Translate this page into:
Isolation of an antitubercular protein from Staphylococcus hominis IS2 from the custard apple and evaluation of its biosafety
⁎Corresponding author. m.palanisamy@mu.edu.sa (Manikanadan Palanisamy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tuberculosis (TB) is one of the leading causes of death throughout the world. Traditional medicine poses a serious challenge to drug resistance against Mycobacterium tuberculosis. Hence, it is an urgent need to develop, novel the anti-mycobacterial compounds. In this study, a potent anti-mycobacterial compound producing Staphylococcus hominis IS2 was isolated from the custard apple exhibiting novel anti-mycobacterial activity than other isolates. The bacterial strain was cultured and anti-mycobacterial compound was purified by the combination of ammonium sulphate precipitation and DEAE-cellulose column chromatography. The purified protein corresponding to 10 kDa presented anti-mycobacterial activity. It was further characterized and the difference in activity towards temperature and pH was observed (p < 0.01). The purified protein was analyzed using FT-IR and MALDI-TOF MS/MS analysis. Enzymatic treatment revealed the absence of carbohydrate moieties in the purified protein. The purified anti-mycobacterial protein presented anti-cancer activity against cervical cancer cell lines (HeLa) and was dose-dependent (p < 0.01). Haemolytic assay and Zebra fish embryo studies revealed that the isolated protein is safe at lower doses.
Keywords
Drug-resistant bacteria
Anti-mycobacterial
Drug
Anticancer
Bio-safety
1 Introduction
Tuberculosis (TB) is one of the contagious diseases caused by the drug-resistant bacterial pathogen Mycobacterium tuberculosis (Mtb) and approximately 1.3 million death was reported in 2020 (WHO, 2020). To treat TB, antibiotics such as pyrazinamide, ethambutol, isoniazid (INH), rifampicin (RIF), and aminoglycosides were prescribed (Fatima et al., 2020). The continuous administration of these antibiotics for six months – two years leads to the development of multidrug-resistant bacterial pathogens and decreased efficiency. With the development of extensively drug-resistant (XDR) and multidrug-resistant (MDR) bacterial strains, the use of existing drugs is highly questionable. Hence, there is an urgent need to develop an effective anti-TB drug to prevent the development of drug-resistant bacterial pathogens. In recent years, various attempts were made to analyze drugs to treat Mtb infection (Mosaei et al., 2018). The new therapeutic options include a mycobacterial gene responsible for bacterial growth, inhibition of fatty acid biosynthesis, and DNA replication (Pradhan and Sinha, 2018). Moreover, recent research reported that the major mechanisms of drug resistance in Mtb are biofilm formation and gene mutation (Saba et al., 2021).
The peptidoglycan of Mtb and the related bacterial genera consists of a mixture of muramic acid residues, encompassing, N-acetyl and N-glycolyl modifications. The modification of peptidoglycan in antibiotic susceptibility was reported previously and the N-glycosylation of peptidoglycan involves the development of drug resistance to lysozyme and beta-lactams. In addition, the peptidoglycan cell wall layer involves immunogenicity in the Mtb pathogenic strains (Hansen et al., 2014). Despite being a highly successful antibiotic group, beta-lactam drugs are not considered therapeutic drugs because of the resistance of Mtb against this antibiotic class, mainly attributed to a beta-lactamase enzyme produced by the chromosomally encoded genes (Saba et al., 2021). The drug resistance was attributed to the impermeable nature of the mycobacterial cell wall and the abundance of non-classical peptidoglycan cross-links, and these two phenomena affect the activity of carbapenems antibiotics (Wivagg et al., 2014). Cervical cancer is one of the most common malignancies affecting humans throughout the world.
Antimicrobial peptides or antimicrobial proteins have an inhibitory effect against various bacterial pathogens (Wu et al., 2020). Bacteria from the genus Staphylococcus produce diverse groups of antimicrobial peptides or proteins (Al-Dhabi et al., 2020). These antimicrobial peptides or proteins have inhibitory effects against bacteria not only against the genus Staphylococcus, but also against several pathogens. Recently, the antimicrobial peptides or proteins have been isolated and antagonistic activity was characterized against Staphylococcus aureus (Arasu et al., 2013), Listeria monocytogenes (Matikevicˇiene et al., 2017). In addition, the isolated peptides and proteins from Staphylococcus sp. have the potential against various cancer cell lines, including, colon cancer, lung cancer, cervical cancer and breast cancer cells (Arasu et al., 2017). The beneficial role of peptides or proteins synthesized by gram-positive Staphylococcus sp. was not completely elucidated. In this study, anti- tubercular and anti-cancer proteins producing Staphylococcus hominis was isolated and characterized.
