Kinetic study on the sedimentation behavior of Na- and Ca-kaolinite suspension in the presence of polyethyleneimine
*Corresponding author nadis@ksu.edu.sa (N. Al Andis)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Flocculation properties (settling rate, sediment thickness, supernatant clarity) of Na- and Ca-kaolinite suspension have been studied in the presence of cationic polyethyleneimine (PEI) and mixtures of NaCl or CaCl2 with PEI under controlled conditions of pH. Two extreme kinds of qualitative sedimentations have been observed: flocculation–sedimentation (at 0, 10 and 50 ppm PEI) and accumulation–sedimentation (at 200, 600 and 1000 ppm PEI). It is concluded that the highest sediment thickness and lowest turbidity were observed for Na- or Ca-kaolinite suspension at 10 ppm PEI, that is to say the critical flocculation concentration occurred at 10 ppm PEI. The sedimentation behaviors of Na- and Ca-kaolinite suspension in the presence of PEI were found dependent on both pH and nature of electrolytes, while their flocculation was enhanced by the addition of low electrolyte concentration (0.001 mol/l) with PEI (10 ppm).
Keywords
Flocculation
Sedimentation
Polyethyleneimine
Kaolinite
Turbidity
1 Introduction
Flocculation is important in many diverse fields, including paper manufacturing, mineral processing, brewing, food processing, pharmaceutical manufacturing, water (drinking and waste) purification, soil conditioning and erosion control. Particles may be flocculated so that they can be more easily removed as solids such as a “filter-cake” or as a “sludge” as in wastewater and sewage treatment. The formation of sufficiently large flocs facilitates separation of solid particles from the suspension by means of sedimentation, the formation of dense flocs allows the removal of a minimal volume of solid phase, and the formation of open flocs facilitates their removal by filtration. Because kaolinite is a major raw material for the above applications, the flocculation and dispersion behavior of kaolinite in aqueous media has been studied extensively (Ma and Pierre, 1992, 1999; Besra et al., 2002; Goldberg and Glaubig, 1987; Rossi et al., 2003).
Kaolinite is a hydrous aluminosilicate of layered structure comprising silica and alumina sheets stacked on top of each other (Hu and Liu, 2003). Kaolinite has different surface structures between base planes and edge planes. The basal surfaces of kaolinite are believed to carry a constant structural charge due to the isomorphous substitution of Si+4 by Al+3, whereas the charge on the edges is due to the protonation/deprotonation of exposed hydroxyl groups. Depending on the solution pH, the edge surfaces can bear net negative, or positive or no charge. The pH where the net total particle charge is zero is called the point of zero charge (PZC), which is one of the important parameters used to describe variable charge surfaces (Pefferkorn et al., 1985). The ability of kaolinite to flocculate is predominantly controlled by Coulombic forces between the negative charges on the basal planes and positive charges at the edges surface (Blockhaus et al., 1997).
When the electrical repulsion of identically charged double layers around kaolinite particles is strong enough, the kaolinite suspension remains dispersed (Amandarajiah and Chen, 1994). Flocculation is brought about by the decrease in this repulsion. The use of polymer to control the stability and flocculation behavior of clay suspension has been widely investigated (Luckham and Rossi, 1999; Bradenburg and Lagaly, 1988; Perezrodriguez et al., 1977). The nature of the flocculation of kaolinite obtained in polymer solution depends upon a number of factors including polymer characteristics and solvent properties as well as the hydrodynamic conditions used during the flocculation and preflocculation stages (Luckham and Rossi, 1999). Such factors are important for achieving the desired flocculation state of particular fine particles which are not easy processes (van Olphen, 1991). Many investigators (KJellin, 1997; Ma and Pierre, 1997; Oades, 1984) had characterized the behavior of several cationic polymers for flocculation of kaolinite. Polyethyleneimine (PEI) is a cationic polymer and is known to be an excellent cationic flocculant. The adsorption of PEI on SiO2, SiC (Pugh and Bergstrom, 1994), Fe2O3 (Radeva and Petkanchin, 1997), ZrO2 (Wang et al., 1999), bentonite (Alemdar et al., 2005) surfaces were investigated.
The different properties of the flocculated systems can be the determining criterion in different processes. For example, whereas supernatant clarity and sediment thickness will be important when water is to be important in effluent treatment. It is well known that the flocculation of fine particles is very difficult. Therefore in this study the effect of PEI adsorption on selected flocculation parameters, such as settling rate, sediment thickness and supernatant clarity, is studied for kaolinite fines. The settling rate is mainly a measure of the size of the flocs and in later stages the compressibility of flocs and floc networks, and supernatant clarity is a measure of the size distribution of flocs and size-dependent capture of the particles and flocs by the polymer. Also the study aims to investigate the influence of pH and electrolyte (NaCl and CaCl2) in the presence of PEI on flocculation behavior of Na- and Ca-kaolinite suspension.
