Translate this page into:
Larvicidal activity and biochemical effect of some essential oils and indoxacarb against peach fruit fly, Bactrocera zonata (Diptera: Tephritidae)
⁎Corresponding author at: Department of Economic Entomology and Pesticides, Faculty of Agriculture, Cairo University, Giza 12613, Egypt. s.sayed@tu.edu.sa (Samy M. Sayed)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Bactrocera zonata (Saunders) (Diptera: Tephritidae) is an important insect pest of fruits in Egypt. The desire for fresh fruits free of insecticides drives the adoption of environmentally friendly biopesticides to manage pests. The current study sought to estimate the toxicity and biochemical alterations of 3rd larval instar of B. zonata affected by clove, neemazal T/S, garlic, ginger and orange oils compared with indoxacarb at LC50 under laboratory conditions. Results after 24 h of treatment indicated that neemazal T/S is more toxic than other tested oils compared with higher toxicity of indoxacarb with LC50 values of 86.09, 174.72, 4514.38, 7224.01, 9387.73 and 2590.2 μg/mL for indoxacarb, neemazal T/S, ginger, clove, garlic and orange oils, respectively. Moreover, after 24 h of treatment, the larval content of digestive enzymes drastically decreased. All compounds caused a remarkable reduction in protease (except clove oil), aspartate aminotransferase (AST), alanine aminotransferase (ALT), phenol oxidase enzyme, α-esterase (except indoxacarb and neemazal T/S) and β-esterase (except ginger oil and neemazal T/S) activity. Also, total carbohydrates, total lipids and total proteins take the same trend. All treatments at LC50 increased the activity of cytochrome p450 and glutathione-s-transferase against 3rd larval instar of B. zonata compared with control. Our findings recommended that the tested botanicals could be an effective substitute for conventional insecticides as well as their safety for human beings and beneficial organisms.
Keywords
Botanical insecticide
Bioassay
Larvicidal effect
Biochemical properties
Bactrocera zonata
1 Introduction
Fruit flies pose serious hazards to horticulture crops, including both fruits and vegetables and are among the most destructive agricultural pests in the world (Hendrichs et al., 2015). There are roughly 4,000 different species in the Tephritidae family of fruit flies, of which 350 are extremely important (Ashfaq et al., 2020). The peach fly fruit Bactrocera zonata (Saunders) is one of the serious insect pests attacking tropical and subtropical fruits (Hosni et al., 2011). Originally from South and South-East Asia, B. zonata has spread to and established itself in several nations throughout the Arabian Peninsula, North Africa, and parts of the Indian Ocean (Zingore et al., 2020). B. zonata feeds on more than 50 domesticated and untamed host plants, including fig, peach, mango, guava, and citrus fruits (Allwood et al., 1999). Fruit flies resulted in both a direct loss in yield and an indirect loss in commerce and export opportunities (Sharma et al., 2015). The mated female fruit flies put their eggs in ripening fruit. The eggs are later followed by larvae and other opportunistic secondary bacteria feasting on the pulp of the fruit, which causes decomposition. Larvae damage ranges from unsightly look owing to egg punctures resulting in decreased marketability to fruit drops resulting in decreased yields (Delrio and Cocco, 2010). Egypt suffers annual financial losses from the fruit fly estimated at EUR 190 million (OEPP/EPPO, 2005). Therefore, B. zonata is considered one of the insects that causes a big problem in the world.
Farmers frequently choose to use synthetic pesticides as quick-fix pest management options to defend their crops against pest attacks and damage (Nkechi et al., 2018). However, the effectiveness and widespread misuse of synthetic pesticides cause a number of concerns, particularly contributing to insect resistance and health-related problems (Shabana et al., 2017). Moreover, the use of pesticides in agricultural crops has been controlled and amended by some European legislation (Reg. CE396/2005; 1095/2007; 33 and 299/2008 and 1107/2009). Thus, there is a great need for environmentally friendly management measures such as botanicals. Plant extracts may offer environmentally benign substitutes for synthetic insecticides in integrated pest management (IPM) of fruit fly populations.
Alternatively, botanical insecticides were commonly used for millennia in both subsistence and commercial cultivation before the invention of synthetic pesticides. Ingredients in botanical pesticides are chemically derived from plants that operate as attractants, antifeedants, and growth inhibitors (Stankovic et al., 2020). Botanicals are more dependable, easily biodegradable, and less likely to cause fruit flies to develop resistance (Saeed et al., 2022, Sayed et al., 2022). Interestingly, producing botanicals is economically inexpensive (Ahmed et al., 2022, Sayed et al., 2022). The majority of botanicals are specialized and have little bearing on the survival of natural adversaries (Potts et al., 2016). Consequently, botanical pesticides are more effective at controlling Bactrocera species in an IPM program.
The biochemical changes in the other insect enzymes such as carbohydrate hydrolyzing enzymes, protein hydrolyzing enzymes, phenoloxidase, protease and acetylcholinesterase caused by botanical pesticides reported in many studies (Ahmed et al., 2022; Bezzar-Bendjazia et al., 2017).
The main hypothesis of this study is that botanicals would control B. zonata and as a result, lower the danger of pesticide pollution in the environment. The aim of this research is to evaluate the larvicide activity of five botanicals (neemazal formulation, ginger, clove, garlic and orange oils) compared with indoxacarb against 3rd instar larvae of B. zonata and their efficacy on hemolymph physiological changes and chemical components activity under controlled conditions.
