Isolation and purification of starch hydrolysing amylase from Streptomyces sp. Al-Dhabi-46 obtained from the Jazan region of Saudi Arabia with industrial applications
⁎Corresponding author. naldhabi@ksu.edu.sa (Naif Abdullah Al-Dhabi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Amylase is an important industrially useful enzyme with various applications, widely explored for their applications in food and biofuel industries. In this study, seventeen actinomycetes strains were isolated from the soil sample and screened for amylase biosynthesis. Among the 17 isolates, nine actinomycetes showed activity on starch agar plates. The strain Al-Dhabi-46 showed more hydrolytic zone (19 mm) on starch agar plates. It was identified by biochemical characters and 18S rDNA sequencing and characterized as Streptomyces sp. Al-Dhabi-46. Amylase production was found to be maximum after 5 days of incubation (108 ± 4.1 U/ml) and at pH 8.0 (118.1 ± 2.3 U/ml) and at 40 °C (124 ± 12.1 U/ml). Amylase synthesis was high in the medium containing starch (208 ± 11.4 U/ml). Amylase production was 241 ± 18.1 U/ml in the culture medium supplemented with beef extract and 0.1% Mn2+ ions significantly enhanced amylase production. The molecular weight of purified amylase from Streptomyces sp. Al-Dhabi-46 was 44 kDa. Amylase was highly active up to pH 8.0 and at 40 °C. Amylase activity was enhanced by the addition of Mn2+ at 10 mM concentrations. Purified amylase from Streptomyces sp. Al-Dhabi-46 digested cassava starch at 25% and 55%, 78% and 95.4% level after 15 min, 30 min, 45 min and 60 min of enzymatic reaction. Enhanced production of amylase enzyme from the Streptomyces sp. Al-Dhabi-46 attract for the production of industrially important products.
Keywords
Streptomyces sp.
Amylase
Optimization
Bioethanol
1 Introduction
Amylases are industrial enzymes and used mainly in starch processing industries. These enzymes have various uses in starch hydrolysis and syrup production (Tonkova, 2006). These cleave 1–4 linkages between glucose units and formation of maltotriose, maltose and glucose. In recent year’s enzymatic hydrolysis of starch has effectively replaced chemical methods (Vidyalakshmi et al., 2009). Amylases have wide application, including, processing of fruits like banana, mango, citrus and papaya (Sharanappa et al., 2011). In dish washing and used in laundry (Vidyalakshmi et al., 2009), food fermentation, paper and textile industries (Sanghvi et al., 2011). These enzymes were derived from various sources, including actinomycetes and bacteria. Among these amylases microbial source is highly preferred than other sources because of its vast availability and its plasticity (Li et al., 2011). Optimization and production of this microbial source of amylases were investigated by submerged fermentation and solid state fermentation. Many cheap substrates were used in solid state fermentation; however in submerged fermentation various synthetic medium has been used. These synthetic culture medium is much expensive and not economical, so it is an urgent need to be replaced with highly economically available industrial and agricultural by products, as they are broadly considered as potential substrates to produce various enzymes in solid state fermentation. pH and temperature are well know process parameters in enzymes production from microorganisms (Pérez-Guerra et al., 2003). Amylases from various microbial sources with some novel properties have many industrial applications. These unique properties include optimum temperature, thermo tolerance, optimum salinity and pH optima (Gupta et al., 2003). Bacteria from the genus Bacillus produce various amylases. These amylases have various applications in the industry. α-amylases synthesized by the Bacillus sp. have potent applications, and in recent years much interest has been focused on culture medium optimization. Bioprocess parameter optimization is an important strategy because these include enzyme yield. Generally, the process parameters were optimized to increase the yield of enzymes (Nielsen and Borchert, 2000, Sivaramakrishnan et al., 2006). Actinomyces such as, Streptomyces sp. SLBA-08, S. avermitilis, S. rochei BTSS 1001 and Streptomyces strain A3 have been used for the production of amylases (Boovaragamoorthy et al., 2019; Gurusamy et al., 2019; Ilavenil et al., 2015; Roopan et al., 2019; Valsalam et al., 