2 Experimental work
2.1 Material
The kaolinite samples for our study were chosen from Ymama regions in Saudi Arabia. X-ray analysis of kaolinite showed the characteristic distance (the distance between a certain plane in the unit layer of kaolinite and corresponding plane in the next unit layer) of about 7.2 Å, which indicates that the sample is mainly kaolinite, not another clay. Chemical analysis obtained from X-ray fluorescence is represented in Table 1. The cationic polyethyleneimine (PEI) was used. It has a molecular weight of 2 × 104 g mol−1. Analar salts of NaCl and CaCl2 from VWR were used.
| Polymer concentration (ppm) | Turbdity (Ntu) | |
|---|---|---|
| Na-kaolinite | Ca-kaolinite | |
| 0 | 23 | 9.03 |
| 10 | 12.5 | 6.595 |
| 50 | 12.9 | 8.91 |
| 200 | 755 | 303.5 |
| 600 | 875.5 | 370 |
| 1000 | >1000 | 754 |
3 Methods
3.1 Clay separation and saturation
This work aims to study the effect of PEI adsorption on the flocculation of fine kaolinite particles (the average particle size was 2 μm) which are not easily flocculated, flocculation being important in water treatment. Therefore, about 1 kg of kaolinite was suspended in a graduated cylinder with a volume of 5 l of deionized water, and the 2 μm fraction was separated by choosing a height of 10 cm to collect a decant and by applying Stoke’s law:
3.2 Sedimentation test
To carry out the sedimentation tests, each suspension of Na-kaolinite or Ca-kaolinite with a median size of 2 μm was prepared in a graduated cylinder with an inner diameter of 30 mm, which was large enough to neglect the wall effect (Ma and Pierre, 1992). Then, distilled water and proper amounts of different concentrations of a polymer (1000, 600, 200, 50, 10 ppm) were added to get an initial suspension volume of 100 ml each, having pH value of 6.5. The cylinders were turned upside down many times to uniformly distribute the components inside the suspension. To study the effect of pH on flocculation of Na- and Ca-kaolinite suspension, the pH in all the suspensions was adjusted with dilute HCl or NaOH to get a pH range from 3 to 12. Also the sedimentation tests were carried out for Na- or Ca-kaolinite in the presence of PEI with NaCl or CaCl2. All experiments were performed at room temperature and cylinders were covered with a piece of Para film to prevent water evaporation.
In all sedimentation experiments where a clear interface occurred, the position of this interface was read every 10 min during the first hour, then every hour for the following 2 h, and finally every 24 h until 360 h. The rate at which this interface moved was used to measure the accumulation rate, or the settling rate (depending on sample behavior, in mm/hr). After 24 h of sedimentation experiments, the obtained supernatant was taken and turbidity was measured by using turbidimeter.
4 Results and discussion
4.1 Effect of polyethyleneimine (PEI)
Figs. 1a and 1b shows the relationship between sediment thickness of Na- and Ca-kaolinite suspension and time, at different polymer concentrations. The curves corresponding to polymer concentration of 0, 10 and 50 ppm show that the onset of flocculation is not instantaneous after the addition of PEI. Solutions of high-molecular weight polymer are viscous at typical operational concentrations and this can lead to a distinct period of time for thorough distribution through the suspension (Ma and Pierre, 1992). Therefore there is a lag time of about 10 min between the addition of the polymer and the onset of flocculation. At this moment, flocs was formed and these flocs joined to each other and created an apparently uniform sediment, separated by a sharp interface from a clear supernatant, liquid. The settling rate of kaolinite suspension after 10 min is fast. Later on, the settling rate began to slow down progressively, until it reached an aging stage (after 24 h). This behavior represents the flocculation–sedimentation (Ma and Pierre, 1992).
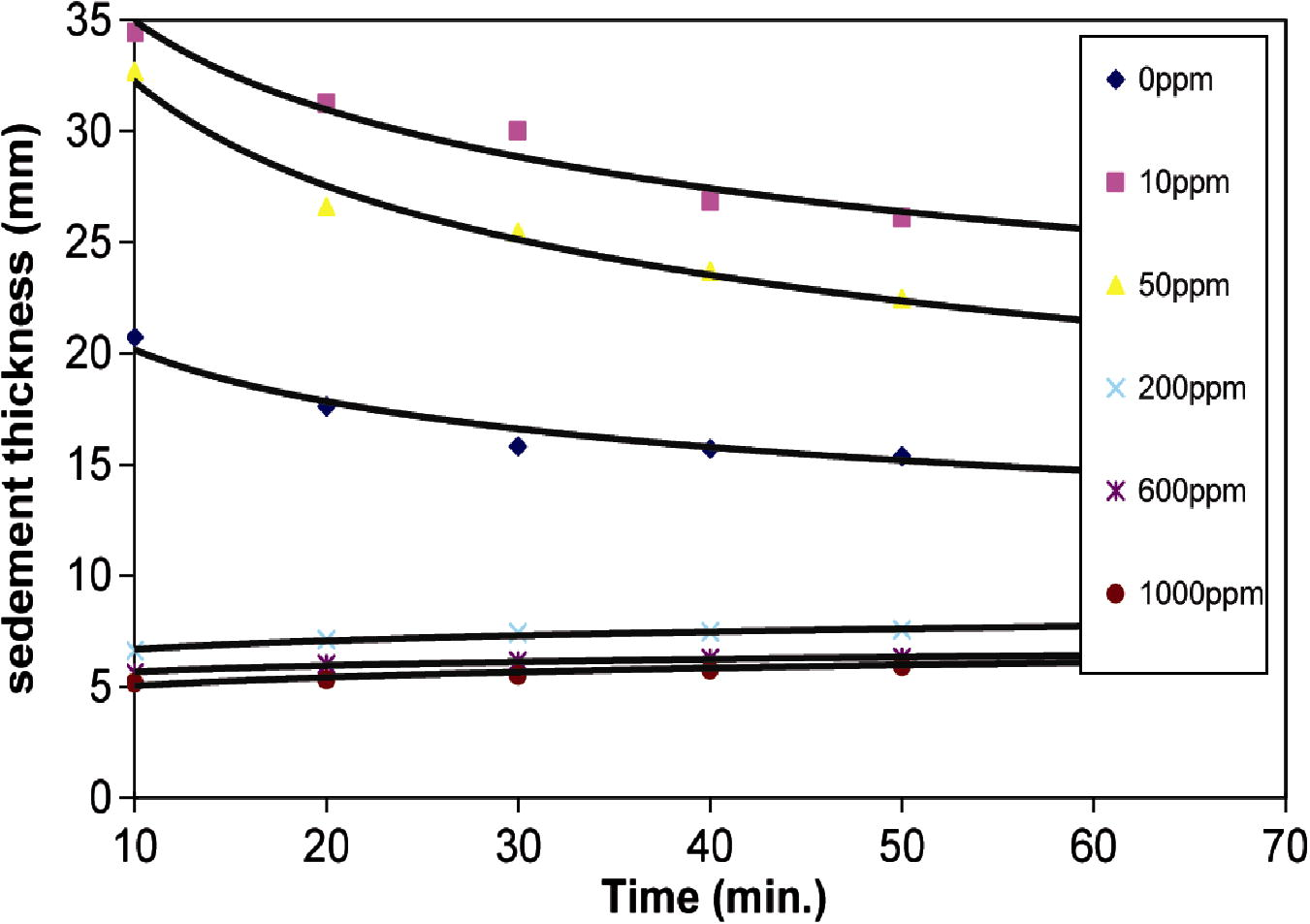
- Relationship between sediment thickness of Na-kaolinite suspension and time at different PEI concentrations (ppm).
