Culex pipiens L
⁎Corresponding author. nabutaha@ksu.edu.sa (Nael Abutaha)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aqueous extract was prepared from the Aloe ferox, and Commipora abyssinica combined to develop a botanical mosquito larvicide. The aqueous extract and the solvent fractions obtained using liquid–liquid extraction were tested against Culex pipiens larvae for larvicidal potential. The maximum larvicidal activity was recorded for the ethyl acetate (EtOAc) extract with LC50 values of 28.24 µg/mL followed by hexane (104.42 µg/mL), water (140.24 µg/mL), and chloroform (211.41 µg/mL) extract against the Cx. pipiens third instar 24 h post-treatment. In midguts of EtOAc extract-treated larvae, Longitudinal sections showed edema between the degenerated epithelial cells and degraded microvilli. The extract caused a dose‐dependent decrease in the percentage of cell viability of HUVEC cells suing MTT (3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay. The IC50 value of the EtOAc extract was 143.6 µg/mL and displayed chromosomal condensation. The total phenolic and flavonoid contents calculated were 15.9 GAE/g (gallic acid equivalent per gram) and 3.69 QurE/g (Qurcetin equivalent per gram), respectively. GC–MS analysis showed that the major chemical components of the EtOAc extract were methyl ester of hexadecanoic acid (28%), 3-benzylbutanoate (9.4%), methyl ester of octadecanoic acid (8.6%), and alpha-muurolene (5.3%). The current investigations revealed the possible use of this botanical combination as a larvicide against Cx. pipiens larvae.
Keywords
Aloe ferox
Aqueous extract
Ethyl acetate extract
Commipora abyssinica
Culex pipiens
Larvicidal
1 Introduction
Different emerging diseases are transmitted by the mosquitos’ bites such as Dengue Virus, Chikungunya Virus, Eastern Equine Encephalitis Virus, Japanese Encephalitis Virus, La Crosse Encephalitis, Malaria, St. Louis Encephalitis, Yellow Fever, West Nile Virus, and Zika Virus (CDC, 2020).
Around 3500 mosquito species are identified worldwide (Harbach and Howard, 2007); only a small number play a role in arboviruses transmission. Cx. pipiens play a critical role in the transmission of viruses, mainly Sindbis Virus (SINV) infection that causes rash and polyarthritis (Lundström, 1999), West Nile Virus that causes a febrile illness that generally resolves without complications (Campbell et al., 2002) and Usutu virus, that causes mortality to Old World blackbirds (Weissenböck et al., 2013) and encephalitis (Pecorari et al., 2009). Vector control is a crucial strategy for managing diseases transmitted by mosquitoes. Larvicides that kill the mosquitoes bound to their habitats (juvenile stages) are much easier before adulthood.
The repeated use of synthetic larvicidal agents resulted in environmental pollution and resistant mosquitoes (Liu, 2015). Insecticide resistance reduces the possibility of eradicating diseases transmitted by vectors (WHO, 1976). Extensive use of mosquitocides to eliminate dengue fever in 2004 (Ayyub et al., 2006), and Rift Valley fever in 2000 (Jupp et al., 2002) increased the level of resistance in mosquitoes. Research conducted by Al-Sarar, 2010 to detect resistance in Cx. pipiens populations in Riyadh City found two populations from Wadi Namar were resistant to bifenthrin, deltamethrin, beta-cyfluthrin, and lambda-cyhalothrin. No resistance to fenitrothion was observed (Al-Sarar, 2010). Thus, there is a necessity to search for new Larvicides.
Plant secondary metabolites are a promising source of larvicidal agents for mosquito management/control. Secondary metabolites such as steroids, terpenes, alkaloids, and phenolic compounds are reported for larvicidal without causing deleterious effects to human and animal health and the environment (Hari and Mathew, 2018; Mathew, 2017).
Earlier research indicated the potential of synergistic plant extract combinations (Benelli et al., 2017). No investigation has been conducted to evaluate the effects of the Aloe ferox and Commipora abyssinica combination against mosquito larvae. Therefore, this encouraged us to investigate the combined effect of these two plants against the Cx. pipiens larvae and chemical constituents analysis. Hence, the outcome of this report could provide a perspective for the development of eco-friendly plant-based alternatives for mosquito control.
