Translate this page into:
Larvicidal activities of family Anacardiaceae on Aedes mosquitoes (Diptera: Culicidae) and identification of phenolic compounds
⁎Corresponding author at: School of Biological Sciences, Universiti Sains Malaysia, Pulau, Pinang, Malaysia. wfatma@usm.my (Wan Fatma Zuharah),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
The mosquito vector control program still relies on the use of synthetic insecticides causes resistance in vector species. The application of alternative strategies using phytochemical sources is a possible way to eliminate the vector of dengue, Chikungunya and Zika viruses. In this study, we tested the larvicidal efficacy of Anacardium occidentale (Cashew), Mangifera indica (Mango), Gluta renghas (Rengas) and Melanochyla fasciculiflora (Rengas) methanolic extract from stems against Aedes albopictus and Ae. aegypti.
Methodology
The stem of A. occidentale, Mn. indica, G. renghas and Ml. fasciculiflora was extracted using Soxhlet extraction using methanol as a solvent. These crude extracts were tested on Ae. aegypti and Ae. albopictus using WHO larval bioassay. The chemical compounds were screening, and the fractions were confirmed using UPLC.
Results
Our study found Gluta renghas exhibited the strongest larvicidal efficacy toward Ae. albopictus at 607 mg/L and 976 mg/L for Ae. aegypti. In terms of order, Gl. renghas exhibited significantly best effectiveness, followed by Mn. indica, Ml. fasciculiflora and An. occidentale on both Aedes species. Screening results on chemical compounds found the presence of saponins, tannins, alkaloids, reducing sugar, flavonoid, and steroid but the absence of alkaloids in all four plants. The fraction by UPLC confirmed the existence of five prominent peaks of quercetin at a retention time of 4.171–4.358 min and apigenin derivatives at 5.010 min in Gl. renghas. However, the higher concentration of apigenin and quercetin in the plant is not a contributing factor in the effectiveness of phytochemical on Aedes mosquitoes. Thus, suggested the larvicidal effects were derived from the combination and synergistic action between cumulative quercetin and apigenin compounds.
Conclusion
Gluta renghas had shown its ability to become the promising bio-larvicides due to the lower dose needed to cause the lethal effect to mosquito larvae. This finding is valuable for bio-larvicides to be integrated with the vector control program.
Keywords
Anacardiaceae
Aedes
Mosquito
Larvicidal
Plant extract
Phenolic compound
1 Introduction
Mosquito-borne diseases have economic impacts in the form of health and commercial losses, specifically in countries within the subtropical and tropical regions (Panneerselvam and Murugan, 2013). More than 100 countries are reported to be endemic to mosquito-borne diseases, and every year about two million people die due to these diseases (Subramaniam et al., 2011). Four countries, particularly Philippines, Cambodia, Malaysia, and Vietnam are confronting annual epidemics, which are more than 90% of the total reported dengue cases in the region (Chang et al., 2011). Aedes albopictus and Aedes aegypti and are reported as potential vectors of several viruses, including dengue, Yellow Fever, Zika and Chikungunya. To date, these species have become the leading vectors in the transmission of dengue hemorrhagic fever and dengue in subtropical and tropical regions (Centers for Disease Control and Prevention, 2005). Dengue is endemic in Malaysia, spreading irrespective of the urbanization level (SaifurRahman, 2012) transmitted by Ae. albopictus and Ae. aegypti (Smith, 1956). Aedes aegypti is a domestic mosquito in urban areas, whereas Aedes albopictus is a semi-domestic mosquito (Mendenhall et al., 2017) and an anthropic in nature (Huber et al., 2008). Most of these dengue vectors species are common in natural and artificial containers such as gutters, pools, septic tanks, the tree holes, leaf axils, fruit peels, discarded and unused tires, water jars, old boats and others (Cadena, 2013).
With the introduction of organic insecticides in the 1940s, chemical control became the most popular approach for controlling mosquito (Yap and Zairi, 2003), and the control effort of mosquitoes is mainly relying on chemical insecticide. Long term exposure to the chemical insecticides will lead to selecting mutation, conferring a level of resistance to chemical insecticides, and indeed, the insecticide-resistance population of mosquitoes is now affecting the success of control programs (Dusfour et al., 2019). Biological products such as plant extracts offer boundless possibilities for a new phytochemical discoveries due to the higher availability of a vast range of chemicals (Cos et al., 2006). Family Anacardiaceae is comprised of economically important edible plants like cashew nut (Anacardium occidentale), pistachio nuts (Pistacia Vera), and mango (Mangifera indica), along with other regional imperative plants (Garcia et al., 2000). Anacardiaceae consists of 76 genera comprised of 600 species, with the majority of which are found in tropical and subtropical regions (Hartley, 1998) and affirmed for their medicinal properties (Eloff, 2001). Some of these plants, especially Mn. indica have been used as herbal medicine in some countries to cure warts, ulcers, fever, corns, dysentery, tumour, piles, insect repellent and fungicidal (Olotu et al., 2020).
Compounds and extracts derived from Mn. indica is numerously reported for antifungal, antifeedant, antioxidant, antimicrobial, antidiarrheal, hepatoprotective, hypoglycemic and larvicidal activities (Rahuman et al., 2009). Cashew nut shell liquid (CNSL) from Anacardium occidentale contains flavonoids, steroids, tannins, glycosides, phenols, terpenoids, triterpenoids, anacardic acid, cardanol, cardol, carbachol, orcinol and quercetin (Oliveira et al., 2011).