2 Material and methods
2.1 Insect rearing
The culture of B. zonata used in this research was supplied as pupae by Plant Protection Research Institute in Dokki, Giza, Egypt. B. zonata adults were raised in a cage (100 × 30 × 30 cm) with hardwood frames and a metal screen covering all four sides. The ratio of sucrose to reinforced protein hydrolysate fed to the flies in the rearing cage was 5:1, respectively. Moreover, water was added to a plastic bottle. False plastic fruits with numerous tiny apertures were provided to fill the cage (as oviposition receptacles). Three centimeters of water are placed into these plastic fruits to hold the eggs and keep them from drying out. Furthermore, Small plastic vials holding cotton wicks wet with guava juice to improve egg lay within these fake fruits were placed on top of these plastic fruits. The artificial diet that the larvae were raised on included 500 mL tap water, 3.00 g sodium benzoate, 3.00 g citric acid, 84.50 g sugar, 84.50 g brewer’s yeast and 330 g wheat bran. Large plastic containers were used to gently combine these materials. Then, using elastic bands to secure the muslin fabric to the plastic trays measuring 20x10x8cm, eggs were strewn across the top of the diet. These trays were then put inside a wooden cage with sand at the bottom so that the jumping larvae could develop into pupae. All pupae were isolated from sand by sifting. (Younes et al., 2009).
2.2 Tested compounds
Fresh leaves of orange, ginger, clove, and garlic were obtained from Plant Protection Research Institute in Dokki, Giza, Egypt. One kg of fresh leaves was washed, dried, and ground to a fine powder. Then, the powder was kept in cool dark conditions. To extract the essential oils, each 30 g of powder was inserted inside a 1.0 L flask with 0.5 L of distilled water. Then, the hydrodistillation using a Clevenger-type apparatus for 6 h was done (Oosta et al., 1978). Anhydrous sodium sulfate was used for drying the oil to remove traces of moisture. Oils were stored at 4 ◦C until use. Whilst, neemazal and indoxacarb (oxadiazine insecticide, known commercially as Avaunt, 30 % SC, DuPont, USA) were obtained from Plant Protection Research Institute at Giza, Egypt as a positive control.
2.3 Bioassays
Neemazal, orange, ginger, clove, garlic oils and indoxacarb at different concentrations were tested against 3rd larva instar of B. zonata for starved 12 h using slice dip method (Nazir et al., 2022). Apple fruits were cut into slices of 3 cm in diameter by a slicer cutter. Five slices were submerged for 10–15 s in each concentration, along with their successive dilutions and controls, then surface dried on tissue paper. Treated slices were then put in Petri plates with filter sheets underneath. In each petri dish, ten larvae of the third instar were released at each concentration level. As a control, slices were dipped in distilled water that had not been treated. Both treated and control larvae were kept at 25 ± 2 °C and 70–80 % relative humidity under a 12:12 h light to dark cycle in the insectary. All Petri plates were placed in controlled lab settings and covered with tight lids to prevent larvae from escaping Larvae were considered to be dead if they cannot move after being touched by a brush. Deaths were noted 24 h after treatment. This experiment was carried out three times. The insecticidal activities of these compounds were displayed by LC50 value, as well as a 95 % confidence interval.
2.4 Biochemical assays
Depending on the bioassay assay, the enzymatic activities of the investigated substances were studied using three repetitions of 10 third-instar larvae, which were treated with tested compounds solutions. Distilled water was used to treat the larvae in the control group. Biochemical assay has been done to evaluate sublethal concentrations LC50 effect of the tested compounds on total protein, total carbohydrates and total lipids. Moreover, amylase, invertase, trehalase, α-esterases and β-esterases, aspartate aminotransferase (AST) and alanine aminotransferase (ALT), protease and phenol oxidase enzyme. Additionally, nitrogen, potassium and phosphorus. Insects were homogenized in distilled water (50 mg/1 mL) using a chilled glass Teflon tissue homogenizer (ST-2mechnic- Preczyina, Poland). Centrifuging homogenates at 8000 rpm at 5 °C for 15 min in a refrigerator. The deposits were thrown away, and the supernatants were stored until use at −20 °C in a deep freezer.
2.4.1 Total protein
Total protein content of the same samples was determined by Oosta et al. (1978) method using bovine serum albumin (BSA) (Merck) as standard.
2.4.2 Total carbohydrates
Carbohydrate (glucose) was determined by Nelson (1944) method. The tissue homogenate was processed to remove the proteins, and the filtrate, which solely contained glucose as a reducing substrate, was heated with an alkaline copper reagent before being subjected to an arsenomolybdate reagent treatment. The blue color was measured at 540 nm.
2.4.3 Total lipid
Total lipids were determined by Knight et al. (1972). The reducing action of fatty acids on a sulphuric acid dichromate combination was used to quantify the amount of lipids in the aqueous phase after they were separated from non-lipid components using a chloroform–methanol solution. The resulting green color was then read at 600 nm.
2.4.4 Carbohydrate hydrolyzing enzymes (Amylase, invertase and trehalase activity)
Digestive enzymes were determined according to Amin (1998). In general, 230 uL of phosphate buffer (pH 5.4, 0.1 M), 250 µL of 4 % sucrose solution for invertase activity, and 20 uL of diluted enzyme solution were incubated for 10 min at 30 ˚C. When 250 µL of DNS reagent was added to each tube in boiling water for five minutes, the reaction was stopped.