2 Materials and methods
2.1 Plant collection and extraction
The leaf Aloe ferox Mill. (Asphodelaceae) and resin of Commipora abyssinica (O.Berg) Engl. (Burseraceae) were collected from a herbal shop in Riyadh, Kingdom of Saudi Arabia. Voucher specimens were deposited in the department of botany for A. ferox (KSU-Bio-225) and C. abyssinica (KSU-Bio-226).
2.2 Aqueous extract
Ten grams of each plant powder were mixed and soaked in 500 mL of boiled distilled water and kept on the shaker (250 rpm) (GFL, Germany) for 24 h. Later, the extract was centrifuged (Centurion, UK) at 5000 rpm for 10 min. Three hundred milliliters of the extract were later extracted using the liquid–liquid extraction method in hexane, chloroform, and ethyl acetate (EtOAc). The aqueous extract and the fractions obtained were concentrated using a rotary evaporator (Heidolph, Germany) at 40 °C. All the extracts were reconstituted in DMSO at 50 mg/mL concentration and stored at 4 °C until analysis.
2.3 Collection and maintenance of Cx. Pipiens
The Cx. pipiens were collected from Bio-product Research Chair Insectary, King Saud University, Riyadh. The larvae were reared in the standard condition (25 ± 2 °C and 85 ± 5% humidity), maintained in a bowl filled with tap water, and fed on a mixture of fish feed.
2.3.1 Dose-response bioassays on Cx. pipiens
The larvicidal bio-efficacy of the extract was evaluated against the third instar of Cx. pipiens. The extract was introduced into 6 well plates containing water (8 mL), and 20 Larvae were released into each well. A well containing water and another well containing DMSO served as control (0.5%). Each test was carried out in triplicate. The number of dead larvae was counted 24, 48, and 72 h post-treatment, and the mortality percentage was calculated. The LC50 and LC90 values of extracts were evaluated, and the results were analyzed using the SPSS Software.
2.3.2 Histopathology alterations
The treated and control larvae were kept in buffered (pH 7.2) formalin. Ethanol dehydrated larvae were embedded in paraffin wax, sectioned using a microtome (Leica, Germany), stained with hematoxylin and eosin, and mounted. The midgut region was assessed under a microscope (Al-Mekhlafi et al., 2020).
2.4 Cytotoxicity
Noncancerous HUVEC cell lines were purchased from the American Type Cell Culture Collection (ATCC, USA). About 50,000 cells/well was seeded for 24 h in Dulbecco's modified Eagle's medium with 10% FBS in a 24-well plate at 37 °C with a 5% CO2 atmosphere. Later, different test concentrations (100,200,300,400, and 500 µg/mL) were added to the cell lines separately and incubated as before. MTT reagent (Thermo, USA) was then added to each well and incubated for 3 h. The formazan crystals formed were solubilized in 1 mL dimethyl sulfoxide, and the absorbance (n = 3) was read using a microplate reader at 570 nm.
2.5 Hoechst 33258 staining
Cells were seeded as prepared in the previous section and treated with IC50 of the extract. Later the cells were washed twice with phosphate-buffered saline (PBS) before and after fixation and then stained for 20 min at 25 °C with 1 µg/ml Hoechst dye (Invitrogen, USA) and observed under a fluorescent microscope (EVOS, USA).
2.6 Total phenol content
The estimation of total phenol was assessed by the Folins-phenols reagent (FC) method (Abutaha et al., 2021). Extract (12.5 μL) was mixed with 125 μL of 25% FC reagent and 12.5 μL of sodium carbonate and incubated for 1.5 h for color development. The absorbance was read at 725 nm (Thermo Fisher Scientific, USA). The calibration curve was constructed using Gallic acid (5–100 μg/ml). The total phenol of extract content was expressed as gallic acid equivalent per gram (GAE). The results were analyzed in triplicate.