2 Materials and methods
2.1 Isolation of Staphylococci strains
A total of four fruit samples (pineapple, apple, orange, and custard apple) were subjected to the isolation of Staphylococci strains. All fruits were collected from the local market between February 2022 and July 2022. Two grams of sample was mixed with 98 mL peptone water and 0.2 mL of sample was spread on Baird-Parker agar medium.The culture plates were incubated at 37 ± 1 °C for 48 h and pure culture was prepared after continuous plating. The coagulase-negative staphylococci were characterized as suggested previously (Soge et al., 2009).
2.2 Analysis of anti-tubercular activity
The isolated Staphylococci strains were cultured in MRS broth (Himedia, Mumbai, India) medium for 48 h at 37 ± 1 °C. After 48 h, the culture was centrifuged at 12000 rpm for 10 min at 4 °C. It was further filtered using a membrane filter (0.22 µM). The cell-free extract was neutralized using 0.1 N NaOH solution. The neutralized supernatant of the isolates was subjected to analyze the anti-tubercular properties against Mycobacterium tuberculosis. The antitubercular property of the neutralized extract was tested as suggested previously (Khusro et al., 2018).
2.3 Characterization of anti-tubercular protein producing Staphylococcus strain ST20
The morphological, and biochemical characteristics of potent anti-tubercular protein-producing Staphylococci strain ST20 was analyzed. It was grown on blood agar plates and haemolytic activity was analyzed. It was cultured on Mueller Hinton Agar plates and the antibiotic susceptibility pattern wasanalyzed. The universal primers were used for the amplification of 16S rDNA gene sequences and an accession number was assigned (Al-Dhabi et al., 2020).
2.4 Purification of anti-tubercular proteins from Staphylococci strain ST20
The selected Staphylococci strain ST20 was inoculated (0.1 mL) in MRS broth medium (100 mL) for 48 h at 37 ± 1 °C. It was further centrifuged at 12000 rpm for 10 min at 4 °C. It was further filtered sterilized, neutralized and considered as crude protein. The crude sample (80 mL) was purified using the salting-out method using solid ammonium sulphate (35% − 80% saturation). After 2 h incubation, the sample was centrifuged at 10000 rpm for 10 min at 4 °C. The precipitated pellet was suspended in 1 mL buffer A (Sodium phosphate buffer, pH 7.2, 0.1 M) and the total protein content was analyzed (Bradford, 1976). The sample showing maximum anti-tubercular activity was further dialyzed and the total protein content was further tested. The dialyzed active fraction was loaded on DEAE–Cellulose matrix packed in a glass column. A total of 1.5 mL sample was loaded on the equilibrated column and removed unbound using buffer A. Then, it was eluted in buffer A containing 0 – 1 M NaCl (gradient elution). All fractions were assayed for antitubercular activity by LRP assay (Khusro et al., 2018). The purified active fraction was lyophilized and loaded on sodium dodecyl sulphate polyacrylamide gel electrophoresis (12.5%) and the molecular weight was determined.
2.5 FT-IR spectroscopy analysis
The purified sample was used for FT-IR analysis. Briefly, 2 mg protein was mixed with potassium bromide (200 mg) and analyzed (Esther Lydia et al., 2019).
2.6 Characterization of anti-tubercular protein
The effect of pH on enzyme activity was determined by incubating anti-tubercular protein (0.1 mL) with 0.9 mL buffer at various pH values (4.0 – 11.0). The sample was incubated for 1 h at 28 ± 1 °C and an LRP assay was performed. To determine the optimum temperature for anti-tubercular activity, the sample was incubated at various temperatures (20 – 80 °C) at pH 7.0 for 15 min. After 30 min incubation, LRP assay was performed. The results were compared with control. The effect of enzymes on anti-tubercular activity was assayed. The sample (0.1 mL) was mixed with 0.4 mL enzymes (pepsin, trypsin, protease K and α-amylase) and incubated for 30 min at pH 7.0 and 35 °C. Enzyme activity was disrupted by boiling and LRP assay was performed (Khusro et al., 2018).