Four plants from this family Anacardiaceae, were selected for this study due to their natural abundance in tropical regions and potential as a larvicidal agent, namely, An. occidentale, Mn. indica, Gluta renghas and Melanochyla fasciculiflora. This study aimed to (i) evaluate larvicidal efficacy on Ae. albopictus and Ae. aegypti larvae, and (ii) detect the phenolic compounds. Till now, only Mn. indica and An. occidentale had been studied for their chemical compounds. This is the first report on the chemical compounds of two other endemic plants of Malaysia (Ml. fasciculiflora and Gl. renghas).
2 Methodology
2.1 Plant materials and extraction
2.1.1 Plant species
Mature stem parts of An. occidentale, Mn. indica, Ml. fasciculiflora and Gl. renghas were selected for the study due to stability of complex reactions, which leads to a broad range of biochemical properties. These plant parts were collected from the Penang National Park, Penang (5°27′38.56″N, 100°12′18.69″E) and Taman Negeri, Bukit Panchor (5°10′10.607″N, 100°32′37.291″E), Malaysia. The temperature for these places is between 25.8 °C and 31.3 °C and receive rainfall on average of 220 mm each year. Herbarium staff of the School of Biological Sciences, Universiti Sains Malaysia had verified and confirmed the plant samples from this study.
2.1.2 Plant extract preparations
The stem was left in the laboratory to dry under normal environmental condition. Stem required about 20–25 days to completely dry. Dried stem was mashed by using a tabletop hammer mill. The powdered samples were then extracted using methanol solvent in the Soxhlet apparatus. For the extraction, 2000 ml of methanol and 50 g of powdered sample was used. The powdered sample was placed in a cellulose thimble (Favorit cellulose extraction thimbles: size 43 × 123 mm) and inserted in the extraction tube of the Soxhlet apparatus. The solvent was boiled at the methanol boiling point at 66 °C using a heating mantle. The process was repeated for three cycles which took about 3 h. The complete extraction happened when the colour of the solvent became semitransparent. The process was repeated three times to have enough crude extracts for the larval bioassay study. To remove the excess solvent, the crude extracts were subjected to an evaporation process using the rotary vacuum evaporator machine under reduced pressure for about 25–30 mins at 66 °C with a speed of 100 RPM. The crude extract was placed in the oven at 40 °C for 24 h to remove all of the excess solvent. The crude extract obtained after removing the excess solvent was stored in a refrigerator at 4 °C until further use. This procedure was done separately for both plant part and plant species.
2.2 Mosquitoes strain
The field strain of Ae. aegypti and Ae. albopictus were obtained from two locations located at Bukit Jambul (5o20′06.7″ N 100o17′26.0″ E) and Flat Hamna (5o20′53.9″ N 100o18′02.8″ E) residential apartments using ovitrap method. Eggs and larvae from ovitraps were brought back to the laboratory and reared until reaching the late 3rd and early 4th instar larvae. Larvae collected from the field were identified into species level using the identification key by Farajollahi and Price (2013). Larvae were fed with finely powdered food, a mixture of dog biscuit, beef liver, yeast, and powdered milk by weight at a ratio of 2:1:1:1 at 0.01 g daily.
2.3 Larvicidal bioassays
Late 3rd and early 4th instar larvae of Ae. aegypti and Ae. albopictus were used in this experiment as in WHO standard larvicidal bioassay (World Health Organization, 2005). Twenty larvae was placed in a 250 ml paper cup containing 200 ml of the different serial concentrations of extracts. A range of concentrations between 50 and 1300 mg/L was prepared and set to 11 concentrations and replicated for three times. Controls comprised 1 ml of 100% of methanol in 199 ml of distilled water. Mortality observations were made after 24 h post-exposure for all the treatments. No food was provided to larvae during the experiment. Larvae were considered dead if they were motionless after touching with a needle. All the culture and experiments were maintained under laboratory conditions at a temperature of 28 ± 3 °C and relative humidity of 70 ± 10%.
2.4 Phytochemical analysis
Methanolic extracts of the stem from An. occidentale, Gl. renghas, Mn. indica and Ml. fasciculiflora were analyzed for the presence of saponins, steroids, flavonoids, tannins, reducing sugars and alkaloids. Detection was then followed by the phenolic compound detection using UPLC. All tests were run separately for each plant stem extract.
2.4.1 Saponins analysis
A total of 0.5 g of each plant extract was taken separately and mixed 300 ml of water in test tubes. Test tubes were made warm in a water bath at 50 °C and then shaken vigorously. The presence of foam or bubbles was observed as initial evidence for saponins. Few drops of olive oil were added to 0.5 g plant extract and then continuous shaking, resulting in the soluble emulsion, which also indicates the occurrence of saponins (Ngbede et al., 2008).
2.4.2 Tannins analysis
Methanolic stem extract of 0.5 g of each extract was added with 10 ml of 15% ferric chloride test solution and tested against all four plants. The dark blue colour indicated the presence of tannins (Aiyelaagbe and Osamudiamen, 2009).
2.4.3 Steroids analysis
About 100 mg of each of the plant extract was dissolved separately in 50 ml of chloroform. Few drops of sulfuric acid was added to the solution to form a lower layer. A reddish-brown colour is indicative of the presence of the steroid ring (Sofowora, 1993).