2.7 Total flavonoids
Estimating total flavonoids was carried out by the Aluminium chloride method (Abutaha et al., 2021). An equal volume of the extract and 100 μL of Aluminium Chloride (2%) was mixed and incubated for 10 min at 25 °C for color development, read at 368 nm. The total flavonoid content of the extract was expressed as quercetin equivalent per gram (QURE). The results were carried out in triplicates.
2.8 IR and GC–MS analyses of bioactive principle
As previously reported, a dried sample containing active EtOAc extract was subjected to infrared (IR) spectroscopy and GC–MS analysis (Abutaha et al., 2021).
2.9 Statistical analysis
The results of mortality are means of three replicates. Analysis of variance (ANOVA) was compared using Duncan’s multi-range test with SPSS 10.0 software. The significance level was p < 0.05.
3 Result
3.1 Bioassays on Cx. pipiens
The larvicidal efficacy of aqueous extract, hexane, chloroform, and ethyl acetate (EtOAc) fractions were tested against the third instar of Cx. pipiens (Table 1). All the extracts showed larvicidal potentials against Cx. pipiens after 24, 48, and 72 h of treatment (Table 1). No mortality was recorded in the control group. The EtOAc extract showed the highest toxicity when compared to other extracts tested. After 24 h of exposure, the EtOAc extract demonstrated an LC50 value of 28.24 µg/mL and an LC90 value of 101.67 µg/mL against the Cx. pipiens. The larvicidal activity of EtOAc extract was followed by hexane (104.42 µg/mL), water (140.24 µg/mL), and chloroform (211.41 µg/mL) extract (Table 1). Small horizontal letters indicate significant differences between concentrations. Significant differences were assessed using one-way ANOVA followed by Tukey’s test, with p < 0.05 considered to indicate significant differences.
Extract Type
Time
Mortality%
LC50 (µg/mL)
LC90 (µg/mL)
Concentration (µg/mL)
15.63
31.25
62.5
125
250
Hexane
24
-
10.00±5.77d
36.67±3.33c
73.33±3.33b
100.00±0.00a
104.42
206.62
48
-
40.00±5.77b
76.67±8.82a
90.00±5.77a
100.00±0.00a
34.18
116.22
72
-
50.00±5.77b
83.33±6.67a
100.00±0.00a
100.00±0.00a
8.33
97.6
Chloroform
24
-
0.00±0.00b
6.67±3.33b
36.67±8.82a
56.67±3.33a
211.41
362.18
48
-
0.00±0.00b
16.67±3.33b
76.67±6.67a
93.33±26.67a
186.37
217.86
72
-
20.00±5.77c
76.67±3.33b
86.67±3.33ab
100.00±0.00a
44.5
184.07
Ethyl acetate
24
16.67±3.33d
53.33±3.33c
73.33±3.33b
100.00±0.00a
-
28.24
101.67
48
23.33±3.33c
70.00±0.00b
93.33±3.33a