2.4.5 Protein hydrolyzing enzymes
2.4.5.1 Transaminases enzymes
The activity of both enzymes AST and ALT were estimated by Reitman and Frankel (1957) method, using Diamond Diagnostic kit (Diamond Co. Egypt). In this approach, 500 μL of 100 mM phosphate buffer, pH = 7.2, 80 mM L-aspartate as a substrate for AST or 80 mM D-L-alanine as a substrate for ALT, and 4 mM α-ketoglutarate were added to 100 μL of enzyme source. This mixture was incubated at 37˚C for 30 min. The solution was then mixed with 500 μL of the developing color reagent (4 mM 2, 4-dinitrophenylhydrazine), and the mixture was allowed to incubate at room temperature for 20 min. The final step involved adding 5 mL of 0.4 N NaOH, mixing it, and left at room temperature for 5 min. Using a Sequoia-Turner Model 340 Spectrophotometer, the absorbance was measured at a wavelength of 546 nm using an assay combination devoid of an enzyme source as the blank. As the blank, an assay mixture devoid of enzyme was utilized. The specific AST and ALT activities were measured in IU/mg protein/hr and expressed as a percentage of the control. The specific AST and ALT activities were measured in μg/g.
2.4.5.2 Protease activity
Total protease activity was estimated by Yang et al. (2020) method. Azocasein substrate 2 % (w/v) was used in 0.1 M Tris-HCl buffer, pH 8.0, 37 °C. 250 µL of a substrate and 300 µL of enzyme extract made up the reaction mixture, which was incubated at 37 °C for 30 min. The reaction was halted with 1.2 mL of 10 % trichloroacetic acid (v/v). It was then let left for 15 min on ice. A solution of 1.4 mL of 1.0 M NaOH was added before reading at 440 nm.
2.4.6 Phenol oxidase enzyme (PO) activity
PO activities were carried out by Cornet et al. (2009) using a spectrophotometric assay. 5 μL of supernatant extract was added to a microplate well along with 20 μL of PBS buffer and 140 μL of dH2O to detect active PO in the experiment. The mixture was then mixed with 20 μL of L-Dopa solution (the substrate for the PO, Sigma D-9628, 4 mg/mL of dH2O), and the colorimetric reaction was monitored at 30 °C in a microplate reader (Versamax, Molecular Devices). Readings were recorded at 490 nm for 30 min. PO was measured in μg/g.
2.4.7 Non-specific esterase determination
α- and β-esterase were estimated according to the method of Van Asperen (1962), using α- and β-naphthol acetate as substrates. The reaction mixture consisted of 5 mL substrate solution (3 × 10−4 M α-or β-naphthyl acetate, 1 % acetone, and 0.1 M phosphate buffer, pH 7) and 20 µL of homogenate. The mixture was incubated for 15 min at 27˚C, and 1 mL of diazo blue color reagent was added; the reagent was prepared by mixing 2 parts of 1 % diazo blue B and 5 parts of 5 % sodium lauryl sulfate. When the substrate was hydrolyzed to yield α- and β-naphthol, the absorbance was measured at 555 or 600 nm. α- and β-esterase were measured in μg/g.
2.5 Statistical analysis
LC50 values and 95 % confidence intervals were calculated by probit analyses using SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA, 2003). The enzymatic activity data were plotted using GraphPad Prism 5.0 (GraphPad Software Inc., La Jolla, CA, USA), and the significance of the data was assessed using a student's t-test.
3 Results
3.1 Tested compounds produced insecticidal toxicity against B. zonata larvae
The response of B. zonata larvae showed a significant difference between neemazal, orange oil, ginger oil, clove oil, garlic oil and indoxacarb treated using the slice dip method. As shown in Table 1, larvae were more susceptible to indoxacarb and neemazal than other tested botanicals. The LC50 values of neemazal, orange oil, ginger oil, clove oil and garlic oil were 174.72, 2590.2, 4514.38, 7224.01 and 9387.73 μg/mL respectively compared with 86.09 μg/mL of indoxacarb. LC50 mean the compound concentration when killing (24 h) insects to 50 %.
