Translate this page into:
Isolation of antimicrobial peptides from Apis florae and Apis carnica in Saudi Arabia and investigation of the antimicrobial properties of natural honey samples
*Corresponding author. Tel.: +966 014771548; fax: +96614767296 ayaad_t@hotmail.com (Tahany H. Ayaad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 22 January 2011
Abstract
As part of the ongoing search for novel antimicrobial agents and their use in singular or combined drug therapy, peptide fractions of molecular weights about 14.500 and 15.00 kDa were isolated from the hemolymph of wild (Apis florae) and carniolan (Apis carnica) bees of Saudi Arabia obtained from different regions in Riyadh (variable plant sources) during the honey seasons (spring and summer) 2008–2009. Following experimental infection with 1.1 × l06 viable Escherichia coli cells (ATCC 25922), the antimicrobial peptides were purified to homogeneity by reversed-phase high performance liquid chromatography. Antibacterial activity of the isolated peptide was evaluated in vitro by an agar well diffusion method for E. coli strain (ATCC 25922) and Klebsiella pneumoniae strain (ATCC 11678), the major Gram negative pathogens causing urinary tract infections, and Staphylococcus aureus (ATCC 6538) as Gram positive bacteria. A total of 10 honey samples collected from bee hives selected arbitrary at different floral areas of south Riyadh were also investigated for their antimicrobial activities against the yeast, Candida albicans (ATCC 10231) and four standard bacteria strains, E. coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), S. aureus (ATCC 6538) and Bacillus subtilis (ATCC 6633) using standard antimicrobial assays. The isolated antibacterial peptides and the different honey samples revealed comparable marked variations in antimicrobial activities and their sensitivity might be depending on their variable floral sources.
Keywords
Antimicrobial peptides
Honey
Apis florae
Apis carnica
Antimicrobial properties
1 Introduction
Antibiotic-resistant bacteria continue to be of major health concern world-wide. Since the use of antibiotics became widespread over 50 years ago, bacteria have progressively developed resistance (Hsueh et al., 2005). Consequently, scientific efforts have been made to study and develop new compounds to be used beyond conventional antibiotic therapy. Honey has been used since ancient times for the treatment of some respiratory diseases and for the healing of skin wounds. It has been proposed that the healing effect of honey could be due to various physical and chemical properties (Snow and Manley-Harris, 2004). Along with the rapidly increasing interest and research into natural health remedies and supplements, is a resurgence of interest in the therapeutic use of honey. Honey as most natural products, may have a large variance in therapeutic components depending on its origin. Thus, the floral source of honey plays an important role on its biological properties (Molan, 2002). In consequence, it would not be surprising that the provenance of honey could determine its antibacterial properties. Honey produced by honeybees (Apis florae) is one of the oldest traditional medicines considered to be important in the treatment of respiratory, gastrointestinal infection and various other diseases due to the absence of sufficient modern health care system, particularly in rural areas.
Often during pathogenic invasion, the first line of defense involves the innate mechanisms of immunity which in turn is followed by acquired immune responses involving the activation of T and B cells against specific antigens (Fearon and Locksely, 1996; Medzhitov and Janeway, 2000). In contrast to these acquired immune mechanisms, endogenous peptides, which are constitutively expressed or induced, provide a fast and effective means of defense against the pathogen. This group of molecules termed ‘antimicrobial peptides’ (AMPs) constitutes a primitive immune defense mechanism and is found in a wide range of eukaryotic organisms, from humans, plants and insects (Lehrer and Ganz, 1999). AMPs are an important component of the natural defenses of most living organisms against invading pathogens. During the past two decades several AMPs have been isolated from a wide variety of animals, both vertebrates and invertebrates, and plants as well as from bacteria and fungi. These peptides exhibit broad-spectrum activity against a wide range of microorganisms including Gram-positive and Gram-negative bacteria, protozoa, yeast, fungi and viruses, they have potential to overcome bacterial resistance makes them promising candidates for therapeutic drugs (Bals, 2000). AMPs are classified based on the three-dimensional structural studies carried out with the help of NMR. Most of these peptides are believed to act by disrupting the plasma membrane leading to the lysis of the cell. AMPs have been found to be excellent candidates for developing novel antimicrobial agents and few of these peptides show antimicrobial activity against pathogens causing sexually transmitted infection. Few peptides have already entered clinical trials for the treatment of impetigo, diabetic foot ulcers and gastric helicobacter infections (Reddy et al., 2004). One of the most promising among these antimicrobial peptide families are the cell-free immune repertoire of honeybees (Apis mellifera) that are induced by bacterial infection provide broad-spectrum antibacterial defense, such as apidaecin, hymenoptaecin, abaecin, and bee defensin. These peptides represent a viable treatment option for the major pathogens in urinary tract infections, that is, Escherichia coli and Klebsiella pneumoniae, causing 90–95% of all urinary tract infections (Czihal et al., 2007).
The purpose of the present study aimed at the isolation of antimicrobial peptides of wild Saudi Arabian Apis florae and carniolan Apis carnica. Evaluating scientifically the in vitro antimicrobial potential of these peptides and investigating the properties of ten natural honey samples produced by honeybees (Apis florae) against standard microorganisms among those commonly involved in causing diseases.