100.00±0.00a
-
21.4
90.07
72
50.00±5.77b
90.00±3.33a
100.00±00a
-
-
5.21
46.88
Aqueous
24
-
10.00±5.77b
10.00±5.77b
63.33±6.67a
83.33±3.33a
140.24
250.83
48
-
13.33±6.67b
16.67±3.33b
76.67±3.33a
90.00±0.00a
119.42
226.43
72
-
30.00±5.77b
36.67±3.33b
93.33±3.33a
100.00±0.00a
72.51
191.63
3.2 Histopathology alterations
The larval exposure to LC50 of the EtOAc extract showed changes in the midgut architecture of Cx. pipiens larvae. The larval exposure to EtOAc extract induced lysis of epithelium layer in the midgut, vacuolization, and destruction of the peritrophic membrane in some regions, loss of epithelial cell, edema between the degenerated epithelial cells, and degraded microvilli (Fig. 1).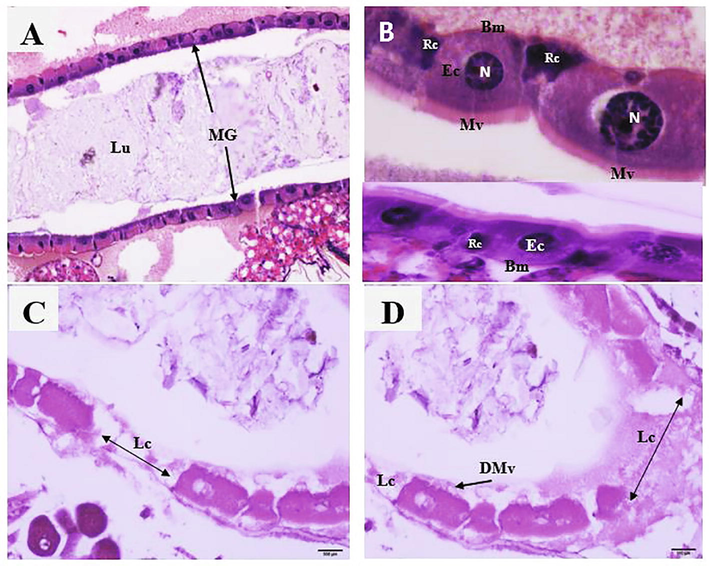
Photomicrographs of midguts of Cx. pipiens larvae treated with EtOAc fraction obtained from aqueous extract of Aloe ferox and Commipora abyssinica combined. Longitudinal sections in the midguts (MG) of control larvae with normal and healthy epithelial cells (Ec), microvilli (Mv), nuclei (n), and regenerative cells (Rc). Note the absence of the lesions. {C-D}: Longitudinal sections in midguts of EtOAc-treated larvae, with loss of some epithelial cells (Lc) and degraded microvilli (DMv). H&E stain.
3.3 Cytotoxicity
Fig. 2 shows the dose‐dependent decrease in the percentage of cell viability in the presence of EtOAc extract in HUVEC cells. The LC50 value of the extract was 143.6 μg/mL as compared with the control. We also investigated the morphological alternation in HUVEC cells exposed to EtOAc extract using fluorescence microscopy. The nuclei in the control cells were round with normal chromatin and evenly stained. Cells incubated with the EtOAc extract displayed chromosomal condensation. In the control wells, the nuclei of HUVEC cells appeared evenly stained and round (Fig. 2).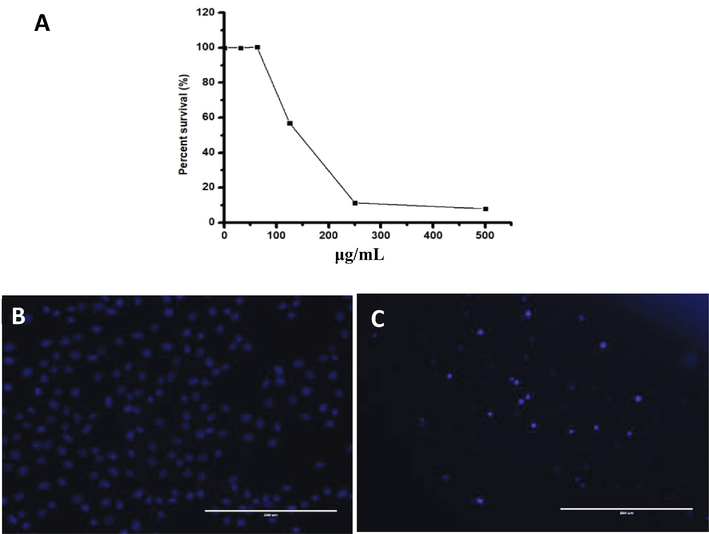
A; Cytotoxicity of EtOAc fraction obtained from aqueous extract of Aloe ferox and Commipora abyssinica combined against Noncancerous HUVEC cell lines using MTT assay showing dose-dependent toxicity. Fluorescence microscopy of cells treated with EtOAc extract for 24 h, followed by DAPI staining and then imaged to assess the morphological changes. (B) Untreated control cells showed normal nuclear morphology; (C) treated cells showed fragmented chromatin.
3.4 Total phenol and content
The total phenolic and flavonoid contents calculated were 15.9 GAE/g and 3.69 QURE/g, respectively.