Compounds
Toxicity regression equation
LC50 (μg/mL)
95 % Fiducial limits (μg/mL)
r
P
df
Ginger oil
y = 0.0404x + 4.116
4514.38
4020.27–4989.65
0.93
0.70
4
Clove oil
y = 0.0073x + 4.759
7224.01
6832.47–7449.82
0.95
0.86
4
Garlic oil
y = 0.0091x + 4.321
9387.73
8836.20–9637.74
0.99
0.95
4
Orange oil
y = 0.0421x + 3.9855
2590.2
2130.33–2896.31
0.95
0.91
4
Neemazal
y = 0.054x + 3.4555
174.72
139.75–155.12
0.99
0.65
4
Indoxacarb
y = 0.0168x + 4.638
86.09
78.45–92.41
0.96
0.51
4
3.2 Effect of tested compounds on total protein, total carbohydrates and total lipids in B. zonata larva
The concentration of total protein, total carbohydrates and total lipids in hemolymph of B. zonata 3rd instar larvae were studied (Fig. 1). A significant decrease (p < 0.05) in total protein content was noticed in indoxacarb compared with other treatments. Total protein level was recorded in indoxacarb (1.79 mg/mL) followed by neemazal (13.03 mg/mL), orange oil (21.17 mg/mL), garlic oil (25.40 mg/mL), ginger oil (28.06 mg/mL) then clove oil (30.49 mg/mL) compared with control (40.87 mg/mL) (Fig. 1a).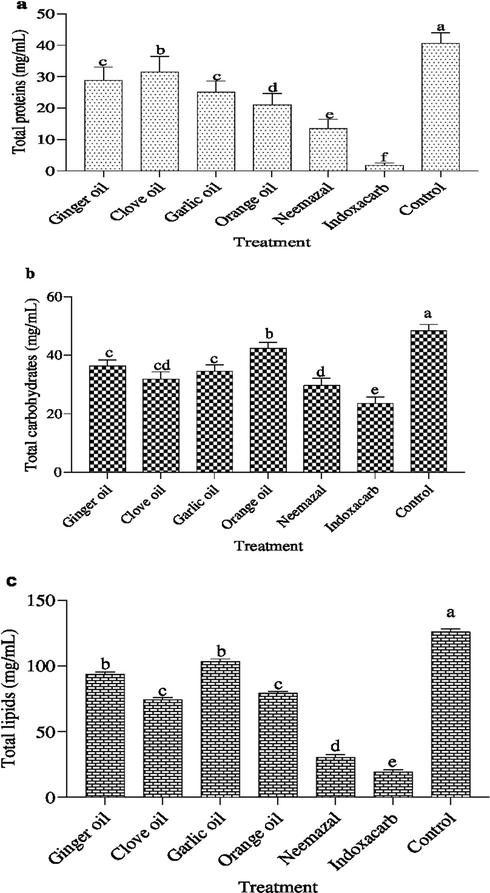
Effect of tested botanical compared to indoxcarb at LC50 value on (a) total proteins, (b) total carbohydrates and (c) total lipids concentration (mg/ml) in the supernatant of B. zonata 3rd instar homogenate larvae under laboratory conditions. Different letters on top of the bar indicate significant differences (p < 0.05).
Fig. 1b showed statistically significant differences in the levels of total carbohydrates in botanicals and indoxacarb compared with control. Total carbohydrates decreased gradually from 48.62 mg/mL in control to 29.12 mg/mL in neemazal, then terminated by indoxacarb (23.93 mg/mL). The other botanical extract sited between control and indoxacarb and recorded decreasing total carbohydrates in descending order (42.48 mg/mL) for orange oil, ginger oil (36.68 mg/mL), garlic oil (34. 76 mg/mL) and clove oil (32. 22 mg/mL).
Changes in lipids content in 3rd instar larvae treated with different extracts are shown in (Fig. 1c). Tested botanical extracts showed a significant reduction (p < 0.05) and recorded descending order in lipids content 19.27 mg/mL for indoxacarb, 30.40 mg/mL for neemazal, 93.59 mg/mL for ginger oil, 103.3 mg/mL for garlic oil, 79.31 mg/mL for orange oil and 74.34 mg/mL for clove oil compared control recording 125.91 mg/mL, respectively.
3.3 Effect of tested compounds on some biochemicals of B. zonata larva hemolymph
3.3.1 Digestive enzymes
All treatments reduced amylase, invertase and trehalase activity than in control Fig. 2. Indoxacarb and neemazal were the most effective and occupied (p < 0.05) the first category from the side of reducing amylase representing 353.31 and 359.03 μg/g as compared control (2013.36 μg/g), respectively. However, the tested botanical extracts reduced amylase in descending order according to concentrations and their effects ginger oil (717.27 μg/g), garlic oil (470.51 μg/g), orange oil (1592.72 μg/g) and clove oil (2008.69 μg/g) occupied the last category in reducing amylase in 3rd larvae B. zonata, respectively.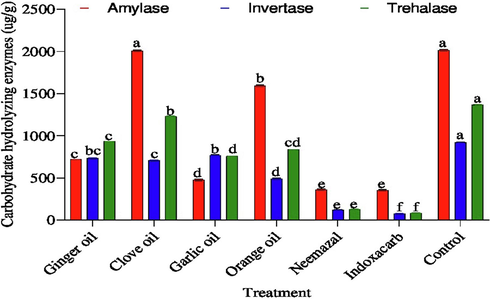
Effect of the tested compounds on carbohydrate hydrolyzing enzymes (Amylase, invertase and trehalase activity) 3th instar larvae of B. zonata after treatment with LC50. Different letters on top of the bar indicate significant differences (p < 0.05).
As for invertase enzyme, all treatments can be arranged to descend according to their effects as follow indoxacarb (75.50 μg/g), neemazal (116.27 μg/g), garlic oil (768.39 μg/g), ginger oil (734.95 μg/g), clove oil (708.42 μg/g) and orange oil (489.37 μg/g) at LC50 value compared with control (918.54 μg/g), respectively.
The highest trehalase enzyme activity was recorded in control (1361.09 μg/g), clove oil (1227.73 μg/g), ginger oil (929.82 μg/g), orange oil (834.95 μg/g), garlic oil (755.88 μg/g) at LC50 value, respectively. Whist, the lowest activity was recorded in indoxacarb (80.59 μg/g) and neemazal (124.50 μg/g) as compared to control.