2.7 Anticancer activity of cell-free extract of strain ST20
The strain ST20 presented maximum anti-tubercular activity and was selected for the determination of the anticancer activity. Cervical cancer cell line (HeLa) was used and anticancer property was established by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay. The sample was loaded on well-grown cell lines at various concentrations (20 – 100 µL) and cytotoxicity was assayed (Khusro et al., 2018). A 96-well microtiter plate was used for the analysis and the sample was read at 620 nm against reagent blank. Doxorubicin (Sigma, USA) was used as a standard. The percentage of cells viability was determined using the following formula:
2.8 MALDI-TOF MS/MS spectrum analysis
The purified anti-tubercular protein was excised from the SDS-PAGE and stored in 50% HPLC grade methanol. The target protein was trypsinated for 12 h at 37 °C. To the sample (0.1 mL), 0.1 mL cyano-4-hydrocinnamic acid solution (10 mg/mL in 70% acetonitrile) was added and mixed using a vortex mixture. MALDI-TOF MS/MS spectrum was performed and the peptides of proteins were characterized. The peptide sequences of protein were performed and peptide mass fingerprinting was carried out. The instrument was set to 100–2250 m/z and mass spectra were analyzed. The obtained sequences (N-terminal amino acid sequence) wereanalyzed and compared to NCBI databases.
2.9 Haemolytic activity
To analyze the haemolytic activity of the protein in whole blood, a purified IS2 sample was added at various concentrations (0.125, 3.75, 6.25, 12.5 and 25 µM) in a sterile vial containing 0.2 mL freshly collected blood sample. The vials were stirred continuously for 15 min at 35 ± 2 °C, and blood cells were removed by centrifugation at 4000 rpm for 10 min at 4 °C. The sample was diluted with phosphate buffered saline (10-fold dilution) and the absorbance of the sample was measured at 540 nm. Fresh blood (0.1 mL) was diluted with MilliQ water (0.9 mL) and considered a positive control for lysis assay (Vijayaraghavan et al., 2016).
2.10 Safety evaluation using Zebra fish embryos
Toxicity analysis was performed using Zebrafish (Danio rerio) embryos. The fertilized eggs were allowed to deposit in 96-well microtiter plates. The protein sample was added at various concentrations (0.125, 3.75, 6.25, 12.5 and 25 µM). Water was used as a negative control, while 3,4-dichloroaniline was used as a positive control. The microtiter plates were incubated for 24 h and 48 h after post-fertilization and the embryos were tested for toxic effects.
3 Results
3.1 Anti-tubercular activity of Staphylococci strains
The anti-tubercular activity of the strain was tested against M. tuberculosis. The cell-free extract of strains showed RLU reduction activity. The RLU reduction of Staphylococci strains was described in Fig. 1. The strain IS2 and IS4 showed maximum activity and RLU reduction percentages were 94.4 ± 1.5%, and 89.7 ± 2.2%, respectively. A total of seven isolates showed > 70% RLU reduction activity, whereas only 4 strains showed < 5% RLU reduction activity.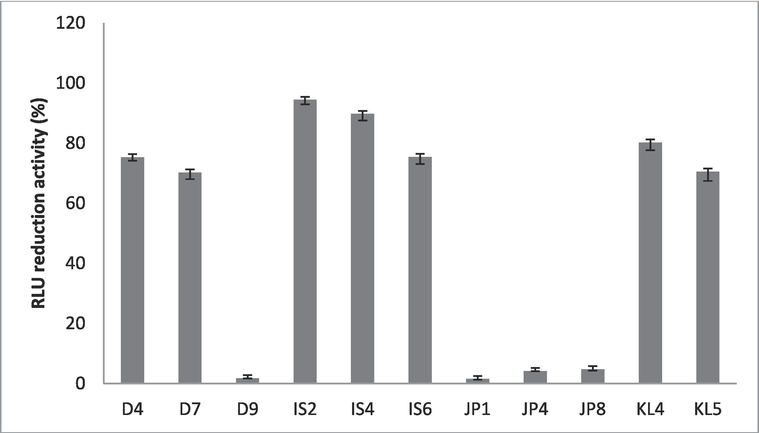
Anti-tubercular activity of Staphylococcal strains against M. tuberculosis. Error bar presented standard deviation.