3.5 GC–MS and IR spectra analysis of EtOAc extract
The IR spectrum of EtOAc extract (Fig. 3) showed a hydroxyl peak at ∼3430 cm−1 and the peaks situated at 2913 and 2996 cm−1 belong to the C H stretching vibration of methylene and methyl group. The FTIR spectrum is dominated by monoterpenes' vibrational modes, seen at 952, 1436, and 1654 cm−1. The band in the 1020 cm−1 region belongs to the C-O stretching vibration of alcohol. The band at 1654 cm−1 could represent amide I, or carboxylic acid. GC–MS analysis showed that the major chemical components of the EtOAc extract were methyl ester of hexadecanoic acid (28%), 3-benzylbutanoate (9.4%), methyl ester of octadecanoic acid (8.6%), and alphamuurolene (5.3%) (Table 2).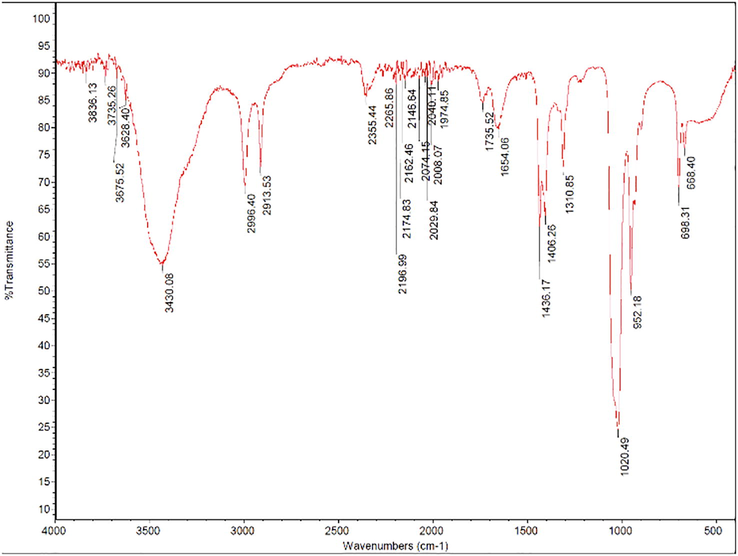
FTIR spectra of EtOAc fraction obtained from aqueous extract of Aloe ferox and Commipora abyssinica combined.
#
Name
Retention time
Area %
1
Octanal
13.31
0.960
2
Methyl ester of octanoic acid
14.03
2.020
3
4-Methyl-benzaldehyde
17.52
0.340
5
alpha.-Muurolene
31.14
5.350
6
Methyl cantharate
31.73
1.190
7
Methyl 2-(1-cyclopentanol)mandelate
32.83
1.980
8
iso-Geraniol
34.00
0.550
9
Methyl ester of hexadecanoic acid
34.27
28.000
10
4,4-Dimethyl-1-phenyl- 2,7-octadien-1-one
34.43
1.520
11
(2-phenylethenylidene)cyclohexene
34.74
0.980
12
Mansonone
34.85
3.640
13
Erythro-2,2-dimethyl-6-nitro-5-Phenylhept-3-ene
35.15
3.110
16
2,5-Dimethylamphetamine
35.95
1.300
17
Methyl 2-phenyl-2,3-dihydroxy-3-Chloromethylbutanoate
36.19
4.230
20
Methyl cantharate
37.29
2.940
21
Methyl ester of 6-octadecenoic acid
37.60
3.220
22
Methyl ester of 11-octadecenoic acid
37.72
2.700
24
Methyl ester of octadecanoic acid
38.09
8.600
25
1,1-Diphenylprop-2-en-1-ol
38.35
4.020
27
Methyl 2-(1-cyclohexanol)mandelate
38.94
2.650
29
Methyl 2-phenyl-2,3-dihydroxy-3-benzylbutanoate
40.10
9.410
4 Discussion
Larviciding is a successful method of managing mosquitoes' spread in their breeding habitant post emerging into adults. Although synthetic larvicides are promising and effective in controlling mosquitos, their frequent use has caused an extensive development of resistance in mosquitoes (Addiss and Lammie, 2013; Kumar et al., 2011). Studies on secondary metabolites of natural origin against mosquito vectors are promising alternative agents to manage the threat of vector-borne diseases that act on mosquitoes' physiological and behavioral. The combination of extracts is more advantageous than individual isolated compounds that prevent mosquito resistance development (Maurya et al., 2007). Though, bioactivity plant extracts vary based on plant species and part used, geographical origin, extraction solvent, chemical nature, mosquito species, and developmental stages (Ghosh et al., 2012; Shaalan et al., 2005).