3.3.2 Protein hydrolyzing enzymes
Fig. 3 showed the changes in the activities of AST and ALT enzymes in hemolymph of 3rd instar B. zonata larvae. The activity of enzymes AST was less in both indoxacarb and neemazal than in control recording 62.97, 192.29, and 362.96 μg/g respectively. While no significant different has observed between clove, ginger, orange and garlic oils compared with control and recording (341.88, 337.83 and 331.88, 317.57 μg/g), respectively. Whereas, indoxacarb, neemazal, garlic, ginger and orange oils decreased the activity of ALT than control, where it reached the minimum activity and significantly recorded 19.53, 223.69, 272.9, 297.91, 327.75 and 360.27 μg/g, respectively. On the other hand, clove oil was increased in the hemolymph of the 3rd instars larvae and recorded 410.72 μg/g.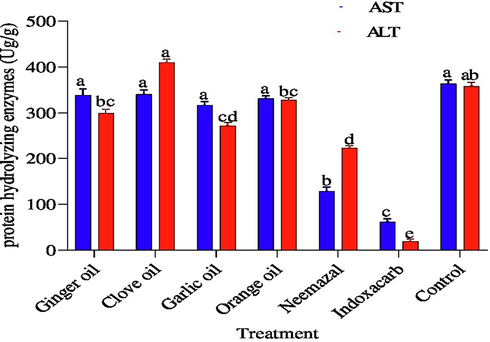
Effect of the tested compounds on aspartate aminotransferase (AST), alanine aminotransferase (ALT) 3th instar larvae of B. zonata after treatment with LC50. Different letters on top of the bar indicate significant differences (p < 0.05).
The effect of tested botanical extracts and indoxacarb on protease enzyme is shown in Fig. 4. indoxacarb and neemazal effective treatment through the reduction in enzyme recording 246.3 and 23.29 U/g. While, garlic, ginger and orange oils affected the reduction of protease recording 252.6, 270.3 and 307.6 U/g compared with control 455.5 U/g respectively. On the other hand, clove oil increased the activity of protease enzyme and recorded 564.8 U/g compared with control.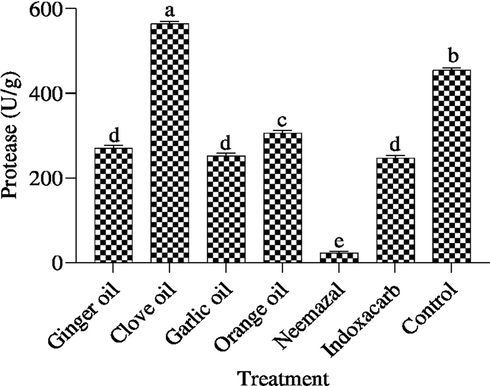
Effect of the tested compounds on protease 3th instar larvae of B. zonata after treatment with LC50. Different letters on top of the bar indicate significant differences (p < 0.05).
Our data shown in Fig. 5, the activities of phenol oxidase enzyme were dramatically decreased in all treatments compared with that of the control. Obviously, the phenol oxidase enzyme activities were significantly inhibition (p < 0.05) in indoxacarb and neemazal, garlic oil, orange oil, ginger and clove oil compared with control, and recording 29.16, 61.64, 71.87, 89.39, 338.69 and 351.27 U/g compare with 429.01 μg/g in control, respectively. Meanwhile, no significant different has observed between ginger and clove oil (338.69 and 351.27 μg/g, respectively) compared with control.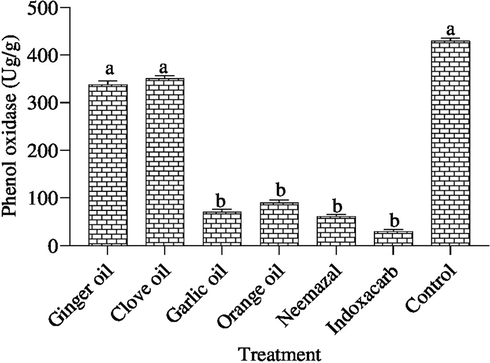
Effect of the tested compounds on phenyl oxidase 3th instar larvae of B. zonata after treatment with LC50. Different letters on top of the bar indicate significant differences (p < 0.05).
3.3.3 Non-specific esterases determination
All treatments decreased the activity of α-esterase except indoxacarb and neemazal compared with control. As shown in Fig. 6, garlic oil was the most effective in reducing α-esterase representing 22.50 μg/g, followed by orange oil, clove oil and ginger oil recording 28.51, 47.43 and 87.79 μg/g. Whilst, indoxacarb and neemazal were recorded at 104.73 and 111.06 μg/g compared with control (101.20 μg/g), respectively. On the other hand, all treatments inhibited β-esterase activity except ginger oil and neemazal. β-esterase activity showed a significant (p < 0.05) decrease with indoxacarb by 198 μg/g, followed by orange, garlic and clove oils reducing this enzyme and recording 349.94, 522.42. 956.78 μg/g. However, β-esterase activity showed a tendency to increase with ginger oil 2174.7 μg/g and neemazal 1310.8 μg/g compared with control (1013.73 μg/g), respectively.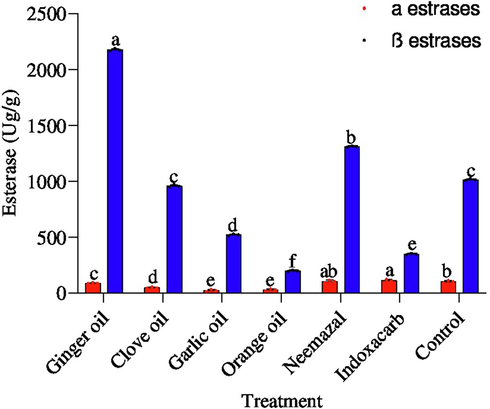
Effect of the tested compounds on α and β esterase 3th instar larvae of B. zonata after treatment with LC50. Different letters on top of the bar indicate significant differences (p < 0.05).