2.4.4 Reducing sugar analysis
A total of 100 mg of each of the extract was re-dissolved in 2 ml of water in the water bath. Four millilitres of the solution were taken in test tubes. A total of 1 ml of each Fehling’s solution A and Fehling’s solution B was added to the extract solution in test tubes. The mixture was shaken and heated in the water bath for ten minutes. Reducing sugar was noticed by red brick precipitation (Aiyelaagbe and Osamudiamen, 2009).
2.4.5 Flavonoids analysis
About five grams of the powdered stem of each of the four plants were soaked in 50 ml acetone. Acetone was evaporated in the oven at a temperature of 40 °C. The residue was extracted in warm water and then filtered while still warm. The filtrate was left to cool. A total of 5 ml of 20% of NaOH solution was added to the filtrate. The yellow colour indicates the presence of flavonoids (Aiyelaagbe and Osamudiamen, 2009).
2.4.6 Alkaloids analysis
Five grams of an extract of each of the four plants was dissolved in 5 ml of ethanol containing 3% tartaric acid. The solution was then filtered. 50 ml of filtrate was poured into two test beakers for alkaloids test as follows: 10 ml Hagar’s reagent in the first beaker and 10 ml Marquin’s reagent in the second beaker. Precipitation in either of the two beakers is indicative of the presence of alkaloids (Aiyelaagbe and Osamudiamen, 2009).
2.5 Detection of phenolic compounds by UPLC
Phenolic compounds in each stem extract were detected using an Acquity Ultra Performance Liquid Chromatography (UPLC) system (Waters, Milford, MA) equipped with a reverse-phase Acquity UPLC BEH C18 column, 1.7 µm (100 mm × 2.1 mm i.d.), and a photo-diode array detector. The mobile phase consisted of solvent A (2% acetic acid) and solvent B (methanol: acetic acid: water, 18:1:1). Each extract was separated using a gradient mode initially set at A: B ratio of 85:15 and then linearly increased to 65:35 at 1.5 min, 40:60 at 2 min to 6.3 min. The detector was set at 320 µL, with a flow rate of 0.20 ml/min and an injection volume of 5.0 µL. Phenolic compounds in the stem extracts were identified by comparing their UV spectra (detected by the photo-diode array detector) with those of authentic standards.
2.6 Statistical analysis
The LC50 and LC95 values for larvicidal activities were calculated using Probit analysis of SPSS version 20. A multivariate analysis of variance was analyzed using SPSS version 20 to examine the effects of the different mosquito strain, concentration and plant species on Aedes larval mortality. The number of larval mortality was considered as the dependent variable, whereas concentration, Aedes species and plant species were considered as the fixed factors. Data were log-transformed to fulfil MANOVA assumption. The level of significance was set at P < 0.05.
3 Results
3.1 Larvicidal activity of family Anacardiaceae
The larvicidal activity of Family Anacardiaceae was tested against field strain mosquitoes collected from dengue hotspot areas has been found to exhibit the lowest 100% mortality when treated with Gl. renghas of 900 mg/L for Ae. albopictus and 1000 mg/L for Ae. aegypti (Fig. 1). Among the four stem extracts listed in Table 1, Gl. renghas showed the strongest larvicidal effects towards both Ae. albopictus and Ae. aegypti at 607 mg/L and 976 mg/L, respectively. Significantly lower concentration of Gl. renghas were needed for Ae. albopictus as compared to Ae. aegypti (MS = 24.26, df = 1, P = 0.002, Table 2). The weakest larvicidal effects were given by An. occidentale with LC50 of 1200 mg/L for Ae. albopictus and 1347 mg/L for Ae. aegypti. In terms of the order, Gl. renghas exhibited the best effectiveness, followed by Mn. indica, Ml. fasciculiflora and An. occidentale for both Aedes species. Significant differences were detected between the mortalities caused by the crude extracts of the four plants against both Aedes species (MS = 12.65, df = 1, P = 0.002, Table 2). *df = degree of freedom, MS = Mean Square values. *Significant values are in bold at 5% significance level.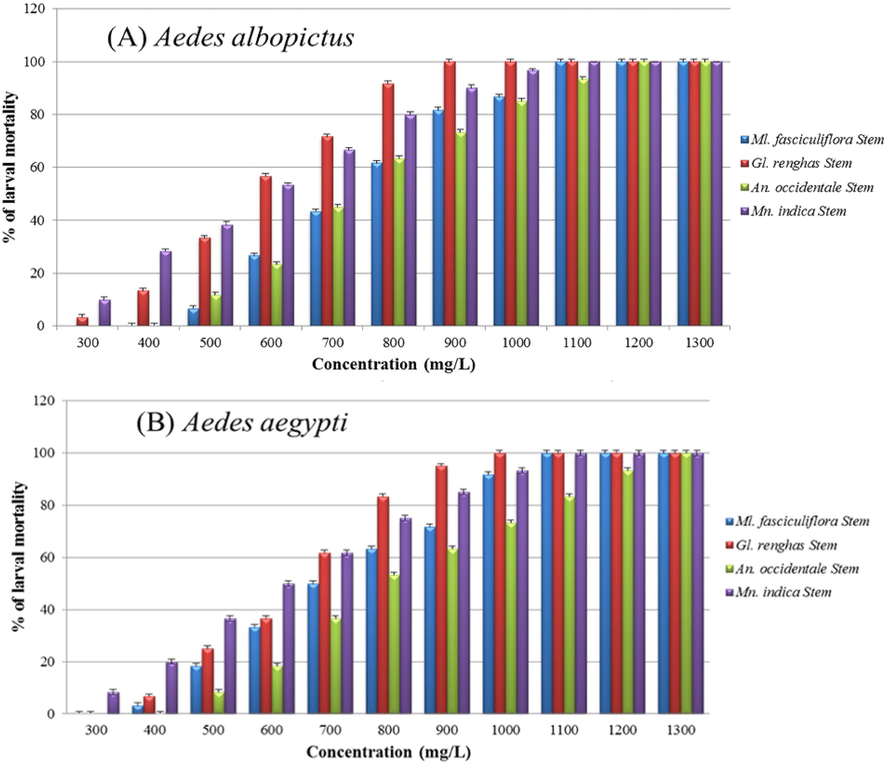
Larval mortality of (A) Aedes aegypti, and (B) Aedes albopictus after 24 h exposure to methanolic stem extracts of Anacardium occidentale, Gluta renghas, Mangifera indica and Melanochyla fasciculiflora.