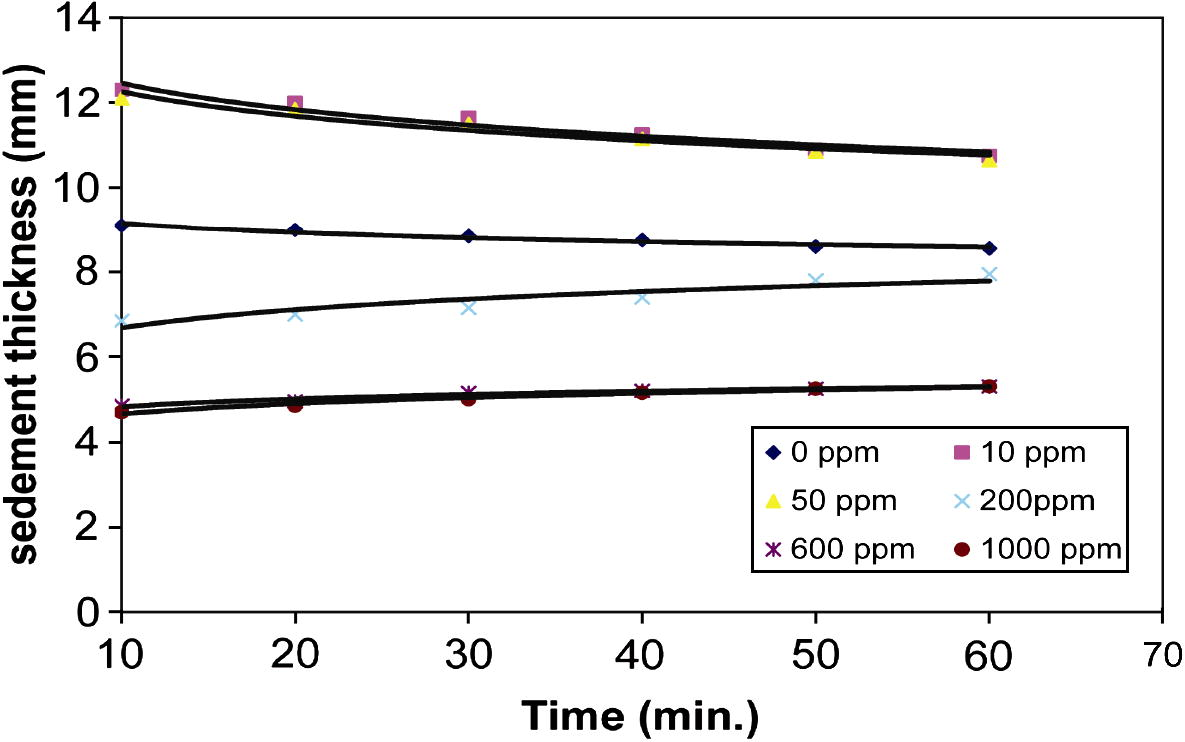
- Relationship between sediment thickness of Ca-kaolinite suspension and time at different PEI concentrations (ppm).
The sedimentation data with high PEI concentrations (200, 600 and 1000 ppm) are also represented in Fig. 1. The sedimentation behavior of Na- or Ca-kaolinite suspension at high polymer concentration is in a reverse trend to that at low polymer concentration and represents accumulation–sedimentation behavior (Ma and Pierre, 1999). The initial volume of the sediment was zero and it increased slowly by the accumulation of new particles. The suspension that remained on top of the accumulated layer was composed of smaller, slower-settling clay particles. The final sediment volume in the accumulation–sedimentation was much lower than that in flocculation–sedimentation. Therefore, the accumulated–sediment was more densely packed than flocculated–sediment (Ma and Pierre, 1992).