Results revealed that LC50 values decreased with the exposure time, recording the lowest value at 72 h of incubation. Dose-dependent mortality was observed and positively correlated with the concentrations used. In our study, the LC50 value of the EtOAc extract was 28.24 µg/mL; this is within the promising range (LC50 less than 100 µg/mL) of reported classification (Thangam and Kathiresan, 1996).
Eradicating mosquitoes at the larval stage is considered the best target for mosquitoicides because they breed in water, and therefore, they are easily controlled in this habitat. The use of synthetic mosquitoicides in aquatic sources may pose risks to the environment. Plants' secondary metabolites are very promising sources of compounds that are used in a different fields. Green mosquitoicides may serve as an alternative to synthetic insecticides. The blending of insecticidal agents is practiced and recommended to optimize the effectiveness of the insecticides to solve the issue of insecticidal resistance and preserve the insecticidal efficiency for a longer period. In the current investigation, the binary blending of different plant extracts have been reported in many research articles such as Callitris glaucophylla and Khaya senegalensis against Aedes aegypti (Shaalan et al., 2005), E. camaldulensis and C. rigidus against A. gambiae larvae (Ríos et al., 2017), Canarium schweinfurthii, Aucoumea klaineana, and Dacryodes edulis against A. gambiae (Obame et al., 2016).
Histopathological changes in the midgut of cx. pipiens larva post-treatment with EtOAc extract help to understand the mode of action of the extract. The midgut is composed of epidermal cells and muscle. Epidermal cells are responsible for the oxidation process, ionic and osmotic regulation, storage of carbohydrate and lipid, pH control, nutrient absorption, and secretion of the digestive enzyme (Silva et al., 2020; Wang et al., 2019). The third instar of Cx. pipiens exposed to EtOAc extract showed morphological deformities. EtOAc extract acted upon epidermal cells and showed damaged epithelial cells, microvilli, muscles, and adipose tissue (Fig. 1C, D). In control, the larval tissues revealed undamaged epithelial cells and adipose tissue (Fig. 1A, B). The results are similar to the previous reports (Abdel-Salam et al., 2018) (FARAG et al., 2018). The toxicity of the plant extract as an insecticide depends on detoxification ability at the developmental stage, target enzymes, and ability to penetrate the insect body (Wang et al., 2019). The third instar of Cx. pipiens exposed to EtOAc extract showed histological deformities. The damage in the larval midgut cells treated with EtOAc extract may be attributed to the remarkable feature of compounds; non-polar extract can easily penetrate the larval body and interrupt the intake of feed, cell division, and breathing.
The IC50 value of Huvec cells treated with EtOAc extract was 145.5 µg/mL. Plant extract with IC50 higher than 30 µg/mL is considered safe based on American National Cancer Institute (Rosidah, 2014). When applying this information to our data, PH2 extract is deemed safe because the concentration needed to be used as larvicides (LC50: 28.2 µg/mL) is 5 times less than the IC50 value Huvec cells (145.5 µg/mL). However, the data cannot be generalized, and different models should assess its toxicity.
The biological activity of extracts is mainly attributed to their major compounds (Bakkali et al., 2008; Gbolade and Lockwood, 2008; Vani et al., 2009). Although, in some times, major compounds may not be accountable for the overall activity, the combination of minor and major compounds could have resulted in synergistic, additive, or antagonistic interactions (Bakkali et al., 2008). The present study demonstrated tissue damage in the larvae treated with extract, as it contains several compounds that are common in many plants that have been reported for their insecticidal activity (Mozaffari et al., 2014. Some compounds were individually isolated, such as methyl ester of hexadecenoic acid. This compound was isolated from Millettia pinnata and demonstrated potent larvicidal activity against the third instar larvae of Cx. pipiens pallens, A. aegypti and A. albopictus and the acetylcholinesterase was the site of action (Perumalsamy et al., 2015). However, the potent larvicidal activity of EtOAc extract could be attributed to the various compounds present in the extract, such as phenols, flavonoids, terpenoids, aldehyde, and esters.