4 Discussion
There is no doubt that the effectiveness and frequent misuse of synthetic pesticides cause a number of issues, namely causing bug resistance and environmental contamination (Shabana et al., 2017). Therefore, the world is currently turning to the use of plant-based pesticides as a safe method for the environment and effective against pests (Sayed et al., 2021). In our study, all tested botanicals (neemazal, ginger, clove, garlic and orange oils) exhibited insecticidal activity against B. zonata larvae. This result might be attributed to the chemical constituents contained in tested botanicals probably caused the major factor in dead insects (Sayed et al., 2022). This result was consistent with a previous study Mahmoud and Shoeib (2008) that reported neem formulation considerably outperformed controls in terms of insecticidal activity against B. zonata. Additionally, Garlic oil was contacting toxic (LD50 = 1.42 g/adult) to Calyptraea chinensis adult in winter (Zhao et al., 2013). Although indoxacarb causes serious environmental problems for humans. However, it was the highest insecticidal activity against B. zonata larvae with LC50 = 86.09 μg/mL. Our data could be the result of sodium channel blockage in the insect nervous system, and the entry points are through the stomach and touch routes (Qie et al., 2020). Our findings in harmony with Aioub et al. (2021) that reported LC25 and LC50 values of indoxacarb decreased the numbers of 3rd and 4th larval instars of Pieris rapae. Based on the assay data, plant-based pesticides have an important role in insect control.
Our data showed all treatments significantly reduced total protein, total carbohydrates and total lipids with the LC50 of tested compounds. Proteins are crucial for fitness-related features at the individual level, like body size, growth rate, and fecundity. At higher organizational levels, they have also been connected to population dynamics, life histories, and even biological diversity (Fagan et al., 2002). Larval protein level may have decreased as a result of decreased protein synthesis or increased protein breakdown to detoxify the active compounds found in plant extracts (Vijayaraghavan et al., 2010). This conclusion was in agreement with Ahmed et al. (Ahmed et al., 2022) reported that neemazal T/S, clove and ginger oils decreased the total protein level in Tuta absoluta. Carbohydrates are a vital source of energy for insects. Carbohydrates can be transformed into lipids and can help produce amino acids. The decrease in carbohydrates could be attributed to the enhanced metabolism brought on by toxicant stress as well as the potential for active glycogenolysis and the glycolytic pathway to provide additional energy under stressful circumstances (Franeta et al., 2018). Our results were concurrent with Ahmed et al. (2022) reported that total protein level in T. absoluta decreased by neemazal T/S, clove and ginger oils. Lipids are made up of phospholipids in living things, free and bound fatty acids, short- and long-chain alcohols, steroids and their esters, and other types of chemicals. The detoxification process in larvae, which necessitates the conversion of a significant amount of eaten food into energy following treatment with pesticides, may be to blame for the decrease in total lipid concentrations (Xu et al., 2016). Our findings are generally in agreement with those reported by Ahmed et al. (2022) who mentioned that garlic oil and neemazal formulation decreased the total lipid levels in Ceratitis capitata.
Our findings revealed a significant decrease in carbohydrate hydrolyzing enzymes (amylase, invertase and trehalase) in treated larva compared to untreated larva after 24 h from treatment. This outcome could be attributed to amylase, the most significant digestive enzyme found in many insects that only consume plants as larvae or adults. Energy is reduced as a result of the organism's poor nutrition when the action of the amylases is impeded. The current decline is consistent with work by Mojarab-Mahboubkar et al. (2015) who found that treatment with A. annua essential oil significantly reduced the activity of the enzyme amylase in the midgut of Helicoverpa armigera larvae. Trehalase is an inverting glycosidase that encourages the conversion of trehalose into two molecules of glucose, which is crucial for insect flight and necessary for the resistance of larvae to stress stimuli (Becker et al., 1996). The present decrease in trehalase may be due to its crucial function in the homeostasis and glycometabolism of insects, and it has been suggested as a potential target for insect pest control. Our result is in harmony with Oladipo et al. (2019) who showed that Xylopia aethiopica and Senna occidentalis extracts decreased trehalase activity on Callosobruchus chinensis. Invertase is an enzyme that facilitates the breakdown of 1,4-glucosidic bonds to produce glucose. This enzyme vigorously hydrolyzes pNP-d-glucopyranoside, maltose, sucrose, and maltodextrin (Ramzi and Hosseininaveh 2010).