2 Materials and methods
2.1 Insects
Two groups of adult honey bee workers (age: 18–45 days and weight: 0.25 g ± 0.01) were either collected from natural environmental locations with different floral origins south Riyadh (Apis florae) or obtained from Department of Agricultural Extension and Rearing Parcels of Bee Queens in Saudi Arabia (Apis carnica). Adult bees were kept in small cages in the laboratory until used for induction by bacteria and isolation of peptides.
2.2 Microorganisms
The standard microorganisms used in this study were the yeast Candida albicans (ATCC 10231) and five different bacteria strains, E. coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), K. pneumoniae (ATCC 11678) as Gram negative and Staphylococcus aureus (ATCC 6538), Bacillus subtilis (ATCC 6633) as Gram positive bacteria.
2.3 Immune induction of honeybees
Humoral immunity was induced in adult honeybees by puncturing a leg with the tip of a hypodermic needle dipped in the 1.1 × l06 viable E. coli cells (ATCC 25922), equivalent to 0.5 McFarland tube suspended in 1 μl phosphate-buffered saline (PBS, 0.15 M, pH 7.2). Intact and induced adult bees were bled by decapitation and oozing lymph samples (2–4 μl/bee) were taken after 24 h post-induction, the collected hemolymph was pooled in tubes containing 100 μl of 2% trifluoroacetic acid (TFA) to prevent proteolytic degradation of the immunoinduced peptides and to precipitate proteins as described by Casteels et al. (1994) (for peptide purification). The collected hemolymph was also pooled in ice-cooled Eppendorf tubes containing few crystals of phenylthiourea to prevent melanization of samples (SDS–PAGE of crude hemolymph samples). Hemocytes were centrifuged (10,000g for 10 min) and the lymph was collected and freeze stored at −70 °C till used.
2.4 Purification of antibacterial peptides: reversed-phase high performance liquid chromatography (RP-HPLC)
The lymph samples were heat-treated (100 °C/5 min). The precipitate was spun down and the clear supernatant was acidified with an equal volume of 0.1% TFA, and fractionated by several rounds of high performance liquid chromatography using reversed-phase column supports, all as described by Casteels et al. (1993) and Lauth et al. (1998).
Sample (50 μl) aliquots of diluted lymph were taken for RP-HPLC analysis using an ABI 150A system (Applied Biosystems Inc., Ramsey, NJ) with a VYDAC C4 (214 TP54) analytical column (The separations group, Hesperia, CA). Solvent A was 0.1% TFA (pH 2.0) and solvent B was 70% acetonitrile (MeCN). Fractions were eluted at 1 ml/min (70 min total times). UV detection was done at 214 nm. All differential peaks between control and immune lymph, including peaks 1 and 2, were collected and further purified on VYDAC C18 (218TP54). Collected fractions were lyophilized and re-dissolved in Milli Q water, Promega (nuclease free water) before being tested for biological activity against standard microorganisms, E. coli strain (ATCC 25922) and K. pneumoniae strain (ATCC 11678). Following this procedure 1.0–10 μg peptides were routinely purified from each separate batch of Apis florae and Apis carnica worker bees.
2.5 Sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) for the isolated Apis florae and Apis carnica peptides
SDS–PAGE of control and purified antimicrobial peptides was carried out by the discontinuous buffer system as described by Laemmili (1970) with some modifications. Electrophoresis was carried out at a constant voltage of 200 V for 90 min. and 12% polyacrylamide gel, under denaturing conditions. The gels were calibrated with standard molecular weight proteins (high and low ranges: 200, 97.4, 68, 29, 18.4 and 8.15 kDa). Protein bands were visualized by Coomassie Brilliant Blue dye staining (CBB). M.Wt calculations were determined by regression analysis using the manufacturers’ software.
All chemicals and buffers are purchased from (Sigma Chemicals Co.–Aldrich Chemicals Co., Sweden).
2.6 Honey samples
This study was carried out on 10 natural honey samples (1 kg each) collected in Saudi Arabia during the honey seasons (spring and summer) 2008–2009. Honey samples collected from Apis florae bee hives were marked randomly as indicated in Table 1. Each honey sample was collected in a sterile universal glass container and kept at 2–8 °C until tested. Each sample was tested at original concentration 100% and diluted to 70% and 30% of its original concentration using physiological saline PBS, pH 7.2 according to the method described by Nzeako and Hamdi (2000) with some modifications. Flora sources are classified according to Migahid (1996) and Chaudhary (2000).
No. of honey sample
Season
Location
Plant cover
1
Summer
Alsomman (plain)
Acacia spp. (Mimosaceae), Ziziphus spina (Rhamnaceae), Rhanterium epapposum (Asteraceae)
2
Summer
Hail (plain)
Acacia spp. (Mimosaceae)
3
Summer
Al-Qasim (plain)
Ziziphus spina (Rhamnaceae)
4
Summer
Horimalaa
Acacia spp. (Mimosaceae), Ziziphus spina (Rhamnaceae)
5
Spring
Roda Eltanhat (plain)
Ziziphus spina (Rhamnaceae), Acacia spp. (Mimosaceae), Peganum harmala (Zygophyllaceae), Rhanterium epapposum (Asteraceae) Wild Shafalah (Mimosaceae)
6
Summer
Horimalaa
Acacia spp. (Mimosaceae)
7
Spring
Toik Mountain
Wild Shaflah (Mimosaceae)
8
Spring
Elkharj
Trifolium spp.