3.2 Characterization of Staphylococcal strain IS2
The bacterial colonies showed orange colour colonies on blood agar plates. It was a Gram-positive organism, with smooth colonies, and opaque. Cells are cocci, non-motile and non-sporulating organism. It grows at broad pH ranges (pH 5.0 to 9.0), between 30 and 35 °C. It was unable to hydrolyze esculin, casein, starch, and gelatin. The growth was inhibited in the presence of citrate and urea.The isolated S. hominis IS2 showed yellow colour colonies of Blood Agar plates. It showed sensitivity against most of the tested antibiotics (Fig. 2).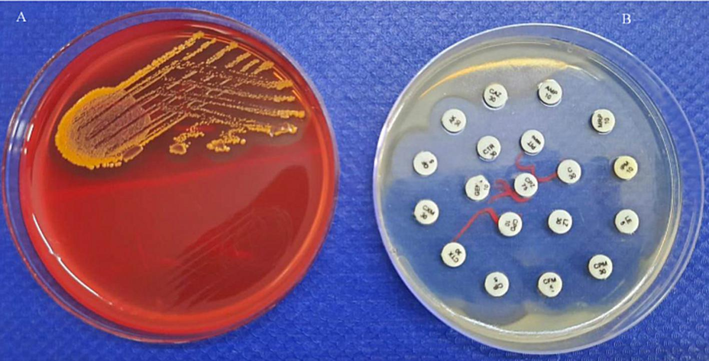
Growth of Staphylococcal strain IS2 on blood agar plates (A) and antibiotic sensitivity (B) on Mueller Hinton Agar plates after 24 h incubation at 37 ± 1 °C.
3.3 Purification of anti-tubercular protein
The anti-tubercular protein was purified by the salting out method and DEAE-cellulose column chromatography. The ammonium sulphate method was a suitable method for the concentration of proteins and the total protein content was 65.3 mg/mL and was found to be maximum between 40 and 50% saturation. After dialysis, the total protein content diluted and was 53.1 mg/mL.The DEAE-cellulose column chromatography purified sample yielded 43.9% with 28.4% total protein recovery. The anti-tubercular activity was maximum in fractions 12 and 13, respectively. These two fractions were combined (12 and 13) and lyophilized. The lyophilized fraction was subjected to SDS-PAGE and the molecular weight of the purified protein was 10.2 kDa (Fig. 3).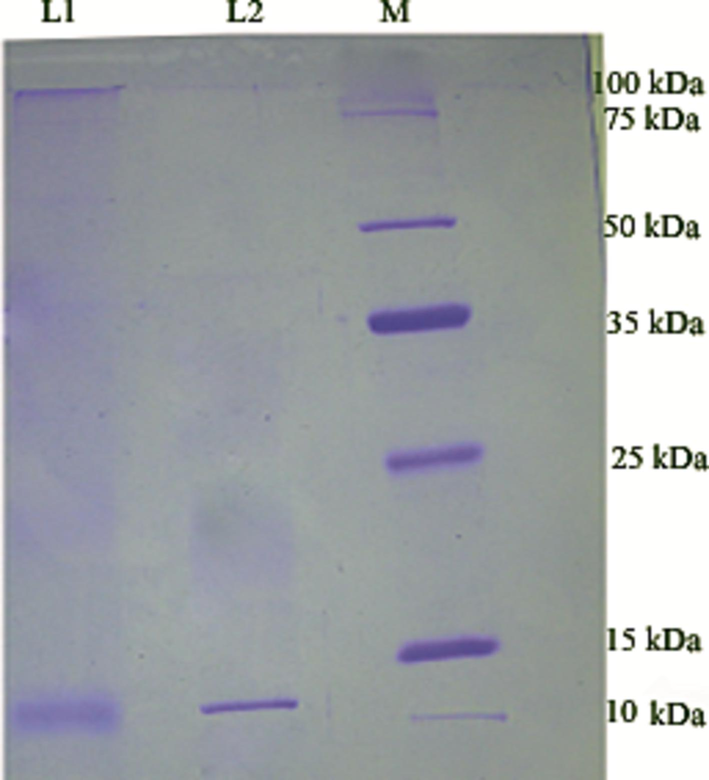
Molecular weight of antitubercular protein from S. hominis IS2. Lane 1: Ammonium sulphate fraction (40–50% saturation), Lane 2: Lyophilized DEAE-cellulose fractions (12 and 13) and Lane M – protein molecular weight marker ranged from 10 to 225 kDa. The molecular weight of S. hominis IS2 purified protein (Lane 2) was approximately 10.2 kDa.