To cause flocculation by reducing the electrical repulsion of identically charged double layers around kaolinite particles, a polymer must necessarily be of ionic character, and, furthermore, must carry a charge opposite in sign to that of the solid surface (Besra et al., 2002). Cationic polyelectrolytes are expected to be active flocculants for such systems (Solberg and Wagberg, 2003). A review by Kitchener (1972) on polymeric flocculants establishes that flocs (aggregated particles) produced with polymers are much stronger than those formed by the addition of electrolytes. Effective bridging flocculation requires that the adsorbed polymer extends far enough from the particle surface to attach to other particles and that sufficient free surface is available for the adsorption of segments of the extended chains. To achieve this, there is an “optimum dosage” for producing the largest flocs where the polymer is practically all adsorbed, but the surface carries only half of the saturation capacity of the polymer. The flocculation of clays by polymers was first studied by Ruehrwein and Ward (1952) and Michaels (1954), who studied the effect of charged polyelectrolytes on the flocculation of clay suspensions. They emphasized the strong adsorption of the polymer on the clay especially at low concentration and the concept of the formation of strong anchor points for bridges. Koksal et al. (1990) found that kaolinite clay was flocculated by very low concentration of polyethylene oxide (PEO) of high molecular weight, this was attributed to the high molecular weight of PEO chain being more amenable to the formation of tails and loops that are required for bridging of flocculation to occur. Recently Alemdar et al. (2005) studied the adsorption of PEI on purified bentonite. They found that the adsorption rate of PEI was very fast and the equilibrium was reached after 5 min, and the adsorption isotherm shows a high affinity L-type. High affinity means a strong electrostatic interaction between clay particles and PEI. Because of the pH-dependent protonation of the imine groups on the polymer chain, the polymer chains gain a net positive charge.
The high molecular weight PEI used in this study has a positive charge over a wide pH range between pHs 3 and 11 (Erim et al., 1995). On the other hand, at the pH values above the point of zero charge (PZC), which is around four for kaolinite under study, both edges and surfaces of clay particles are negatively charged. Therefore, the addition of low PEI concentration resulted in neutralizing the charge on the kaolinite particles and consequently a reduction in the electrical double layer thickness, hence electrostatic repulsions between kaolinite particles decreased and the particles were able to approach each other close enough giving a chance for short range H-bonding forces between
At high PEI concentrations (200, 600 and 1000 ppm), all available adsorptive sites on the surfaces of suspended kaolinite particle can be satisfied by adsorption of oppositely charged PEI, and consequently all faces of kaolinite are positively charged. As a result, the repulsive forces between the positively charged particles prevent them from coming together or flocculate (Alemdar et al., 2005). Thus, sorption of large amount of PEI to kaolinite resulted in increased stability of kaolinite suspension due to increases of positive charge on kaolinite particles (electrostatic stabilization). This may result in steric stabilization caused by a thermodynamic repulsion that occurs between interpenetrating polymer chains attached to the kaolinite surfaces (Hunter, 1986). Therefore, the chance of formation of polymer bridges between kaolinite particles reduced and consequently, the heaviest particles settled immediately under gravity and formed the accumulated sediment at the bottom of the cylinders.
The final sediment thickness of Na- and Ca-kaolinite in the presence of different PEI concentrations at equilibrium (after 24 h) is shown in Fig. 2. The highest sediment thickness was observed for Na- or Ca-kaolinite at 10 ppm PEI. The results of sedimentation were confirmed by measuring the turbidity of the obtained supernatant as shown in Table 1. The result indicates that the turbidity of the supernatant decreased from 23 to 9.03 Ntu for Na- and Ca-kaolinite suspension, respectively, in the absence of PEI and from 12.5 to 6.595 Ntu after addition of 10 ppm PEI. From Fig. 2 and Table 2 it could be concluded that the highest sediment thickness and lowest turbidity were observed for Na- or Ca-kaolinite at 10 ppm PEI. But with increasing polymer concentration the final sediment thickness decreases to a very small value and turbidity increases, that is, the critical flocculation concentration occurs at 10 ppm PEI.
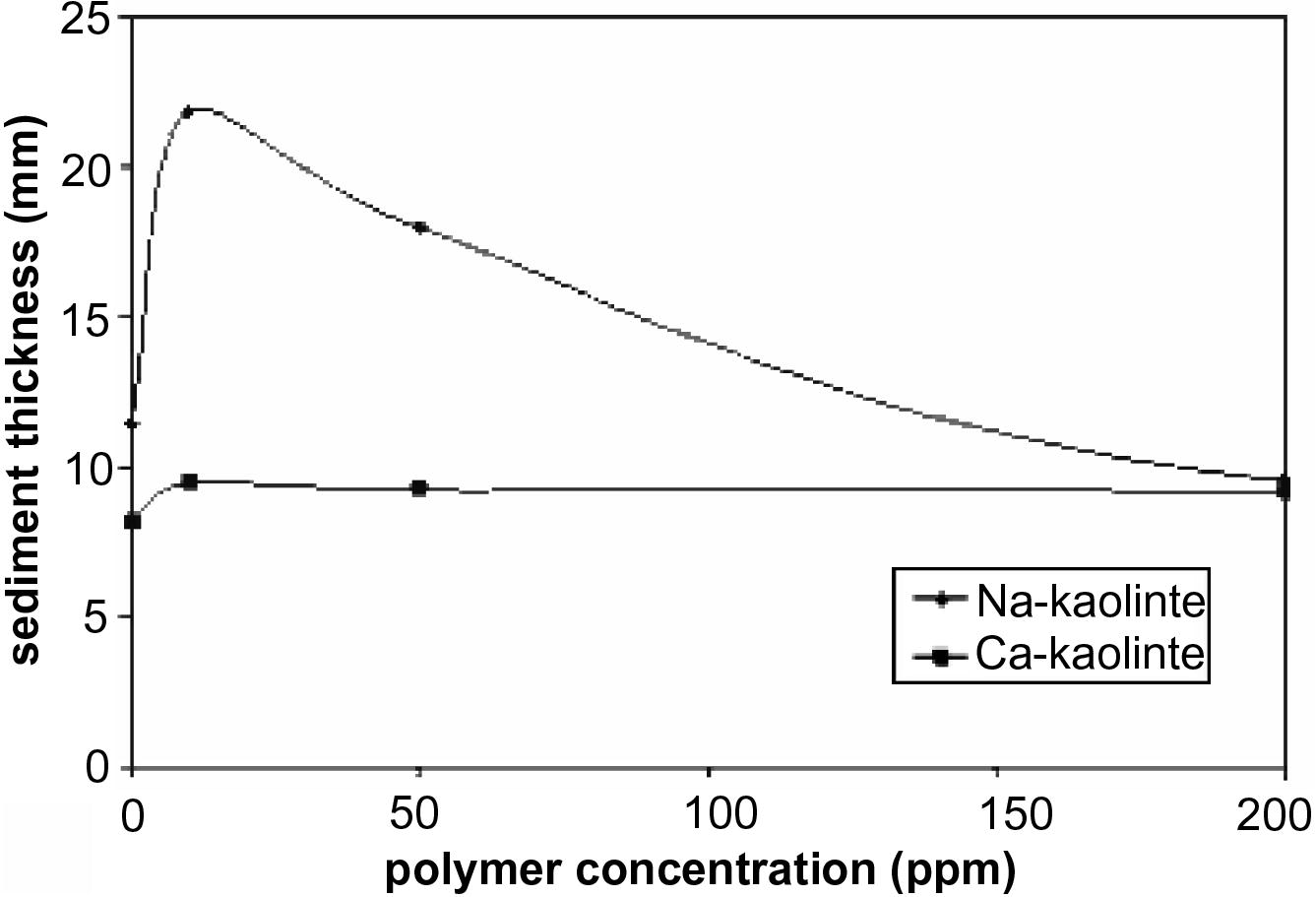
- Sediment thickness of Na- and Ca-kaolinite suspension at different polymer concentrations.