9
Summer
Roda Elkhashm (plain)
Ziziphus spina (Rhamnaceae), Acacia spp. (Mimosaceae)
10
Summer
Horimalaa
Ziziphus spina (Rhamnaceae), Acacia spp. (Mimosaceae)
2.7 Antimicrobial activity of honey samples and purified antimicrobial peptides
An agar well diffusion method was used to assess the antimicrobial activity of the honey samples and purified peptide fractions against certain selected standard microorganisms (NCCLS, 2003). Fifty microliters (50 μl) of each honey dilution (undiluted, 70% and 30%, w/v) were used against E. coli (ATCC 25922), P. aeruginosa (ATCC 27853), S. aureus (ATCC 6538), B. subtilis (ATCC 6633) and C. albicans (ATCC 10231), separately. While 20 μl of twofold serial dilution of purified peptide fractions were tested against each of E. coli (ATCC 25922), K. pneumoniae (ATCC 11678) the major Gram negative pathogens causing urinary tract infections and S. aureus (ATCC 6538) as G-positive bacteria, separately. Each of the bacterial strains was inoculated into nutrient broth and incubated overnight at 37 °C until growth was 1.5 (optical density at 640 nm). Each of the honey sample concentrations and the purified peptide fractions were added, separately into wells of 5 mm diameter of inoculated Mueller–Hinton agar plates (Oxoid) by selected microorganisms, each dilution was done in triplicate. The plates were incubated at 37 °C till the honey seeped into the agar. Zones of growth inhibition were recorded in mm after an overnight incubation at 37 °C. The end point of antimicrobial activity of each determination was defined as the highest dilution (lowest concentration) producing an inhibition zone with the tested organisms. The growth after 24 h incubation at 37 °C was then compared to a control plate that contained no peptide fraction or honey samples. All strains were handled under aseptic conditions and the microorganisms were destroyed by autoclave to ensure bio-safety.
2.8 Statistical analysis
Data analysis were carried out using SPSS for Windows Ver. 17.0.
3 Results
Major antimicrobial peptides were isolated from two groups of Saudi Arabian Apis florae and carniolan bees either unchallenged (intact) or induced. The antimicrobial peptides were initially fractionated under RP-HPLC by using 70% (v/v) acetonitrile containing 0.1% (v/v) trifluoroacetic acid recovered as two peaks 1 and 2 (Fig. 1) in the final RP-HPLC step. Gel electrophoresis analysis indicated apparent