3.4 Functional characterization of anti-tubercular protein using FT-IR analysis
The FTIR spectrum of anti-tubercular protein was illustrated in Fig. 4. The increased absorption bands were observed at 3288.7, 1532.18, 1482.22, 1187.8, and 982.25 cm−1 indicating the presence of amide, CAN, NAH stretch and bond, respectively (Fig. 4).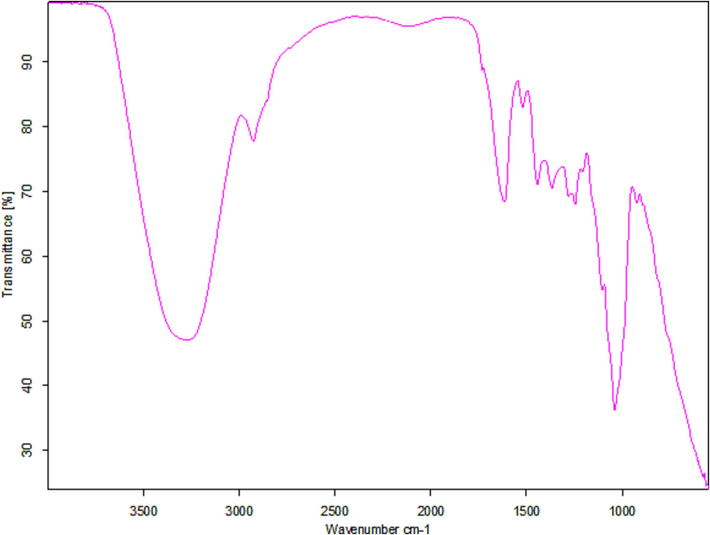
Functional characterization of anti-tubercular protein from S. hominis IS2 using FT-IR.
3.5 Characterization of anti-tubercular protein
The effect of pH on enzyme activity was tested to determine the optimum pH value and maximum activity was presented at pH 7.0 (Fig. 5A). The effect of temperature on enzyme activity was tested and maximum activity was achieved at 40 °C. Anti-tubercular activity was depleted at higher temperatures (>60 °C) (Fig. 5B). Anti-tubercular activity in relation to temperature and pH was statistically significant (p < 0.01). The protein sample treated with trypsin, pepsin and protease K completely degraded protein. Amylase treatment showed 99% residual anti-tubercular activity.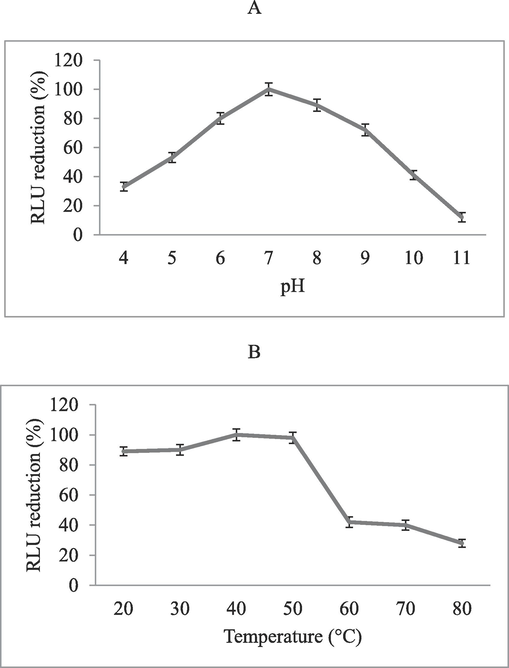
Effect of pH at various pH values (4–11) (A) and temperature at ranges from 20 to 80. °C (B) on anti-tubercular activity.