Species
Plant Extract
N
LC50 (mg/L)
95% CIs
LC95 (mg/L)
95% CIs
Slope
x2 (df)
Aedes
albopictus
An. occidentale
480
638
(605–971)
1200
(1095.72–1355.59)
6.00 ± 0.51
1.25
Gl. renghas
300
240
(185.18–296.59)
607
(448.83–1191.32)
4.08 ± 0.41
5.39
Mn. indica
480
431
(398.29–463.40)
1076
(944.02–1284.15)
4.14 ± 0.35
2.87
Ml. fasciculiflora
300
535.59d
(506.78–562.64)
888.68
(810.82–1018.46)
7.48 ± 0.83
1.10
Aedes
aegypti
An. occidentale
480
792
(758.07–825.06)
1347
(1243.83–1500.46)
7.122 ± 0.6
1.47
Gl. renghas
360
623
(597.01–649.92)
976
(904.69–1082.83)
8.45 ± 0.77
4.15
Mn. indica
480
582
(548.63–615.63)
1198
(1074.67–1385.58)
5.25 ± 0.44
2.30
Ml. fasciculiflora
360
696
(660.91–730.80)
1222
(1101.82–1429.12)
6.73 ± 0.75
3.23
Source
df
MS
F-Ratio
P
Aedes species
1
24.26
9.72
0.002
Plant species
3
219.43
87.94
0.000
Concentration
11
922.77
369.80
0.000
Aedes species × Plant species
3
12.65
5.07
0.002
Aedes species × Concentration
10
0.929
0.37
0.957
Plant × Concentration
23
5.92
2.37
0.001
Aedes species × Plant species × Concentration
20
1.18
0.47
0.972
Error
144
2.50
3.2 Phytochemical and identification of phenolic compounds
Phytochemical compounds found in methanolic stem extracts of An. occidentale, Mn. indica, Ml. fasciculiflora and Gl. renghas were presented in Table 3. Screening results indicated the presence of saponins, tannins, alkaloids, reducing sugar, flavonoids and steroids in all stem extract of An. occidentale, Gl. renghas, Mn. indica and Ml. fasciculiflora from the family Anacardiaceae. However, alkaloids were tested negative for all stem extracts. Phytochemical tests confirmed the existence of the phenolic compound of flavonoid existed in all methanolic stem extracts of An. occidentale, Gl. renghas, Mn. indica and Ml. fasciculiflora.
Phytochemical Constituents
An. occidentale
Gl. renghas
Mn. indica
Ml. fasciculiflora
Saponins
+
+
+
+
Tannins
+
+
+
+
Alkaloids
–
–
–
–
Reducing sugar
+
+
+
+
Flavonoids
+
+
+
+
Steroids
+
+
+
+
Further test under the adjusted UPLC conditions, separation of the methanolic extracts of An. occidentale, Gl. renghas, Mn. indica and Ml. fasciculiflora stem was obtained in 7 min with UV detector recorded at 320 nm wavelength. The stem extracts were run by an appropriate reversed-phase Acquity UPLC separation system. The extract constituents were elated as individual UPLC peaks were trapped on solid-phase extraction cartridges using a Prospekt-2 SPE device (Spark Holland), which handles two trays with 96 cartridges each, and thus enables trapping of a large number of individual UPLC peaks in automation. Phenolic and flavonoid compounds in all extracts were identified by comparing UV spectra detected by photo-diode array detected and retention time. The standard calibration curve used authentic standards of gallic acid for phenolic and quercetin methanolic solution for flavonoid.
The major groups of compounds detected were flavonols from quercetin and apigenin derivatives for all the stem extracts of Anacardiaceae, as listed in Table 4. The highest concentrations of apigenin derivatives were detected in Mn. indica, followed by An. occidentale and Gl. renghas, while in Ml. fasciculiflora with the lowest concentration. Similarly, the highest amount of quercetin derivatives was also found in Mn. indica followed by An. occidentale, Gl. renghas and Ml. fasciculiflora. Conclusively, the average amount of apigenin derivatives was found less than the quercetin derivative.
Fig.