homogeneity and approximate M.Wt of 14.500 and 15.00 kDa for the isolated peaks 1 and 2, respectively. Differential pattern analysis of described peaks are barely detectable in unchallenged (intact) bees but are strongly induced upon induction. All the peptide fractions obtained from chromatography columns isolated from Apis florae and Apis carnica groups either (intact or bacterial induced) presented antimicrobial activity against two Gram negative bacteria E. coli and K. pneumoniae. Fig. 2 represents antibacterial activity of the purified antimicrobial peptide fractions 14.500 and 15.00 kDa for each type of wild and carniolan bees either (induced or intact) against the tested G-negative bacteria. Presented data revealed that the isolated peptides from intact and induced honey bees have significant moderate activities against E. coli and K. pneumoniae. Overall, the activity of the purified peptide showed comparable antimicrobial activity in both groups of honey bees either Apis florae or Apis carnica.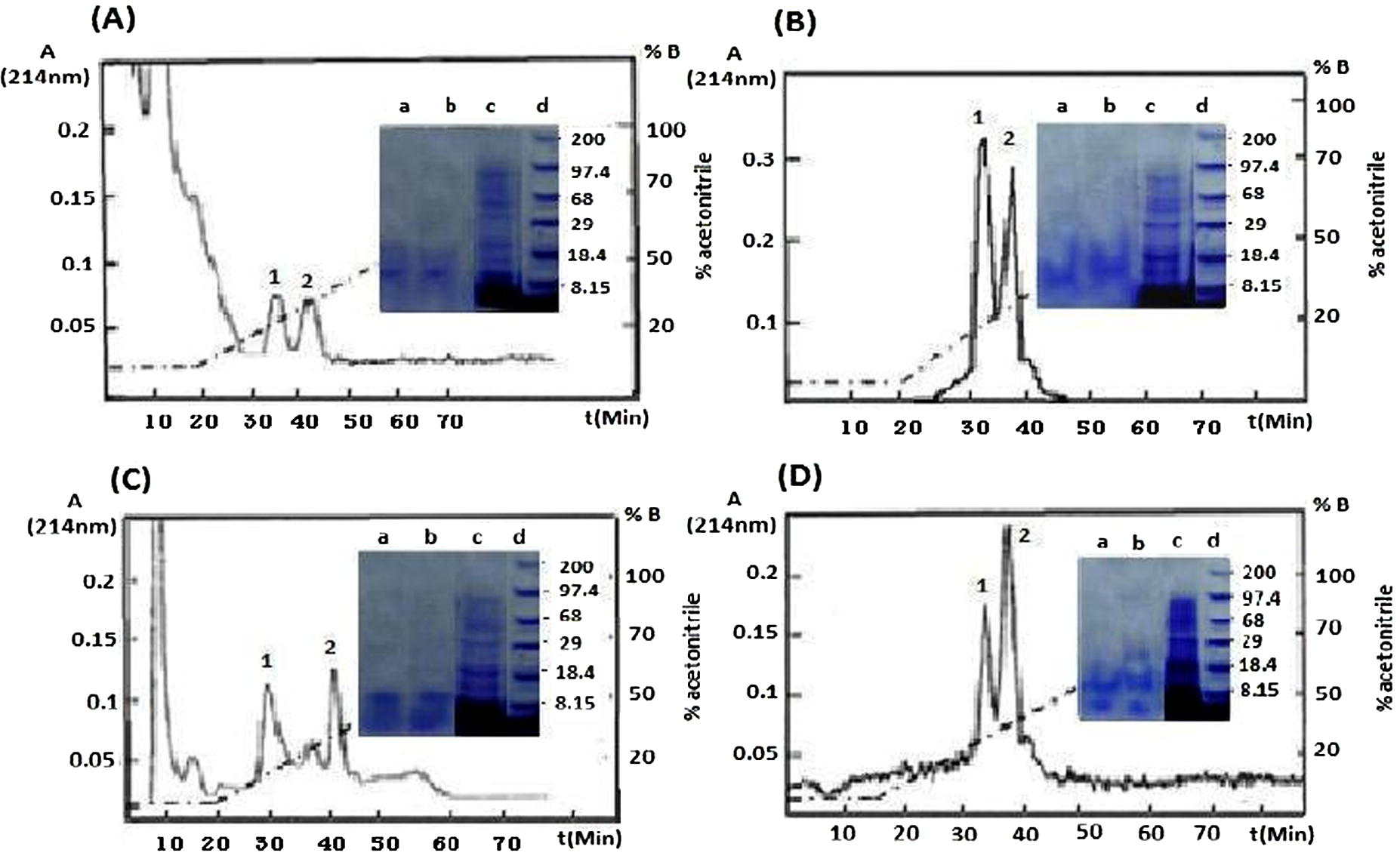
RP-HPLC purification and SDS–PAGE of antimicrobial peptide fractions from wild Apis florae and carniolan Apis carnica bees. Heat treated lymph was fractionated, separately, on a VYDAC® C4 and finally C18 VYDAC® columns. Fractions 1 and 2 developed at a flow rate of 1 ml/min and a gradient of 30–70% B/70 min. Panels A and B show the HPLC patterns of the isolated peptide fractions from, intact (non-induced) and E. coli induced Apis florae bees. Panels C and D intact and induced Apis carnica bees. The dashed line represents the percentage of solvent B (70% acetonitrile) in solvent A (0.1% trifluoroacetic acid). Electrophoretic analysis of pure peptide fractions (lanes a and b) and crude adult bee lymph (lane c) were shown in the center of the figure. The molecular weight of standards is shown in lane d (×103). CBB stained.
Antibacterial activity of Apis florae and Apis carnica purified peptide fractions against (A) E. coli and (B) K. pneumoniae. Comparable inhibition zones observed by Apis florae and Apis carnica bee peptides whether intact (1–4) or induced (5–8 wells) against both gram negative bacteria.
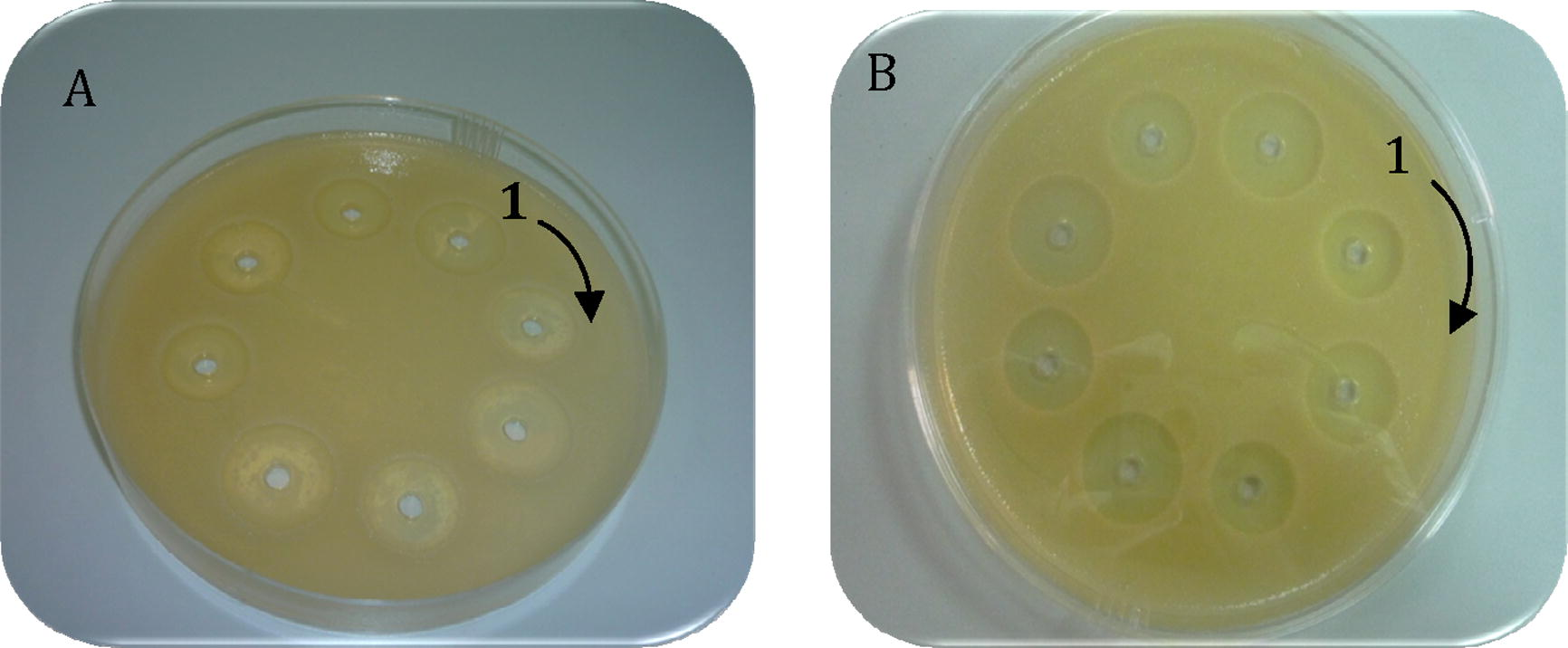
On the other hand, no antimicrobial activity was observed against S. aureus in case of intact fractions while the isolated antimicrobial peptide from immune bees have significant activities as shown in Fig. 3.