3.6 Anticancer activity of anti-tubercular activity
Anticancer activity of the anti-tubercular protein isolated from the bacterial strain IS2 against HeLa cell line was described in Fig. 6. The cell lines were treated with 20–100 µL samples and observed morphological changes of cells, including, cell shrinkage and observed reduced number of viable cells. The viability of the HeLa cells increased at increased concentrations of protein (p < 0.01). The IC50 value of the purified protein was 63 mg/mL.Moreover, the standard anticancer agent doxorubicin showed reduced IC50 value against HeLa cell lines (5.2 mg/mL) (Fig. 6).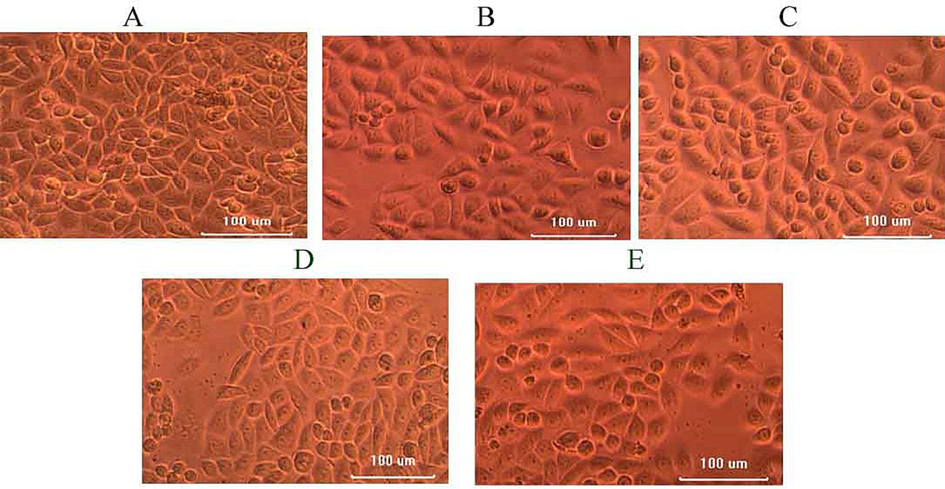
Anticancer activity of protein extract against hela cell lines. the purified protein (20–100 µL) was inoculated on a microtiter plate containing a layer of cell lines and the cytotoxicity was assessed. Morphological changes and a reduced number of HeLa cells were observed. A-20 µL, B-40 µL, C-60 µL, D-80 µL and E-100 µL.
3.7 Mass spectrometry analysis of protein
MALDI-TOF MS/MS spectrum of anti-tubercular protein was described in Fig. 7. The spectrum of each peak represents a peptide of the trypsin-digested excised band protein from SDS-PAGE. The molecular size of the obtained peptide ions ranged between 129.1 m/z and 996.4 m/z. The major abundant peptide ion was determined in 173.1 m/s, 299 m/s, and 424.2 m/s, respectively (Fig. 7).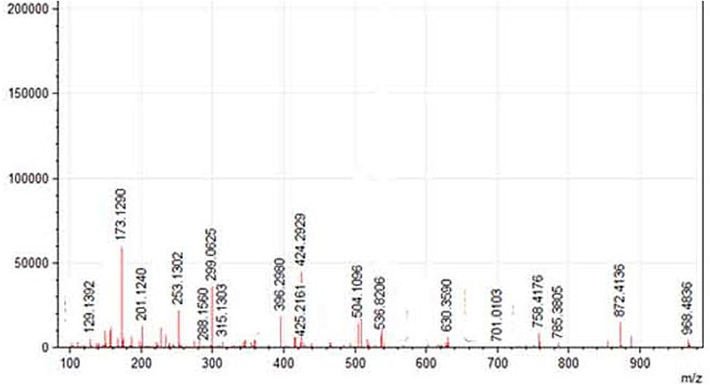
Analysis of trypsin digested SDS-PAGE separated anti-tubercular protein using MALDI-TOF MS/MS. Each peak represents the mass of the peptide fragment.
3.8 Safety evaluation of purified protein
The haemolytic effect of Staphylococcal protein was tested using whole blood. The concentration of the sample influenced the haemolytic potential of the proteins. At lower concentrations, haemolytic activity is very less and considerably increased at higher concentrations. However, haemolysis was not observed at a very low protein sample. The released haemoglobin content (%) was also calculated and depicted in Fig. 8A. Zebrafish embryos are highly sensitive to environmental factors, toxins and pollutants and are frequently used as the model organism. In this study, the purified protein was tested against Zebrafish embryos. Zebrafish embryos treated with proteins at lower concentrations did not show any visual abnormalities up to 24 h treatment; however, treatment after 48 h with a low dose (3.75 µL) displayed 17.5% mortality. The lowest concentration (<3.75 µL) is the bio-safe dose for 48 h (Fig. 8B).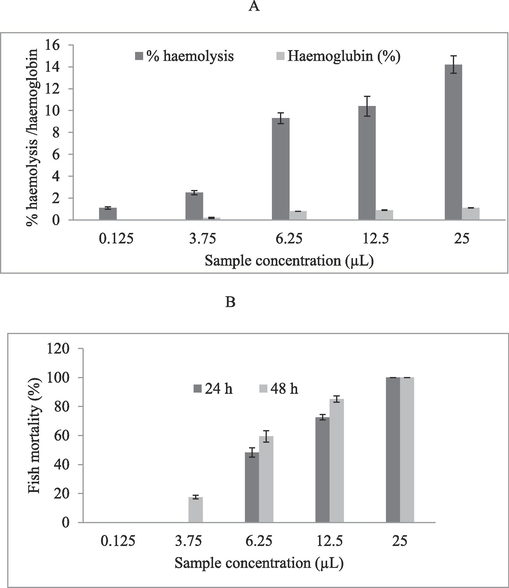
Bio-safety assay of anti-tubercular protein at various concentrations. A haemolytic assay was performed on blood and the percentage of haemolytic activity was determined (A). The same concentration was tested on Zebrafish embryos and safety dose was determined.