| Electrolyte concentration (mol/l) | Na-kaolinite | Ca-kaolinite | ||
|---|---|---|---|---|
| Sediment thickness (mm) | Turbidity (Ntu) | Sediment thickness (mm) | Turbidity (Ntu) | |
| 0* | 25.5 | 12.5 | 10.75 | 6.6 |
| 0.001 | 20 | 8.12 | 9.7 | 5.3 |
| 0.01 | 17.5 | 14.8 | 9.5 | 10.4 |
| 0.1 | 18 | 19.5 | 9.3 | 12 |
Results show that the sedimentation behavior of Na-kaolinite is similar to that of Ca-kaolinite at different polymer concentrations, but there is variation in sediment thickness and turbidity values of Na- and Ca-kaolinite suspension at different PEI concentrations. The behavior is somewhat complex. Particles in Ca-kaolinite settle more slowly at the start than those in Na-kaolinite as shown in Figs. 1a and 1b. For example, the slope of the curve of sedimentation for Na-kaolinite is steeper than the slope of the curve of Ca-kaolinite. After a certain period of time (60 min), the settling rate of particles in Na- or Ca-kaolinite suspension becomes constant.
Sediment thickness of kaolinite suspension decreases with an increase in the valence of saturating cation at the same polymer concentration. This result, due to the double layer of surface face and edge face on Ca-kaolinite, is more compressed and becomes very thin compared to that of Na-kaolinite. Therefore, the repulsion force between the electrical double layer around kaolinite particles decreases and attractive force increases, face–face domains form easily in the sedimentation experiment and these domains associate into aggregates (Luckham and Rossi, 1999). This association leads to lower sediment; voids between the Ca-kaolinite particles, formation of very closed flocs (lower sediment thickness) and consequently lower turbidity values of the supernatant solution compared to the formation of very open flocs (high sediment thickness) and higher turbidity in Na-kaolinite (Ma and Pierre, 1992). Thus, higher valence of saturating cation becomes more effective in reducing sediment thickness and consequently turbidity of Ca-kaolinite in the presence of PEI compared to Na-kaolinite.
4.2 Effect of pH
Fig. 3 shows the sediment thickness for Na- and Ca-kaolinite at 10 ppm of PEI with different pH values (3, 7 and 12) after 24 h of sedimentation. Sedimentation data is similar to those of Fig. 1a (which correspond to what is called flocculation–sedimentation here). As was observed with increasing the pH, the sediment thickness increases, but the reverse trends were observed for turbidity results as shown in Fig. 4. At pH 3, the flocculated sediment thickness was smaller than that at pHs 7 and 12. For kaolinite, the reported values for the point of zero charge (ZPC) are between 2.5 and 3.8, depending on the conditions (Hu and Liu, 2003). Below the ZPC, the edge surfaces (E) of kaolinite particles are considered to be charged positively and the face surfaces (F) are charged negatively. Above the ZPC, the edges also become negatively charged. Depending on the pH values, three different modes of particle association may occur: face to face (FF), or edge-to-face (EF) and edge-to-edge (EE) (Luckham and Rossi, 1999). The electrical interaction energy for the three types of associations is governed by three different combinations of the two double layers. Also, the rate of diffusion of the particles as they approach each other in these ways is not the same. FF association leads to thicker and larger flocs, and EF and EE association leads to three-dimensional voluminous “house-of-cards” structures. The extent to which the particles become flocculated depends upon the degree of compression of the double layer. When the edges are positive, the platelets flex toward a negative face (Zaman et al., 2000). When the edges are negative, the platelets are forced to assume a more parallel type orientation (face-to-face, FF). For this reason, the pH of the medium is an important parameter to flocculation and sedimentation of clay suspension. Bradenburg and Lagaly (1988) proposed that in the pH region 5–7.5 edge-to-face (EF) contacts are destroyed due to the low positive charge density.