Antibacterial activity of Apis florae and Apis carnica bee purified peptide fractions against S. aureus. No activity detected in fractions (1–4) from intact Apis florae and Apis carnica bees. Labels (5, 6 and 7, 8) show inhibition zones of purified fractions from both induced bee types.
3.1 Antimicrobial activity of honey samples
Antimicrobial activity of the 10 honey samples were done with three concentrations 30% (w/v), 70% (w/v) and undiluted used in this study are shown in Fig. 4. By using the agar-well diffusion method, the growth of all five standard microorganisms S. aureus, B. subtilis, P. aeruginosa, E. coli and C. albicans were inhibited (Jorgensen and Ferraro, 1998). Antimicrobial activity was evaluated by measuring the zone of inhibition (mm) against the tested microorganisms. At higher concentrations of honey there was a progressive increase in growth inhibition of the microorganisms. It was observed that S. aureus was the most inhibited bacterial strain by all honey samples. The average diameter of the inhibition zones produced by the undiluted honeys samples was approximately (35 ± 0.1) mm. Our data show that all honey samples tested have some antibacterial action at 30%, 70% and undiluted concentrations. In general, all the five tested microorganisms were variably sensitive to honey up to 30% concentration.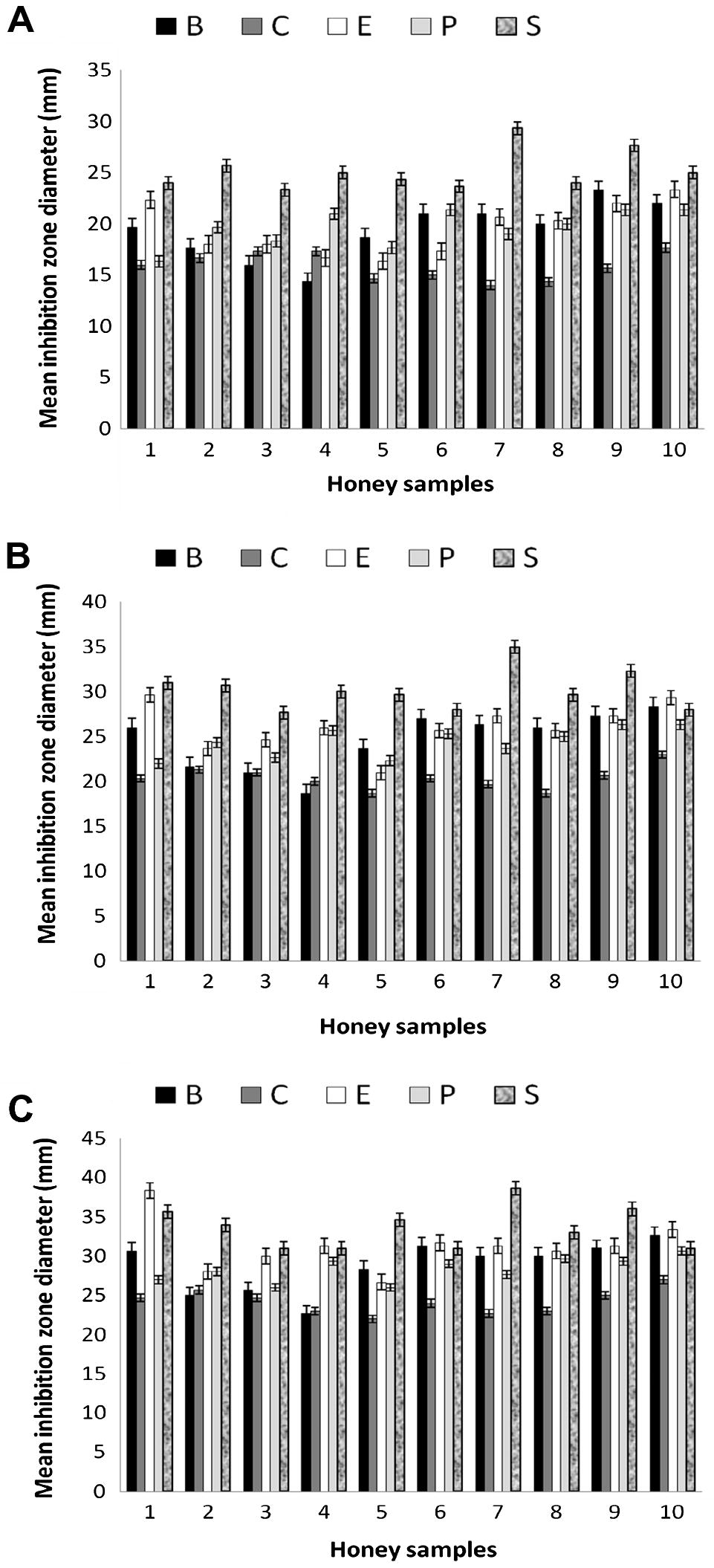
Mean of microbial inhibition zones by different concentrations (mm) of 10 honey samples (30%, 70% and undiluted A, B, C, respectively) against five standard microorganisms (B refers to Bacillus subtilis, C refers to Candida albicans, E refers to Escherichia coli, P refers to Pseudomonas aeruginosa and S refers to Staphylococcus aureus).