The phytochemical and GC–MS analysis are the first step towards understanding the nature of larvicidal constituents present in the extract. Nevertheless, further investigations are needed to explore their potentials. Insecticides derived from herbal extract are less active than synthetic ones (Mohan et al., 2010; Shaalan et al., 2005). However, this is justified for being a mixture of inactive or active compounds. The complexity of the extract with a different mechanism of action could increase the bioactivity or prevent the evolution of resistance populations (Tak and Isman, 2015). Phenolics are widely found in the plant kingdom (Pereira et al., 2009), possessing many therapeutic activities (Fukumoto and Mazza, 2000; Germano et al., 2006). The phenolic compounds inactivate enzymes and form a phenol–protein complex that is hard to digest by mosquitoes (Mello and Silva-Filho, 2002).
5 Conclusion
The findings of the present investigation revealed that EtOAc extract has potent larvicidal activity against Cx. pipiens. Further studies are needed to assess its potential in the field.
Acknowledgement
Researchers Supporting Project number (RSP-2021/112), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Histologicl effects of two chitin synthesis inhibitors on the larval midgut of the cotton leafworm, Spodoptera littoralis (Boisd.)(Lepidoptera: Noctuidae) EJAR. 2018;96(4):1379-1389.
- [Google Scholar]
- Abutaha, N.M., Farooq, M.F., Mohammed, A.-Z., Alotaibi, A., Cordero, M.A.W., Bepari, A., Alarifi, S., 2021. Cytotoxic activity and toxicity study of HF8, a poly-herbal formulation. J King Saud Univ Sci 33(3), 101377.
- Addiss, D.G., 2013. Global elimination of lymphatic filariasis: a “mass uprising of compassion”. PLoS Negl Trop Dis 7(8), e2264.
- Insecticide resistance of Culex pipiens (L.) populations (Diptera: Culicidae) from Riyadh city, Saudi Arabia: Status and overcome. Saudi. J. Biol. Sci.. 2010;17(2):95-100.
- [Google Scholar]
- Inhibition of the growth and development of mosquito larvae of Culex pipiens L. (Diptera: Culicidae) treated with extract from flower of Matricaria chamomilla (Asteraceae) Entomol. Res.. 2020;50(3):138-145.
- [Google Scholar]
- Characteristics of dengue fever in a large public hospital, Jeddah, Saudi Arabia. J. Ayub. Med. Coll.. 2006;18(2):9-13.
- [Google Scholar]
- Biological effects of essential oils–a review. Food Chem. Toxicol.. 2008;46(2):446-475.
- [Google Scholar]
- Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int.. 2017;66(2):166-171.
- [Google Scholar]
- CDC, 2020. https://www.cdc.gov/niosh/topics/outdoor/mosquito-borne/other.html.
- Ultra-structural studies on the midgut of Culex pipiens larvae treated with pomegranate peel extract, Punica granatum. J. Egypt. Soc. Parasitol.. 2018;48(1):77-84.
- [Google Scholar]
- Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agr. Food Chem.. 2000;48(8):3597-3604.
- [Google Scholar]
- Toxicity of Ocimum sanctum L. essential oil to Aedes aegypti larvae and its chemical composition. J. Essent. Oil Bear. Pl.. 2008;11(2):148-153.
- [Google Scholar]
- Frequency and causes of implantable cardioverter-defibrillator therapies: is device therapy proarrhythmic? Am. J. Cardiol.. 2006;97(8):1255-1261.
- [Google Scholar]
- Plant extracts as potential mosquito larvicides. Indian J. Med. Res.. 2012;135(5):581.
- [Google Scholar]
- Index of currently recognized mosquito species (Diptera: Culicidae) Eur. mosq. bull.. 2007;23:1-66.
- [Google Scholar]
- Larvicidal activity of selected plant extracts and their combination against the mosquito vectors Culex quinquefasciatus and Aedes aegypti. Environ. Sci. Pollut. R.. 2018;25(9):9176-9185.