Plants
Peak No
Retention Time
Height
Derivatives
Anacardium occidentale
2
4.136
100,219
Quercetin
5
4.218
31,719
Quercetin
A
8
4.255
16,833
Quercetin
10
4.326
38,966
Quercetin
12
4.373
60,589
Quercetin
15
4.987
153,358
Apigenin
Gluta renghas
3
4.171
77,783
Quercetin
7
4.252
26,644
Quercetin
9
4.291
13,392
Quercetin
B
11
4.358
28,591
Quercetin
13
4.403
47,245
Quercetin
18
5.010
125,544
Apigenin
Mangifera indica
1
4.043
105,930
Quercetin
C
6
4.219
230,236
Quercetin
17
5.007
388,191
Apigenin
19
5.307
124,347
Apigenin
Melanochyla fasciculiflora
4
4.217
35,177
Quercetin
D
14
4.883
10,428
Apigenin
16
4.991
10,462
Apigenin
Methanolic extracts of family Anacardiaceae; An. occidentale, Gl. renghas, Mn. indica and Ml. fasciculiflora were fractioned by UPLC, as shown in Fig. 2. The chromatographic spectrum shows 19 peaks, each peak indicating separate compounds. Anacardium occidentale spectrum displayed six prominent peaks, derived from quercetin with retention time from 4.136 to 4.373 min and apigenin at 4.987 min. The UV spectrum of quercetin was detected at the peaks of 4.171 to 4.403 min and apigenin at 5.010 min for Gl. renghas. Similarly, four main peaks were observed in the spectrum of Mn. indica methanolic extracts were represented by two principal groups of derivative compounds of quercetin and apigenin. Melanochyla fasciculiflora depicted only three noticeable peaks at the retention time of 4.217 min as derivative of quercetin, and apigenin compound was at 4.883 and 4.991 min. Although the spectrum may contain plenty of impurities, however; major compounds were confirmed using their respective standards.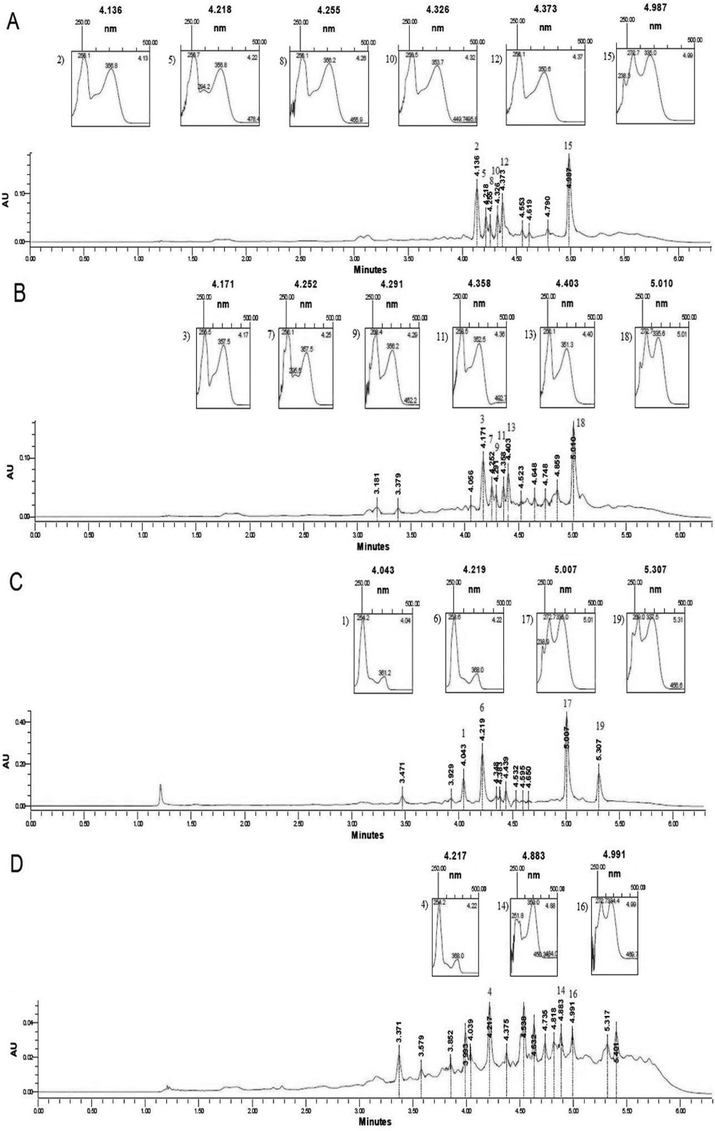
UPLC chromatograms (at 320 nm) of the stem extracts of (A) Anacardium occidentale, (B) Gluta renghas, (C) Mangifera indica and (D) Melanochyla fasciculiflora. The UV spectra of several peaks generated by a photodiode array detector are indicated in all plant samples.