4 Discussion
In recent years there was a dramatically increase in bacteria strains resistance to one or even several antibiotics. Thus, the development of antimicrobial compounds with novel modes of action is a major focus of current pharmaceutical research. A very interesting and promising approach relies on antibacterial peptides, because bacteria do not develop any resistance to these antimicrobial peptide families. One of the most promising among these families are the short, proline-rich antibacterial peptides originally isolated from insects, such as apidaecin, drosocin, formaecin, and pyrrhocoricin. These peptides represent a viable treatment option for the major pathogens in urinary tract infections, that is, E. coli and K. pneumoniae, causing 90–95% of all urinary tract infections (Czihal et al., 2007).
The exact mechanism of action of AMPs remains a matter of controversy, there is a consensus that these peptides selectively disrupt the cell membranes and the amphipathic structural arrangement of the peptides is believed to play an important role in this mechanism. The phospholipids head group charge on cell membranes and peptide charge distribution appears to play an important role in the peptide–membrane interactions (Oren and Shai, 1998; Cudic and Otvos, 2002). There is accumulating evidence suggesting that the antibacterial or self-defense peptides which are usually highly basic, recognize the acidic phospholipids exposed on the surface of the bacterial membrane (Tytler et al., 1995). In the case of microbes, the anionic lipids are present on the outer surface of the membrane, whereas for mammalian cells, anionic lipids are present along the cytoplasmic side of the membrane. This feature might account for their preferential activity against bacteria but not against mammalian cells.
Several structure function studies on AMPs have been published (Hanke and Schlue, 1997; Wieprecht et al., 1997; Mor, 2000). It is well documented that biophysical properties such as secondary structure, overall charge and hydrophobicity influence the interaction of AMPs with model membranes and biological cells.
An ubiquitous polypeptide fractions were purified from the Saudi Arabian honeybee Apis florae and Apis carnica. The isolated polypeptides are naturally detected in the adult bees hemolymph, presenting moderate spectrum of antimicrobial activity against E. coli and K. pneumoniae bacteria the major Gram negative pathogens causing urinary tract infections to human, this is may be due to the nature of G-negative cell wall which characterized by thin layer of peptidoglycans the main target of antimicrobial peptide activity.
On the other hand isolated antimicrobial peptides showed significant inhibition to S. aureus growth with induced fractions and non-significant with intact one as shown in Fig. 3. These results can be explained as G-positive bacteria have thick layer of peptidoglycans in its cell wall which required immune induction for honeybees to produce more peptides.
Apparently an immune induction of the bees increased the polypeptides production as appeared from the purification peaks (RP-HPLC, Fig. 1) and more inhibition of the growth of G-positive bacteria (Fig. 3) during evaluating their antimicrobial activity. Similar results was obtained by Casteels et al. (1993) for the apidaecin as an increase in the transcription level occurred 4 h after experimental infection and very high concentrations were sustained throughout the entire 36 h post-infection. This suggests that there is mounting evidence that activation of insect peptide antibiotic gene is the endpoint of a signal pathway that has bacteria, or more specifically lipopolysaccharide (LPS), as initiating agent (Girardin et al., 2002).