- [Google Scholar]
- The 2000 epidemic of Rift Valley fever in Saudi Arabia: mosquito vector studies. Med. Vet. Entomol.. 2002;16(3):245-252.
- [Google Scholar]
- Multiple insecticide resistance/susceptibility status of Culex quinquefasciatus, principal vector of bancroftian filariasis from filaria endemic areas of northern India. Asian Pac. J. Trop. Med.. 2011;4(6):426-429.
- [Google Scholar]
- Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annu. Rev. Entomol.. 2015;60(1):537-559.
- [Google Scholar]
- Mosquito-borne viruses in western Europe: a review. J. Vector Ecol.. 1999;24(1):1-39.
- [Google Scholar]
- Mosquito repellent activity of volatile oils from selected aromatic plants. J. Parasitol. Res.. 2017;116(2):821-825.
- [Google Scholar]
- Larvicidal efficacy of Aloe barbadensis and Cannabis sativa against the malaria vector Anopheles stephensi (Diptera: Culicidae) Entomol. Res.. 2007;37(3):153-156.
- [Google Scholar]
- Plant-insect interactions: an evolutionary arms race between two distinct defense mechanisms. Brazilian J. Plant Physiol. 2002;14(2):71-81.
- [Google Scholar]
- Combination larvicidal action of Solanum xanthocarpum extract and certain synthetic insecticides against filarial vector, Culex quinquefasciatus (Say) Southeast Asian J. Trop. Med. Public Health. 2010;41(2):311-319.
- [Google Scholar]
- Chemical composition, larvicidal and repellency properties of Cionura erecta (L.) Griseb. against malaria vector, Anopheles stephensi Liston (Diptera: Culicidae) J. Arthropod Borne Dis.. 2014;8(2):147.
- [Google Scholar]
- Ovicidal and larvicidal activities against Anopheles gambiae, antioxidant and antibacterial proprieties of Aucoumea klaineana Pierre, Canarium schweinfurthii Engl and Dacryodes edulis (G. Don) HJ Lam essential oils from Gabon. Int. J. Pharmacol. Res.. 2016;6(1):68-75.
- [Google Scholar]
- First human case of Usutu virus neuroinvasive infection, Italy, August-September 2009. Eurosurveillance. 2009;14(50):19446.
- [Google Scholar]
- Pereira, D.M., Valentão, P., Pereira, J.A., Andrade, P.B., 2009. Phenolics: From chemistry to biology. Molecular Diversity Preservation International.
- Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasit. Vectors. 2015;8(1):1-14.
- [Google Scholar]
- Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae) Rev. Bras. Entomol.. 2017;61(4):307-311.
- [Google Scholar]
- Cytotoxic effect of n-hexane, ethylacetate and ethanol extracts of Plectranthus amboinicus,(Lour. Spreng.) on HeLa and Vero cells lines. Int. J. PharmTech. Res.. 2014;6(6):1806-1809.
- [Google Scholar]
- A review of botanical phytochemicals with mosquitocidal potential. Environ. Int.. 2005;31(8):1149-1166.
- [Google Scholar]
- Essential oil from leaves of Eugenia calycina Cambes: Natural larvicidal against Aedes aegypti. J. Sci. Food Agr.. 2020;101:1202-1208.
- [Google Scholar]
- Enhanced cuticular penetration as the mechanism for synergy of insecticidal constituents of rosemary essential oil in Trichoplusia ni. Sci. Rep.. 2015;5(1):1-10.
- [Google Scholar]
- The prey consumption and prey preference of the larvae of the mosquito Culex (Lutzia) raptor on the larvae of Culex quinquefasciatus. Experientia. 1996;52(4):380-382.
- [Google Scholar]
- Comparative study of volatile compounds from genus Ocimum. Am. J. Appl. Sci.. 2009;6(3):523-528.
- [Google Scholar]
- Toxicity and possible mechanisms of action of honokiol from Magnolia denudata seeds against four mosquito species. Sci. Rep.. 2019;9(1):1-19.
- [Google Scholar]