4 Discussion
Mosquito control using the plant-based insecticide has been known for a long time with their ability to kill the larvae, reduce the number of eggs through oviposition deterrence and ovicidal activity (Zuharah and Yousaf, 2016). Gluta renghas has exhibited the best toxicity effects against both Aedes larvae more than those possessed by Mn. indica. The toxicity of the plants depends on the various bioactive compounds, including phenolics, terpenoids, flavonoids, and alkaloids, either as single or joint compounds (Elumalai et al., 2012). Plant phytochemicals are produced as secondary metabolites which encounter toxic effect to protect the insects from predatory (Ghosh et al., 2012). Gluta renghas has been reported for excellent toxicity against laboratory strains of Ae. albopictus at 600 mg/L (Yousaf and Zuharah, 2015), which might contain flavonoids, phenolic lipids and tripertenes as reported in many Anacardiaceae families (Correia et al., 2006). The variety of larvicidal potentials depends on the composition of chemical compounds in the plant. As to our knowledge, no study has been identified the chemical compound of Gl. renghas. Phenolic identification in the current study found high concentrations of flavonol derivatives of quercetin and apigenin in this plant which might be responsible for the larvicidal activity. Whereas many studies have been reported the potential of Mn. indica (Rahuman et al., 2009) and An. occidentale on various mosquitoes’ species and their chemical compounds (Farias et al., 2009).
Several plant species have been tested for their activities against different vectors and found to be target-specific. Our study has shown a significant different larvicidal effect on Ae. albopictus and Ae. aegypti mosquitoes, even though both species are a sibling. Aedes albopictus was found to be prone to the toxicity of all Anacardiaciae plants as compared to Ae. aegypti. The toxicity of the plant as larvicides on particular mosquito species is due to the synergistic effects from combining compounds existed in the plant extracts (Tawatsin et al., 2006). Other than that, the quality of compounds extracted from the plant is influenced by the extraction method, solvent polarity and also the part of the plant (Oliveira et al., 2010), including the plant species, cultivating condition, plant preparation and plant storage (Tawatsin et al., 2006).
Recently, interest has been raised by the beneficial potential of plants due to their phenolic compound, particularly flavonoids (Pandey and Kumar, 2012). Flavonoids are extensively distributed and most common group of the plant phenolic compounds found in all plants parts, especially the photosynthesizing cells of plants (Kumar and Pandey, 2013) which contain a huge number of compounds, abundant in plants, imparting the antioxidant activities, including anti-inflammatory, anti-carcinogenic, anti-bacterial, anti-viral, and anti-allergic effects (Yong et al., 2018). Other compounds, such as the presence of carbohydrates, saponins, phytosterols, phenols and tannins, including flavonoids in plants having also reported for larvicidal activities against mosquitoes (Khanna and Kannabiran, 2007).
In our study, we found that Gl. renghas had caused mortality to both Aedes mosquitoes at a low concentration which probably associated with a synergist of a cumulative quercetin and apigenin compound found in this plant. Even though UPLC detected low concentration of both phenolic compounds than those detected in Mn. indica and An. occidentale, but this plant species possessed the best toxicity effects on Aedes larvae. Thus, suggested, the synergistic effects between all profound compound are necessary to elicit the larvicidal properties. The high amount of quercetin in the leaves of a locust tree, Robinia pseudoacacia (Nasir et al., 2005) reduced the population of Lymantria dispar (Barbosa and Krischik, 1987) and prefer to attack forest trees in spring when the leaves have a small amount of flavonoids including quercetin (Salminen et al., 2004). No comparative study can discuss as this is considered the first evaluation of phenolic compound in Gl. renghas and Ml. fasciculiflora.
Cashew nut shell liquid (CNSL) derived from An. occidentale was examined for its antioxidant activities against Ae. aegypti and the GC-MS analysis of its constituents detected quercetin as the most active compound, followed by orcinol, cardanol, cardol and anacardic acid (Oliveira et al., 2011). We also detected a higher number of quercetin compounds from the stem part of An. occidentale suggested it’s ability as a potential larvicidal agent. A rich source of flavonoid had been observed to have good larvicidal activity against Cx. pipiens Pallens, Ae. aegypti and Ae. albopictus derived from methanolic seed extracts of Millettia pinnata (Perumalsamy et al., 2015). Similarly, methanolic and ethanolic extract of flowers and leaves of Lantana camara Linn. have demonstrated their larvicidal activities against Cx. quinquefasciatus and Ae. aegypti, which reported in the presence of flavonoids (Kumar and Maneemegalai, 2008).
Peel and pulp of the fruit of Mn. indicia were also reported with the presence of quercetin (Atawodi et al., 2014), similar to the stem extract from our study found two derivatives of quercetin compounds. Few studies also indicated flavonoid potential to inhibit and caused lethal effects on other animals and insects. The flavonoid can act as antiparasitic and causing mortality to parasite due to swollen mitochondria with massive multilobe vacuoles (Yong et al., 2018). Interestingly, the myricetin and Scutellarein from the flavonoid group were found to inhibit SARS-CoV helicase (nsP13) by inhibiting ATPase activity (Mani et al., 2020). The presence of flavonoids and tannins in aqueous extracts of immature fruits of Mn. indica had a higher potential for larval development inhibition against the larvae of Strongyloides stercoralis (El-Sherbini and Osman, 2013).