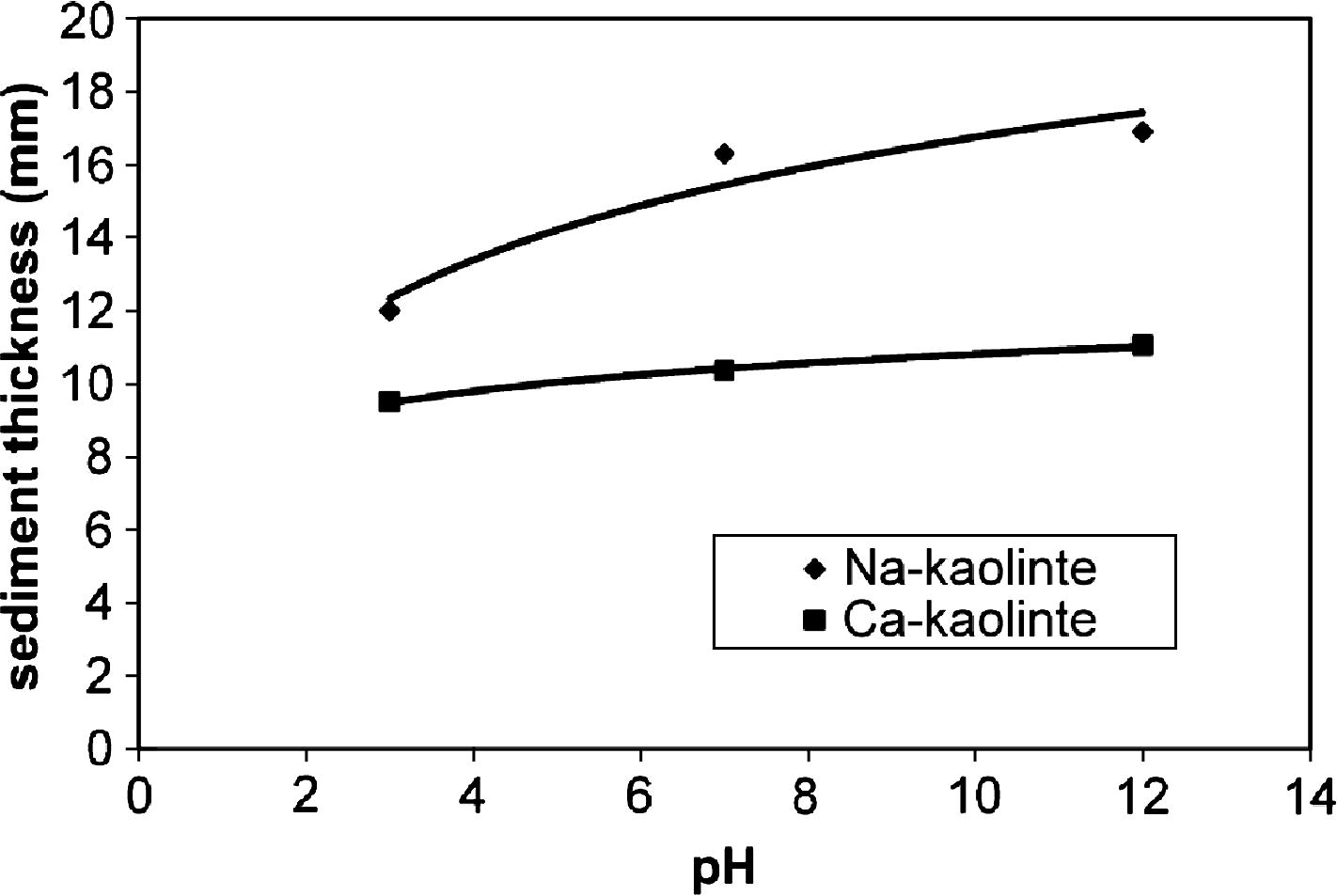
- Sediment thickness of Na- and Ca-kaolinite suspension in the presence of 10 ppm of PEI at different pHs. After 24 h.
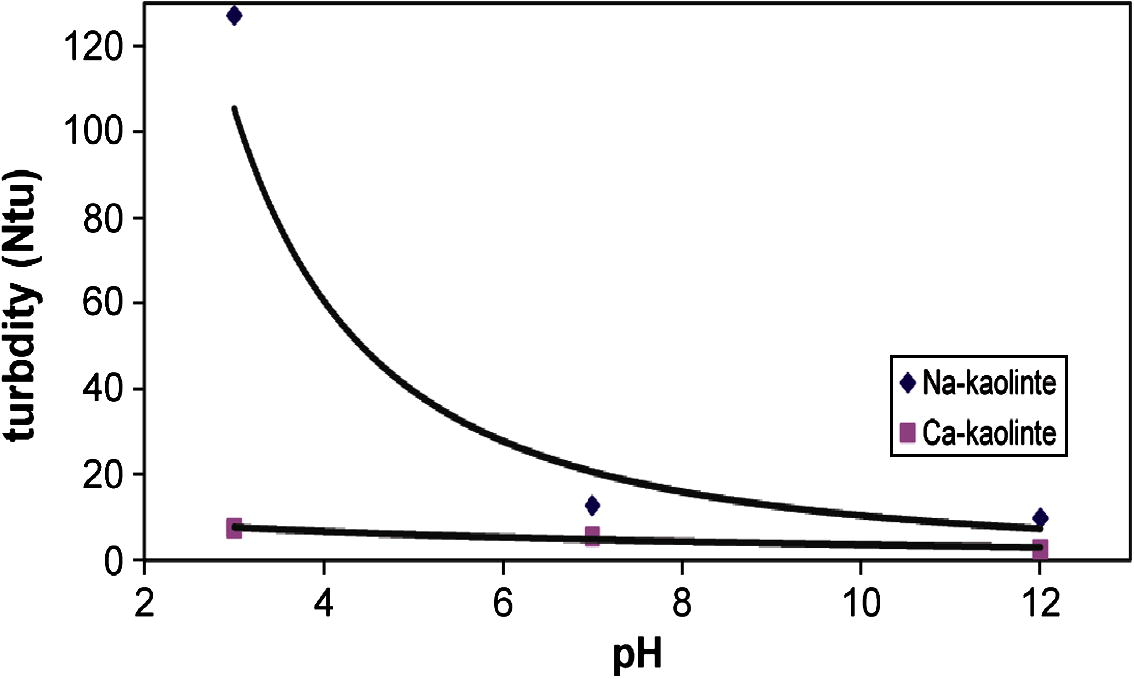
- Turbidity of Na- and Ca-kaolinite suspension in the presence of 10 ppm of PEI at different pHs.