Comparison of the mean inhibition zones of antibacterial activity between the two purified fractions 1 and 2 revealed non-significant differences P ⩽ 0.05 indicating that these peptide fractions are functionally identical. These results are also indicative, as revealed from the electrophoretic profile that showed apparent homogeneity and an approximate M.Wt of 14.500 and 15.00 kDa, for both types of bees under investigation, respectively and this may be attributed to the difference in its amino acid modification and the cDNA genes coding the different antimicrobial families as confirmed by Xu et al. (2009) in the honey bee Apis cerana. Originally the presented data showed that the peptide fractions proved to be effective as an antibacterial agent in the lymph of the intact bees towards the tested standard bacteria (Fig. 2). These results are indicative, as in case of the corresponding hymenoptaecin of Casteels et al. (1993) and defensin of Chernysh et al. (1996), but unlike apidaecin (Casteels et al., 1990). Consequently the isolated peptide holds its place somewhere between the group of peptides that attack G-positive and G-negative bacteria equally well as reported by Boman (1994) and many other antibacterial peptides that seem to have clear preference for either G-negative such as apidaecins (Casteels et al., 1989) and diptericins (Bulet et al., 1995) or G-positive, e.g. insect defensin (Lauth et al., 1998), lysozyme and royalisin (Fujiwara et al., 1990). In addition, as the corresponding results indicates the purified fractions profile proved to be comparably identical for both groups of wild Apis florae and carniolan Apis carnica bees collected from different floral locations.
The variation in the antimicrobial potential of honey samples used in this study as compared to the previous similar studies highlights that the source of the nectars may have contributed to the difference in the antimicrobial activities of honey that is, the flowers from which bees gathered nectar to produce the honey, since flora source determines many of the attributes of honey, for example flavor, aroma, color and composition. As being a natural product, the composition of honey is highly variable (NHB, 1994). Antimicrobial activity of honey is not dependent alone on its phytochemical nature, i.e. tetracycline derivatives, ascorbic acid, peroxidase or amylases, streptomycin, sulfonamides which are claimed as heat labile. On the other hand, the antimicrobial effect of honey is attributed to its phenolic acid, flavonoids, benzyl-alcohol, 2-hydroxy benzoic acid which are heat stable and may be active agents but their concentration in honey appears too low to solely responsible (Heerng, 1998).
The obtained antimicrobial data of 10 honey samples collected from hives of bees from different flora were generally consistent with other reports showing that honey has good antibacterial activity (Patricia et al., 2005). Also, Ceyhan and Ugar (2001) tested 84 honeys against eight bacteria and two fungi showing that honey has broad-spectrum activity. In addition, these authors found that the antibacterial activity of honey was greater than that which could be attributed to the sugar content of the honey. Nzeako and Hamdi (2000) in their study of six commercial honeys found that inhibition of S. aureus, E. coli and P. aeruginosa did not occur at honey concentrations 40%, in contrast to the current study where all the tested bacteria showed growth inhibition up to 30% of natural honey concentrations and have shown an excellent activity against S. aureus. Interestingly, the obtained results of the ten honey samples under investigation revealed that C. albicans sensitivity were less than other bacterial organisms tested and these are consistent with the data proved by Obeseiki-Ebor and Afonya (1984) and Nzeako and Hamdi (2000).
The results shown by honey samples in relation to S. aureus may be important, given that in recent decades there has been a marked increase in difficult to treat skin and underlying tissue infections associated with S. aureus (Halcón and Milkus, 2004). It has been informed that S. aureus has developed resistance against several antibiotics and that it is the principal contaminant agent in many clinical infections (Moreno et al., 2005). Thus, new strategies to treat wounds infected with S. aureus are needed, and the possibility to use honey appears as a convenient and less costly treatment option. Poor activity of the honeys against S. aureus was unexpected as previous reports by Cooper et al. (1999). Part of the explanation for the difference in results from other studies may be due to methodological differences between studies because the agar dilution method used by these authors different from an agar well diffusion method that is used in this study. However it is also likely to be due to variation in the natural floral origin of the honey being produced. Our honey samples also exerted antimicrobial activities on P. aeruginosa, which were resistant to some antibiotics.
5 Conclusions
Honey and antimicrobial peptide produced by honeybees either Apis florae or Apis carnica have antimicrobial activity when tested in vitro against standard microorganisms. However, pharmacological standardization and clinical evaluation on the effect of honey and peptides are essential before using them as a preventive and curative measure to common diseases related to the tested bacterial species. In spite of all the positive facts associated with antimicrobial peptides there have been a few problems. Firstly, there are fewer data available on the unknown in vitro and in vivo toxicities of the peptides. Secondly, the stability of the synthesized compound formulations in vivo has not been studied in detail. Lastly, the cost of the production of these peptides on a large scale has been a major obstacle for quite some time.
Hence, further foci should be undertaken to identify more of such novel peptides, redesign the existing peptides to get rid of their toxicity and develop novel recombinant protocols to obtain greater yield of peptides at a lower cost.
Acknowledgments
The authors wish to express their deep gratitude to the Research Centre of Female Sections for Science and Medical Studies King Saud University, and King Abdul Aziz City for Science and Technology for supporting and funding the present work.
References
- Epithelial antimicrobial peptides in host defense against infection. Resp. Res.. 2000;1:141-150.
- [Google Scholar]
- Cecropins: antibacterial peptides from insects and pigs. In: Hoffmann J.-A., Janeway C.A., Natori S., eds. Phylogenetic Perspectives in Immunity: The Insect Host Defense. Austin, TX: S.R.G. Landes Co.; 1994. p. :43-65.
- [Google Scholar]
- Insect immunity. The inducible antibacterial peptide deptericin carries two O-glycans necessary for biological activity. Biochemistry. 1995;34:7394-7400.
- [Google Scholar]
- Isolation and characterization of abaecin, a major antimicrobial response peptide in the honey bee (Apis mellifera) Eur. J. Biochem.. 1990;187:381-386.