Quercetin was also found toxic as it inhibited Glutathione S-transferases (GST) in Triatoma infestans (Sivori et al., 1999), which was found to be toxic to the insect. Not only death but the active compound of apigenin, quercetin, luteolin and volatile oils derived from P. amboinicus leaf extract would interrupt the development and movement of dengue vectors without interrupting the predation ability, development and survivability of the natural enemy copepod (Murugan et al., 1996). Quercetin compound also increases the mortality rate of L. dispar larvae by decreasing the midgut activities of larvae resulting in decreased body mass and, finally death (Perić-Mataruga et al., 2014). Besides quercetin, flavonoids apigenin, vitexin, isovitexin, sitosterol and campesterol was pointed out for positive larvicidal activities against Ae. aegypti, Cx. quinquefasciatus (Rahuman et al., 2008) and An. arabiensis (Tomass et al., 2011). Quercetin is an interesting compound as it is found safe for other non-target organisms, including human beings and other vertebrates (Perić-Mataruga et al., 2014).
Therefore, this plant is probably safe to be used as one of the larvicidal products with no harm to the human being. The positive response and growing trend and of the community towards phytochemicals and their environment-friendly behaviour builds an open ground for the research and innovation of plant-based insecticides. For future research, it is worth extracting active ingredients of apigenin and quercetin from these Anacardiaceae plants, which had been endorsed the importance of its biological control potential to reduce the use of plant sources. This will promote the utilization of these chemical compounds of botanical origin as a safer option, readily degradable and environmentally safe insecticide for the vector control program.
Authors contributions
WFZ contributed in conceived and designed the study, analyzed data and wrote and corrected the final manuscript. YA collected the field samples, performed the bioassays, analyzed data and helped draft the manuscript. KLO and SFS have helped in analyzing the phenolic compound of the plant extracts. The final manuscript was approved by all authors.
Funding
This study was supported by the Universiti Sains Malaysia under Research University Grant (1001/PBIOLOGI/815079) and Fundamental Research Grant Scheme, Ministry of Higher Education Malaysia (203/PBIOLOGI/6711629).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemical screening of active compounds in Mangifera indica leaves from Ibadan, Oyo state. Plant Sci. Res.. 2009;2:11-13.
- [Google Scholar]
- Review of the antioxidant potential of African medicinal and food plants. In: Dubey N.K., ed. Plants as a Source of Natural Antioxidants. UK: CAB International; 2014. p. :49.
- [Google Scholar]
- Influence of alkaloids on feeding preference of eastern deciduous forest trees by the gypsy moth Lymantria dispar. Am. Nat.. 1987;130:53-69.
- [Google Scholar]
- Mosquitoes of the Southeastern United States. Tuscaloosa, Alabama: University of Alabama Press; 2013. p. :19-30.
- Centers for Disease Control and Prevention, 2005. Third national report on human exposure to environmental chemicals: Executive summary. In: Third national report on human exposure to environmental chemicals: executive summary, NCEH Pub. No. 05-0570.
- Challenges and future perspective for dengue vector control in the Western Pacific Region. Western Pacific Surveillance and Response. 2011;2:9-16.
- [Google Scholar]
- Polyphenolic profile characterization of Agrimonia eupatoria L. by HPLC with different detection device. Biomed. Chromatogr.. 2006;20(1):88-94.
- [Google Scholar]
- Anti-infective potential of natural products: how to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol.. 2006;106:290-302.
- [Google Scholar]
- Management of insect resistance in the major Aedes vectors of arboviruses: advances and challenges. PLoS Negl. Trop. Dis.. 2019;13(10):e0007615.
- [Google Scholar]
- Antibacterial activity of Marula (Sclerocarya birrea (A. rich.) Hochst. Subsp. caffra (Sond.) Kokwaro)(Anacardiaceae) bark and leaves. J. Ethnopharmacol.. 2001;76:305-308.
- [Google Scholar]
- Anthelmintic activity of unripe Mangifera indica L. (Mango) against Strongyloides stercoralis. Int. J. Curr. Microbiol. Appl. Sci.. 2013;2:401-409.
- [Google Scholar]
- Larvicidal, ovicidal and pupicidal activity of Eranthemum roseum (Vahl) R. BR. against malarial vector mosquito, Anopheles stephensi (liston) (Diptera: Culicidae). International Journal of Current Agricultural. Science. 2012;2(7):28-33.
- [Google Scholar]
- A rapid identification guide for larvae of the most common North American container-inhabiting Aedes species of medical importance. J. Mosquito Control Assoc.. 2013;29(3):203-221.
- [Google Scholar]
- Insecticidal action of sodium anacardate from Brazilian cashew nut shell liquid against Aedes aegypti. J. Am. Mosq. Control Assoc.. 2009;25:386-389.
- [Google Scholar]
- Allergy to Anacardiaciae: description of cashew and pistachio nut allergens. J. Investig. Allergol. Clin. Immunol.. 2000;10(3):173-177.
- [Google Scholar]
- Plant extracts as potential mosquito larvicides. Indian J. Med. Res.. 2012;135:581-598.
- [Google Scholar]
- Secondary compound within the Anacardiaceae. Colorado: Colorado State University; 1998.
- Aedes aegypti in Senegal: genetic diversity and genetic structure of domestic and sylvatic populations. Am. J. Tropical Med. Hygiene. 2008;79:218-229.
- [Google Scholar]
- Larvicidal effect of Hemidesmus indicus, Gymnema sylvestre and Eclipta prostrata against Culex qinquifaciatus mosquito larvae. Afr. J. Biotechnol.. 2007;6:307-311.
- [Google Scholar]
- Evaluation of larvicidal effect of Lantana camara Linn against mosquito species Aedes aegypti and Culex quinquefasciatus. Adv. Biol. Res.. 2008;2:39-43.