4 Discussion
Tuberculosis is one of the highly contagious diseases and is termed as “white plague” that kills several million people every year.Several anti-tuberculosis agents were proposed to treat TB for the past few decades (Yuan and Sampson, 2018). Conventional treatment takes about six months to treat effectively and causes side effects to the patients. The continuous use of antibiotics led to the emergence of multidrug resistance, which posesa serious threat to humans (Sander et al., 2020). The antimicrobial peptide and proteins of specific groups of bacteria, particularly Staphylococcus spp. produced various bioactive compounds. Bacteria such as S. xylosus (Matikevicˇiene et al., 2017), S. pseudintermedius strain 222 (Wladyka et al., 2015) and S. hominis MBBL 2–9 (Kim et al., 2010) produced antimicrobial peptides with a molecular mass of 5921.92 Da, 2038.4 Da, and 6 kDa, respectively.
The purified protein showed anti-tubercular activity and increasedactivity was observed at higher doses. The difference in the activity at varying temperatures may be due to the instability of hydrogen bonds. The purified anti-tubercular protein lost its activity at higher temperatures (>60 °C). The purified protein lost activity after treatment with protease K, trypsin, and pepsin. However, treatment with α-amylase treatment did not show any inhibitory effect revealing that the proteins have any carbohydrate moieties and have only amino acid residues. The present finding was similar to previous studies reported earlier (Yi et al., 2016). The molecular weight of the purified protein was 10.2 kDa. LC-MS analysis was performed and the spectrum of the trypsinated peptide was analyzed. The molecular size of the obtained peptide ions ranged between 129.1 m/z and 996.4 m/z.The cytotoxic profile was studied using cervical cancer cell lines (HeLa) and the cytotoxic activity was dose-dependent manner. This finding revealed the anticancer potential of protein isolated from S. hominis IS2. The purified protein is an anticancer agent to manage cervical cancer. FT-IR analysis was used to determine the functional group of antimicrobial peptides and is considered a powerful tool to analyze structural and conformational changes (Esther Lydia et al., 2019). The IR peaks represented bending and stretching vibration in the IR region. The anti-mycobacterial peptides or proteins were characterized from the bacteria-contaminated equipment (Hussain et al., 2022). In this study, Zebrafish embryos were used as the model organism to analyze toxicity. Ismail et al. (2017) used Zebrafish embryos to analyzethe phytotoxicity of medicinal plants.
5 Conclusions
Antimicrobial peptides and proteins produced by bacterial species, especially Staphylococcal microbiota have gained much more attention in recent years because of their significant mechanism of action and stable activity than other bioactive compounds. Thus, the potent peptide or protein is a valuable agent against drug-resistant bacterial pathogens. The present study revealed the production of thermo-stable anti-tubercular and anticancer protein from S. hominis IS2. The purified low-molecular-weight protein was non-toxic on blood cells and did not show any mortality in Zebra fish at very low concentrations. The present study suggests that S. hominis IS2 may be useful to treat Mtb infections.
Acknowledgement
The authors extend their appreciation to the deputyship forResearch & Innovation, Ministry of education in Saudi Arabia forfunding this research work through the project number (IFP-2022-17).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Isolation and screening of Streptomyces sp.Al-Dhabi-49 from the environment of Saudi Arabia with concomitant production of lipase and protease in submerged fermentation. Saudi. J. Biol. Sci. 2020:474-479.
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol. B. 2017;172:50-60.
- [Google Scholar]
- Susceptibility of poultry associated bacterial pathogens to Momordica charantia fruits and evaluation of in vitro biological properties. Microb. Pathogen.. 2019;132:222-229.