- [Google Scholar]
- Functional and chemical characterization of Hymenoptaecin, an antibacterial polypeptide that is infection-inducible in the honeybee Apis mellifera. J. Biol. Chem.. 1993;268(10):7044-7054.
- [Google Scholar]
- Biodiversity of apidaecin-type peptide antibiotics. J. Biol. Chem.. 1994;299(42):26107-26115.
- [Google Scholar]
- Investigation of in vitro antimicrobial activity of honey. Riv. Biol. Forum. 2001;94:363-372.
- [Google Scholar]
- Chaudhary, S.A., 2000. Flora of the Kingdom of Saudi Arabia, vol. 2. Ministry of Agriculture, Riyadh.
- The inducible anti bacterial peptides of the hemipteran insect Palomena prasina: identification of a unique family of Proline-rich peptides and of a novel insect defensin. J. Insect Physiol.. 1996;42:81-89.
- [Google Scholar]
- Antibacterial activity of honey against strains of Staphylococcus aureus from infected wounds. J. R. Soc. Med.. 1999;92:283-285.
- [Google Scholar]
- Intracellular targets of antibacterial peptides. Curr. Drug Targets. 2002;3:101-106.
- [Google Scholar]
- Antimicrobial activity of apidaecin peptides. Eur. Soc. Clin. Microbiol. Infect. Dis.. 2007;29(2):S602.
- [Google Scholar]
- The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50-54.
- [Google Scholar]
- A potent antibacterial protein in royal jelly. J. Biol. Chem.. 1990;265:11333-11337.
- [Google Scholar]
- Intracellular vs extracellular recognition of pathogens – common concepts in mammals and flies. Trends Microbiol.. 2002;1:193-199.
- [Google Scholar]
- Staphylococcus aureus and wounds: a review of tea tree oil as a promising antimicrobial. Am. J. Infect. Control. 2004;32:402-408.
- [Google Scholar]
- Lipid bilayers: methods and applications.Biological Techniques Series. San Diego, USA: Academic Press; 1997.
- Immunochemical screening for antimicrobial drug residue in commercial honey. Analyst. 1998;123:2759-2762.
- [Google Scholar]
- Hsueh, P., Chen, W., Luh, K., 2005. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991–2003 at a university hospital in Taiwan. Int. J. Antimicrob. Agents 26, 463–472.
- Antimicrobial susceptibility testing: general principles and contemporary practices. Clin. Infect. Dis.. 1998;26:973-980.
- [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (London). 1970;227:680-685.
- [Google Scholar]
- Isolation, characterization and chemical synthesis of a new insect desfensin from Chironomus plumosus (Diptera) Insect Biochem. Mol. Biol.. 1998;2:1059-1066.
- [Google Scholar]
- Antimicrobial peptides in mammalian and insect host defense. Curr. Opin. Immunol.. 1999;11:23-27.
- [Google Scholar]
- Migahid, A.M., 1996. In: Flora of Saudi Arabia, vol. 2, third ed. King Saud University Press, Riyadh.
- Not all honeys are the same for wound healing. Bull. Eur. Tissue Rep. Soc.. 2002;9:5-6.
- [Google Scholar]
- Peptide based antibiotics: a potential answer to raging antimicrobial resistance. Drug Dev. Res.. 2000;50:440-447.
- [Google Scholar]
- Tracking methicillin resistant Staphylococcus aureus clones Colombian hospitals over 7 years (1996–2003): emergence of a new dominant clone. Int. J. Antimicrob. Agents. 2005;25:457-462.
- [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS), 2003. Performance standards for antimicrobial susceptibility testing. Fourteenth Informational Supplement, vol. 4(1). NCCL, Wayne, PA.
- National Honey Board (NHB), 1994. Honey definitions document. Am. Bee J. 117–118.
- Antimicrobial potential of honey on some microbial isolates. SQU J. Sci. Res. Med. Sci.. 2000;2:75-79.
- [Google Scholar]
- In vitro evaluation of the anti candidiasis activity of honey distillate (HY-1) compared with that of some antimycotic agents. J. Pharm. Pharmacol.. 1984;36:283-284.
- [Google Scholar]
- Mode of action of linear amphipathic α-helical antimicrobial peptides. Biopolymers. 1998;47:451-463.
- [Google Scholar]
- Bactericidal activity of different honeys against pathogenic bacteria. Arch. Med. Res.. 2005;36:464-467.
- [Google Scholar]
- Antimicrobial peptides: premises and promises. Int. J. Antimicrob. Agents. 2004;24:536-547.
- [Google Scholar]
- On the nature of non-peroxide antibacterial activity in New Zealand Manuka honey. Food Chem.. 2004;84:145-147.
- [Google Scholar]
- Molecular basis for prokaryotic specificity of magainin induced lysis. Biochemistry. 1995;34:4393-4401.
- [Google Scholar]
- Peptide hydrophobicity controls the activity and selectivity of magainin 2 amide in interaction with membranes. Biochemistry. 1997;36:6124-6132.
- [Google Scholar]
- Antimicrobial peptide evolution in the Asiatic honey bee Apis cerana. PLoS One. 2009;4:1-9.
- [Google Scholar]