In our study, the PZC of kaolinite was at about pH 4. This value agreed closely with that of Hu and Liu (2003) and did not agree with PZC of kaolinite edge surfaces reported in the literature that ranges from pHs 5 to 9, depending on the kaolinite used and the method used for its determination (Herrington et al., 1992; Melton and Rand, 1977).
Therefore at pH 3 (below point of zero charge, PZC), the edge (E) surfaces of kaolinite are positively charged. Also at this pH, the protonation degree of PEI increases and consequently the positive charge on the polymer chains increases, the face surface (F) of kaolinite becomes positive due to polymer adsorption. As a result, the association by edge-to-face (EF) contacts are destroyed, due to enhancement of electrostatic repulsion between the edges surface and face surface of kaolinite, thus preventing the kaolinite particles from coming together or flocculated (Conway, 2002). Therefore lower sediment thickness and higher supernatant turbidity for Na- or Ca-kaolinite suspension were obtained at pH 3. But at high pHs 7 or 12 (above point of zero charge), the edges of kaolinite particle become negative, and the face surfaces are positively charged due to adsorption of PEI, therefore the EF association enhances again, leading to an increase in the mean aggregate radius, and consequently higher sediment thickness and lower supernatant turbidity were obtained.
4.3 Effect of electrolyte
Table 2 shows the final sediment thickness and turbidity of Na- or Ca-kaolinite suspension in the presence of 10 ppm PEI at different NaCl or CaCl2 concentrations after equilibrium (24 h). The kaolinite sediments relatively decreased in the presence of 10 ppm PEI, by the addition of NaCl or CaCl2 electrolytes. Lower turbidity was obtained when the electrolyte concentration was 0.001 mol/L. This behavior could be attributed to the polymer–kaolinite interactions, which was affected by the presence of electrolyte. This result can be explained by examining the physicochemical properties of both the adsorbed polymer and the electrolyte. Flocculation of Na- or Ca-kaolinite by PEI was enhanced by the addition of the electrolyte. This electrolyte (coagulant) reduced the thickness of the diffuse double layer of adjacent kaolinite particles, thus reducing the interparticle clay particles, whereas PEI (a flocculent) is able to span the interparticle distances between these aggregated kaolinite particles by the formation of bridges through the hydrogen bond mechanism (Mekhamer and Assaad, 1999). These two processes may act individually or together to affect the sedimentation of kaolinite, leading to the initial stages of aggregation especially at low electrolyte concentration. Then a network structure could be produced through the polymer molecules attachment at many points of adsorption sites on the kaolinite surface. This stage accompanied by the formation a large flocs with minimum voids between the aggregates (Ma and Pierre, 1999), that is, the minimum turbidity for the supernatant of Na- or Ca-kaolinite, could be obtained. But at high electrolyte concentration, NaCl or CaCl (0.01 and 0.1), the kaolinite surface becomes more positive, leading to retardation of PEI adsorption (which has positive charge on its chains) on kaolinite surface. Therefore, the chance of bridge formation between kaolinite particles by the polymer decreased, smallest flocs were obtained (lower sediment thickness and consequently higher turbidity).
4.4 Data fitting
In this section, we address the problem of constructing parametric models which approximate the behavior of the empirically gathered data. If the data fit is sufficiently accurate, it is anticipated that the parametric model will provide further insight into the characterization of the phenomenon from which the measured data came.
A basic approach in obtaining a model for a given data
The parametric model (2) that provides the best fit is selected using the MATLAB routine SIMPLEX SEARCH. In these computations, we have set N = 2. The estimated parameters are given in Table 3. It is evident from the figures that the proposed parametric models well fit the empirically gathered data.
| Polymer concentration (ppm) | 0 | 10 | 50 | 200 | 600 | 1000 |
|---|---|---|---|---|---|---|
| C1 | 0.7022 | 0.0247 | 6.7123 | −2.0025 | 1.5890 | 0.0000 |
| C2 | 9.2215 | 37.2352 | 0.0000 | 7.2642 | 6.0833 | 4.9466 |
| 1λ | 0.6696 | −0.0791 | 3.2645 | 0.1010 | 0.1103 | −0.1662 |
| 2λ | 0.0013 | 0.0083 | 0.0057 | −0.0008 | −0.0007 | −0.0037 |
5 Conclusion
The sedimentation behavior of Na- or Ca-kaolinite suspension in the presence of PEI can be classified into two types: (i) sedimentation by settling of flocculated kaolinite particles which form a wall-to-wall network structure in the cylinder at low PEI concentrations (0, 10 and 50 ppm), (ii) sedimentation by accumulation of individual kaolinite particles at high PEI concentration (200, 600, and 1000 ppm). Highest sediment thickness was observed for Na- or Ca-kaolinite suspension of PEI concentration of 10 ppm, i.e. the critical flocculation concentration occurred at 10 ppm of PEI. The action of Ca+2 as a saturating cation has a more pronounced effect on flocculation of kaolinite than Na+ as a saturating cation. The flocculated sediment thickness of Na- or Ca-kaolinite by PEI was enhanced when the kaolinite has been initially coagulated by the addition of 0.001 mol/l NaCl or CaCl2. The lower flocculated sediment thickness and the higher turbidity were obtained at pH 3 compared to those at pHs 7 and 12.