- [Google Scholar]
- Fatima, S., Kamble, S.S., Dwivedi, V.P., Bhattacharya, D., Kumar, S., Ranganathan, A., Van Kaer, L., Mohanty, S. and Das, G., 2020. Mycobacterium tuberculosis programs mesenchymal stem cells to establish dormancy and persistence. J. Clin. Investigat., 130(2), pp.655-661. doi: 0.1172/JCI128043.
- N-glycolylated peptidoglycan contributes to the immunogenicity but not pathogenicity of Mycobacterium tuberculosis. J. Infect. Dis.. 2014;209:1045-1054.
- [CrossRef] [Google Scholar]
- Anti-mycobacterial activity of heat and pH stable high molecular weight protein (s) secreted by a bacterial laboratory contaminant. Microb. Cell Fact.. 2022;21(1):1-13.
- [Google Scholar]
- Comparative study of herbal plants on the phenolic and flavonoid content, antioxidant activities and toxicity on cells and zebrafish embryo. J. Trad. Complement. Med.. 2017;7(4):452-465.
- [Google Scholar]
- Anti-tubercular and probiotic properties of coagulase-negative staphylococci isolated from Koozh, a traditional fermented food of South India. Microb. Pathogen.. 2018;114:239-250.
- [Google Scholar]
- Characterization and structure identification of an antimicrobial peptide, hominicin, produced by Staphylococcus hominis MBBL 2–9. Biochem. Biophys. Res. Commun.. 2010;399:133-138.
- [Google Scholar]
- Matikevicˇienė , V., Grigiškis, S., Lubytė , E., Dienys, G., 2017. Partial purification and characterization of bacteriocin-like peptide produced by Staphylococcus xylosus. Environ. Technol. Resour. 3, 213–216.
- Mode of action of kanglemycin a, an ansamycin natural product that is active against rifampicin-resistant Mycobacterium tuberculosis. Mol. Cell.. 2018;72:263-274.
- [CrossRef] [Google Scholar]
- High throughput screening against pantothenate synthetase identifies amide inhibitors against Mycobacterium tuberculosis and Staphylococcus aureus. Silico. Pharmacol.. 2018;6:9.
- [CrossRef] [Google Scholar]
- Compromised base excision repair pathway in Mycobacterium tuberculosis imparts superior adaptability in the host. PLoSPathog.. 2021;17:e1009452.
- [Google Scholar]
- Mechanisms of drug-induced tolerance in Mycobacterium tuberculosis. Clin. Microbiol. Rev.. 2020;34:e00141-e00220.
- [CrossRef] [Google Scholar]
- Characterization of methicillin-resistant Staphylococcus aureus and methicillin-resistant coagulase-negative Staphylococcus spp. isolated from US West Coast public marine beaches. J. Antimicrob. Chemother.. 2009;64(6):1148-1155.
- [CrossRef] [Google Scholar]
- Cow dung is a novel feedstock for fibrinolytic enzyme production from newly isolated Bacillus sp. IND7 and its application in in vitro clot lysis. Fronti. Microbiol.. 2016;7:361.
- [CrossRef] [Google Scholar]
- Mechanisms of blactam killing and resistance in the context of Mycobacterium tuberculosis. J. Antibiotics. 2014;67:645-654.
- [CrossRef] [Google Scholar]
- A peptide factor secreted by Staphylococcus pseudintermedius exhibits properties of both bacteriocins and virulence factors. Sci. Rep.. 2015;5:14569.
- [Google Scholar]
- World Health Organization (WHO). (2020). Global Tuberculosis Report. Available online at: https://www.who.int/teams/global-tuberculosis-programme/tbreports/global-tuberculosis-report-2020 (accessed January 30, 2021).
- Characterization of biofilm formed by multidrug resistant Pseudomonas aeruginosa DC-17 isolated from dental caries. Saud. J. Biol. Sci.. 2020;27(11):2955-2960.
- [Google Scholar]
- Purification and characterization of a novel bacteriocin produced by Lactobacillus crustorum MN047 isolated from koumiss from Xinjiang, China. J. Dairy Sci.. 2016;99:7002-7015.
- [Google Scholar]
- Hit generation in TB drug discovery: from genome to granuloma. Chem. Rev.. 2018;118:1887-1916.
- [CrossRef] [Google Scholar]