Treated larvae had decreased levels of protease, AST, and ALT after 24 h of treatment. Proteases are crucial for the digestion of food in insects because they transform protein into the amino acids that the body needs (Dimou et al., 2022). Our result confirms the work of Bezzar-Bendjazia et al. (2017) who reported that botanical insecticides may prevent the creation of certain types of proteases and make them incapable of breaking down ingested proteins. A. annua L. essential oil increased from the mortality percentage of H. armigera and reduced the activity of protease (Mojarab-Mahboubkar et al., 2015). The transaminases are critical enzymes in the catabolism of amino acids, which is primarily concerned with transferring an amino group from one amino acid to another keto acid to create another amino acid. The aspartate and alanine aminotransferases constitute a crucial link between the metabolism of carbohydrates and proteins and are known to change under a variety of pathological and physiological circumstances (Etebari et al., 2005).
Our result showed the tested compounds decreased from Phenoloxidase (PO) activity in B. zonata larvae. Result showed the decrease in PO enzyme may be due to its critical function in the innate immune responses of insects, which catalyzes the manufacture of quinones and other reactive intermediates to obstruct invasive pathogens and parasites (Valadez-Lira et al., 2012). Moreover, it is crucial for the development of immune protection and wound healing on the basis of melanin creation. Furthermore, Calceolaria integrifolia remarkably suppressed phenoloxidase, resulting in a considerable reduction in insect development (Muñoz et al., 2013).
Esterase (EST) detected different attitudes in B. zonata larvae treated with tested compounds at LC50. The response enhancement of EST enzymes to botanical extracts was remarkably referred to the esterase enzymes belonging to detoxifying enzymes responsible for removing any foreign substances from an insect's body (Ahmed et al., 2022). In addition, Esterase is a crucial enzyme for detoxification that hydrolyzes the esteric link in any toxin. Furthermore, Esterase is one of the enzymes responding to environmental stimuli the most strongly. The findings presented here are consistent with those found by Yazdani et al. (2014) who showed that general esterase activity was elevated in Glyphodes pylolais larvae when treated with Thymus vulgaris L. and Origanum vulgare L. compared with control.
5 Conclusion
The present investigation indicated that indoxacarb and neemazal formulation exhibited the highest toxic effect against 3rd larva instar of B. zonata compared with Ginger, Clove, Garlic and orange oils. Additionally, tested compounds affected the activity of total protein, tota; carbohydrates and total lipids as well as carbohydrate hydrolyzing enzymes, protein hydrolyzing enzymes, phenoloxidase and non-specific esterase in this pest. These extracts may thus serve as an alternative to conventional insecticides for B. zonata control.
Acknowledgments
This research was supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R365), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The larvicidal effect of neemazal t/s, clove oil and ginger oil on tomato leafminer, tuta absoluta compared to coragen. Saudi J. Biol. Sci.. 2022;29(3):1447-1455.
- [Google Scholar]
- Compatibility of entomopathogenic nematodes with insecticides against the cabbage white butterfly, pieris rapae l. (lepidoptera: Pieridae) Egypt. J. Biol. Pest Control. 2021;31:1-12.
- [Google Scholar]
- Host plant records for fruit flies (diptera: Tephritidae) in southeast asia. Raffles Bull. Zool.. 1999;47(Supplement 7):1-92.
- [Google Scholar]
- Biochemical and physiological studies of some insect growth regulators on the cotton leafworm, spodoptera littoralis (boisd.). 1998;2(8):587-594. Ph.D. Thesis
- Loss assessment and management of bactrocera zonata (diptera: Tephritidae) in citrus orchards. Pak. J. Agric. Sci.. 2020;57(2):451-456.
- [Google Scholar]
- Azadirachtin induced larval avoidance and antifeeding by disruption of food intake and digestive enzymes in drosophila melanogaster (diptera: Drosophilidae) Pestic. Biochem. Physiol.. 2017;143:135-140.
- [Google Scholar]
- Variation in immune defence among populations of gammarus pulex (crustacea: Amphipoda) Oecologia. 2009;159:257-269.
- [Google Scholar]
- The peach fruit fly, bactrocera zonata: A major threat for mediterranean fruit crops? In: XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on the 940. 2010. p. :557-566.
- [Google Scholar]
- Radial and longitudinal density variations in abies cephalonica and pinus halepensis. J. For. Res.. 2022;12(2):87-94.
- [Google Scholar]
- A study on interaspecific biodiversity of eight groups of silkworm (bombyx mori) by biochemical markers. Insect Sci.. 2005;12(2):87-94.
- [Google Scholar]
- Nitrogen in insects: Implications for trophic complexity and species diversification. Am. Nat.. 2002;160(6):784-802.
- [Google Scholar]
- Effects of different insecticides on the antioxidative defense system of the european corn borer (ostrinia nubilalis hubner) (lepidoptera: Crambidae) larvae. Archiv. Biol. Sci.. 2018;70(4):765-773.
- [Google Scholar]
- Biological aspects of the peach fruit fly, bactrocera zonata (saund.) (diptera: Tephritidae) and its parasitoid species, aganaspis daci weld. (hymenoptera: Eucoilidae). Egyptian Journal of Biological. Pest Control. 2011;21(2)
- [Google Scholar]
- Chemical basis of the sulfo-phospho-vanillin reaction for estimating total serum lipids. Clin. Chem.. 1972;18(3):199-202.
- [Google Scholar]
- Sterilant and oviposition deterrent activity of neem formulation on peach fruit fly bactrocera zonata (saunders)(diptera: Tephritidae) J. Biopest.. 2008;1(2):177-181.