Acknowledgement
The authors would like to thank professor Saleh alshubili for his fruitful discussion.
References
- Effects of polyethyleneimine adsorption on the rheological properties of purified bentonite suspensions. Colloids Surf. A: Physicohem. Eng. Aspects. 2005;252:95-98.
- [Google Scholar]
- Double-layer repulsive force between two inclined platy particles according to the Gouy–Chapman theory. J. Colloid Interface Sci.. 1994;168:111-117.
- [Google Scholar]
- Polymer adsorption: its correlation with flocculation and dewatering of kaolin suspension in the presence and absence of surfactants. Int. J. Miner. Process.. 2002;66:183-202.
- [Google Scholar]
- Electrophoretic studies of turbidity removal by coagulation with aluminum sulfate. J. Am. Water Works Assoc.. 1961;53:438.
- [Google Scholar]
- Adsorption–desorption behavior of acrylic–maleic acid copolymer at clay minerals. J. Colloid Interface Sci.. 1997;186:234-247.
- [Google Scholar]
- pH-independent and pH-dependent surface charges on kaolinite. Clays Clay Miner.. 1980;28:412-418.
- [Google Scholar]
- Rheological properties of sodium montmorillonite dispersions. Appl. Clay Sci.. 1988;3:263-279.
- [Google Scholar]
- Electrical Double-layer and Ion Adsorption Behavior at Solid/Solution Interface; Encyclopedia of Surface and Colloid Science. New York: Marcel Dekker Inc.; 2002.
- Performance of a physically adsorbed high-molecular-mass polyethyleneimine layer as coating for the separation of basic proteins and peptides by capillary electrophoresis. J. Chromatogr. A. 1995;708:356-361.
- [Google Scholar]
- Effect on saturating cation, pH, and aluminum and iron oxide on the flocculation of kaolinite and montmorillonite. Clays Clay Miner.. 1987;35:220-227.
- [Google Scholar]
- Herrington, T.M., Clarke, A.Q., Watts, J.C., 1992. The surface charge of kaolin. Colloids Surf. 68, 161–169.
- Chemical composition and surface property of kaolins. Miner. Eng.. 2003;16:1279-1284.
- [Google Scholar]
- Foundation of Colloid Science. Oxford: Oxford University Press; 1986.
- Interactions between adsorbed layers of a low charge density cationic polyelectrolyte on mica in the absence and presence of anionic surfactant. J. Colloid Interface Sci.. 1997;190:476-484.
- [Google Scholar]
- The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci.. 1999;82:43-92.
- [Google Scholar]
- Sedimentation behavior of a fine kaolinite in the presence of fresh Fe electrolyte. Clays Clay Miner.. 1992;40:586-592.
- [Google Scholar]
- Effect of interaction between clay particles and Fe (super 3+) ions on colloidal properties of kaolinite suspensions. Clays Clay Miner.. 1997;45:733-744.
- [Google Scholar]
- Clay sediment–structure formation in aqueous kaolinite suspensions. Clays Clay Miner.. 1999;47:522-526.
- [Google Scholar]
- Flocculation and coagulation of Ca- and K-saturated montmorillonite in the presence of polyethylene oxide. J. Appl. Polym. Sci.. 1999;73:659-662.
- [Google Scholar]
- Particle interactions in aqueous kaolinite suspensions: II. Comparison of some laboratory and commercial kaolinite samples. J. Colloid Interface Sci.. 1977;60:321.
- [Google Scholar]
- Interactions of polycations of aluminum and iron with clays. Clays Clay Miner.. 1984;32:49-57.
- [Google Scholar]
- Adsorption of polyacrylamide to Na-kaolinite: correlation between clay structure and surface properties. J. Colloid Interface Sci.. 1985;106:94-103.
- [Google Scholar]
- A natural clay organic complex from Andalusian black earth. Clays Clay Miner.. 1977;25:243.
- [Google Scholar]
- Surface and Colloid Chemistry in Advanced Ceramics Processing. New York: Dekker; 1994.
- Electric properties and conformation of polyethylenimine at the hematite–aqueous solution interface. J. Colloid Interface Sci.. 1997;196:87.
- [Google Scholar]
- Influence of non-ionic polymers on the rheological behaviour of Na+-montmorillonite clay suspensions. Part II. Homopolymer ethyleneoxide and polypropylene oxide-polyethylene oxide ABA copolymers. Colloids Surf. A: Physicochem. Eng. Aspects. 2003;215:1-10.
- [Google Scholar]
- Flocculation of kaolinite due to the attraction of oppositely charged crystal faces. Discuss. Faraday Soc.. 1954;18:135-145.
- [Google Scholar]
- Adsorption and flocculation behavior of cationic polyacrylamide and colloidal silica. Colloids Surf. A: Physicochem. Eng. Aspects. 2003;219:161-172.
- [Google Scholar]
- An Introduction to Clay Colloid Chemistry (second ed.). Florida: Krieger Publishing; 1991.
- Surface characterization of NH4 PAA-stabilized zirconia suspensions. J. Colloid Interface Sci.. 1999;213:552.
- [Google Scholar]
- Effect of adsorbed polyethylene oxide on the rheology of colloidal silica suspensions. J. Colloid Interface Sci.. 2000;226:290-298.
- [Google Scholar]