- [Google Scholar]
- Chemistry and biological activities of flavonoids: an overview. Sci. World J.. 2013;2013:1-10.
- [Google Scholar]
- Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res.. 2020;284:197989.
- [CrossRef] [Google Scholar]
- Peridomestic Aedes malayansis and Aedes albopictus capable vectors of arboviruses in cities. PLoS Negl. Trop. Dis.. 2017;11(6):e0005667.
- [Google Scholar]
- Antipupational effect of neem oil and neem seed kernel extract against mosquito larvae of Anopheles stephensi (Liston) J. Entomol. Res.. 1996;20:137-139.
- [Google Scholar]
- Allelopathic potential of Robinia pseudoacacia L. J. Chem. Ecol.. 2005;31:2179-2192.
- [Google Scholar]
- Phytochemical screening for active compounds in canarium schweinfurthii (Atile) leaves from Jos North, Plateau state, Nigeria. Res. J. Biol. Sci.. 2008;3:1076-1078.
- [Google Scholar]
- Antioxidant, larvicidal and antiacetylcholinesterase activities of cashew nut shell liquid constituents. Acta Trop.. 2011;117:165-170.
- [Google Scholar]
- Larvicidal activity of 94 extracts from ten plant species of northeastern of Brazil against Aedes aegypti L. (Diptera: Culicidae) Parasitol. Res.. 2010;107(2):403-407.
- [Google Scholar]
- Varieties of Mangifera indica L. (Anacardiaciae) used as food and medicine by the Idoma people of Eke-Ogadumu in Okpokwu local government area of Benue State, Nigeria. J. Pharmacognosy Phytochem.. 2020;9(5):6-10.
- [Google Scholar]
- Antioxidant, lipo-protective and antibacterial activities of phytoconstituents present in Solanum xanthocarpum root. Int. Rev. Biophys. Chem.. 2012;3:42-47.
- [Google Scholar]
- Adulticidal, repellent, and ovicidal properties of indigenous plant extracts against the malarial vector, Anopheles stephensi (Diptera: Culicidae) Parasitol. Res.. 2013;112(2):679-692.
- [Google Scholar]
- Potential improvement of Lymantria dispar L. management by quercetin. Arch. Biol. Sci.. 2014;66:1125-1129.
- [Google Scholar]
- Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasites Vectors. 2015;8:237-250.
- [Google Scholar]
- Evaluation of indigenous plant extracts against larvae of Culex quinquefasciatus Say (Diptera: Culicidae) Parasitol. Res.. 2009;104:637-643.
- [Google Scholar]
- Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) Parasitol. Res.. 2008;102(5):867-873.
- [Google Scholar]
- Recent ecological, physiological and protein profile of the dengue vector population in Penang Island, Malaysia. Malaysia: Universiti Sains Malaysia. Penang; 2012. PhD Thesis
- Seasonal variation in the content of hydrolyzable tannins, flavonoid glycosides, and proanthocyanidins in oak leaves. J. Chem. Ecol.. 2004;30:1693-1711.
- [Google Scholar]
- Fenitrothion toxicity in Triatoma infestans synergized by quercetin or thymol blue. Pestic. Sci.. 1999;55:18-26.
- [Google Scholar]
- The history of dengue in tropical Asia and its probable relationship to the mosquito Aedes aegypti. J. Trop. Med. and Hygiene. 1956;59:243-251.
- [Google Scholar]
- Medicinal plants and traditional medicine in Africa. In: Screening plant for bioactive agents. Nigeria: Spectrum Books Ltd; 1993. p. :134-156.
- [Google Scholar]
- Bio-efficacy of plant-extracts against the malarial vector, Anopheles stephensi (Diptera: Culicidae) Indian J. Appl. Entomol.. 2011;25:131-135.
- [Google Scholar]
- Repellent of essential oil extracted from plants in Thailand against four mosquito vectors (Diptera: Culicidae) and oviposition deterrent effects against Aedes aegypti (Diptera: Culicidae) Southeast Asian J. Trop. Med. Public Health. 2006;37(5):915-931.
- [Google Scholar]
- Larvicidal effects of Jatropha curcas L. against Anopheles arabiensis (Diptera: Culicidea) Momona Ethiopian J. Sci.. 2011;3:52-64.
- [Google Scholar]
- World Health Organization, 2005. Guidelines for laboratory and field testing of mosquito larvicides. World Health Organization, (WHO/CDS/WHOPES/GCDPP/2005.13). Pp. 10-18.
- Yap, H.H., Zairi, J., 2003. Mosquito control. In: Lee, C.Y., Adanan, C.R., Lee N.C. (Eds.), Urban Pest Control, A Malaysian Perspective, 2nd Edition; Vector Control Research Unit. Universiti Sains Malaysia: Penang, Malaysia, pp. 43–53.
- New insights on bioactivities and biosynthesis of flavonoid glycosides. Trends Food Sci. Technol.. 2018;79(2018):116-124.
- [Google Scholar]
- Lethal response of the dengue vectors to the plant extracts from family Anacardaciae. Asian Pacific J. Trop. Biomed.. 2015;5(10):812-818.
- [Google Scholar]
- Assessment of Gluta renghas and Mangifera indica crude extracts of sublethal effects on dengue vectors. J. Asia Pac. Entomol.. 2016;19(4):1043-1051.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2021.101471.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