- [Google Scholar]
- Effect of artemisia annua l. Essential oil on toxicity, enzyme activities, and energy reserves of cotton bollworm helicoverpa armigera (hübner) (lepidoptera: Noctuidae) J. Plant Protect. Res.. 2015;55(4)
- [Google Scholar]
- Tyrosinase inhibitors from calceolaria integrifolia sl: Calceolaria talcana aerial parts. J. Agric. Food Chem.. 2013;61(18):4336-4343.
- [Google Scholar]
- Distribution, host range and toxicity assessment of different insecticides on bactrocera diversa coquillett, 1904 (diptera: Tephritidae) Braz. J. Biol.. 2022;84
- [Google Scholar]
- A photometric adaptation of the somogyi method for the determination of glucose. J. Biol. Chem.. 1944;51(3):448-460.
- [Google Scholar]
- Effects of aqueous and oil leaf extracts of pterocarpus santalinoides on the maize weevil, sitophilus zeamais pest of stored maize grains. Afr. J. Agric. Res.. 2018;13(13):617-626.
- [Google Scholar]
- Bactrocera zonata. Data sheets on quarantine pests. European and mediterranean plant protection organization. EPPO Bull.. 2005;35:371-373.
- [Google Scholar]
- Toxicity and biochemical mechanisms underlying the insecticidal efficacy of two plant extracts on callosobruchus chinensis (coleoptera: chrysomelidae) infesting cowpea seeds. J. Crop Protect.. 2019;8(3):259-274.
- [Google Scholar]
- Optimization of folin-ciocalteu reagent concentration in an automated lowry protein assay. Anal. Biochem.. 1978;89(1):31-34.
- [Google Scholar]
- Safeguarding pollinators and their values to human well-being. Nature. 2016;540(7632):220-229.
- [Google Scholar]
- Insight into the detoxification of haedoxan a and the synergistic effects of phrymarolin i against mythimna separata. Ind. Crop. Prod.. 2020;158:112967
- [Google Scholar]
- Biochemical characterization of digestive α-amylase, α-glucosidase and β-glucosidase in pistachio green stink bug, brachynema germari kolenati (hemiptera: Pentatomidae) J. Asia Pac. Entomol.. 2010;13(3):215-219.
- [Google Scholar]
- A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol.. 1957;28(1):56-63.
- [Google Scholar]
- Preference and performance of peach fruit fly (bactrocera zonata) and melon fruit fly (bactrocera cucurbitae) under laboratory conditions. Saudi J. Biol. Sci.. 2022;29(4):2402-2408.
- [Google Scholar]
- Suitability of five plant species extracts for their compatibility with indigenous Beauveria bassiana against Aphis gossypii Glov. (Hemiptera: Aphididae) Egypt. J. Biol. Pest Control. 2021;31:11.
- [Google Scholar]
- Toxicity, deterrent and repellent activities of four essential oils on Aphis punicae (Hemiptera: Aphididae) Plants. 2022;11:463.
- [Google Scholar]
- Efficacy of plant extracts in controlling wheat leaf rust disease caused by puccinia triticina. Egypt. J. Basic Appl. Sci.. 2017;4(1):67-73.
- [Google Scholar]
- Practical Approaches to Pest Control: The Use of Natural Compounds. Pests, Weeds and Diseases in Agricultural Crop and Animal Husbandry Production. London, UK: IntechOpen; 2020.
- Comparative evaluation of phenoloxidase activity in different larval stages of four lepidopteran pests after exposure to thuringiensis bacillus. J. Insect Sci.. 2012;12(1):80.
- [Google Scholar]
- A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol.. 1962;8(4):401-416.
- [Google Scholar]
- Effect of plant extracts on biochemical components of cabbage leaf webber, crocidolomia binotalis zeller. J. Biopest.. 2010;3(Special Issue):275.
- [Google Scholar]
- Effects of sublethal concentrations of cyantraniliprole on the development, fecundity and nutritional physiology of the black cutworm agrotis ipsilon (lepidoptera: Noctuidae) PLoS One. 2016;11(6):e0156555.
- [Google Scholar]
- Ph influences the profiles of midgut extracts in cnaphalocrocis medinalis (guenée) and its degradation of activated cry toxins. J. Integr. Agric.. 2020;19(3):775-784.
- [Google Scholar]
- Effect of thymus vulgaris l. And origanum vulgare l. Essential oils on toxicity, food consumption, and biochemical properties of lesser mulberry pyralid glyphodes pyloalis walker (lepidoptera: Pyralidae) J. Plant Prot. Res.. 2014;54(1)
- [Google Scholar]
- Histopathological effects of gamma irradiation on the peach fruit fly, bactrocera zonata (saund.) female gonads. J. Appl. Sci. Res.. 2009;5(3):305-310.
- [Google Scholar]
- Evaluation of acute toxicity of essential oil of garlic (allium sativum) and its selected major constituent compounds against overwintering cacopsylla chinensis (hemiptera: Psyllidae) J. Econ. Entomol.. 2013;106(3):1349-1354.
- [Google Scholar]
- Global risk of invasion by bactrocera zonata: Implications on horticultural crop production under changing climatic conditions. PLoS One. 2020;15(12):e0243047.
- [Google Scholar]