2019b; Rajkumari et al., 2019). Microbial amylases have also been used in the field of medicine (Acharyabhatta et al., 2013; Chakraborty et al., 2012). These amylases have isolated and characterized from various bacterial species including, Streptomyces, Bacillus, Escherichia, Serratia, Proteus and Micrococcus (Shafiei et al., 2011; Van Der Maarel et al., 2002). The genus Streptomyces adopted to survive in various environment and synthesized various metabolites, including enzymes (Al-Dhabi et al., 2016, 2019a). Amylase has also been used in detergent formulation to hydrolyze starches. In Saudi Arabia, about 90% areas are covered by deserts; however the microbial consortium from actinobacterial community not has been exploited (Nithya et al., 2015; Al-Dhabi et al., 2018, 2019b). In recent years, amylase producing actinomycetes have been isolated from various sources (Kar and Ray, 2008; Krishnakumar et al., 2015; Krishnan and Sampath, 2015; Singh et al., 2011). Supplementation of carbon source, nitrogen source and ions induce amylase production. It was also reported that addition of various nitrogen sources such as beef extract and yeast extract enhanced amylase production (Ponnuswamy et al., 2011). Amylases are used in the preparation of digestive aid, pharmaceutical aid, and preparation of bread, used in baking industry. Amylases also have been used in various industries including detergent, brewery, starch liquefaction, textile, saccharification, paper and food (Gupta et al., 2003; Al-Dhabi and Ghilan, 2018; Arasu et al., 2017). The yield of amylase is mainly based on the selected strain, cultivation method, pH, nutrient requirements, incubation time and cultivation temperature. Earlier, various researchers isolated actinomycetes from various sources for the production of amylases. However, studies are limited on thermostable amylase production from Streptomyces sp. Therefore, the main aim of the present investigations is to isolate and screen various Streptomyces sp. for amylase production and to characterize this enzyme for various industrial processes.
2 Materials and methods
2.1 Chemicals and nutrient media
The chemicals and nutrient medium used in the present study were procured from the Somatco, Riyadh, Saudi Arabia. The antibiotics used for the isolation of the actinomycetes were purchased from Himedia, India. All the lab experiments were performed using double distilled water.
2.2 Isolation of Streptomyces sp. for amylase production
In this study, soil sample was collected from Jazan region of Saudi Arabia for the isolation of actinomycetes. About one-gram soil was mixed with 100 ml double distilled water and spread on actinomycetes isolation agar. It was incubated for 7 days at 28 °C. Antibiotics such as nalidixic acid and nystatin were added to minimize the contamination of microbes. Morphologically different actinomycetes were purified using starch casein agar plates.
2.3 Screening and identification of actinomycetes isolates for amylase production
The isolated actinomycetes were screened for the production of amylase. For amylase screening, 1% soluble starch was incorporated with minimal medium and incubated at 28 °C for 5 days. Then Gram’s iodine was flooded and clear distinct zone around actinomycetes indicates amylase production. The characteristic features such as casein hydrolysis, tyrosine and xanthin were used for the determination of the genus Streptomyces. Also, utilization of various nitrogen sources, carbon sources, production of melanin were analyze for the determination of Streptomyces. Further 16S rDNA sequence analysis was also performed to identify the organism in molecular level (Korn-Wendisch and Kutzner, 1991).
2.4 Amylase production from Streptomyces sp. Al-Dhabi-46
In this study Streptomyces sp. was grown in basal medium. To this basal medium 1% soluble starch was added to induce amylase production. About 1.0 ml of 5 days Streptomyces sp. Al-Dhabi-46 culture was inoculated in to 100 ml of culture. Then, the culture was centrifuged for 10 min at 10,000g at 4 °C to separate the supernatant. The supernatant was assayed for the determination of amylase using soluble starch.
2.5 Optimization of fermentation conditions for amylase production
The process parameters such as, incubation time (1–6 days), medium pH (6–10), temperatures (20–50 °C), carbon sources (glucose, lactose, fructose, maltose, arabinose, starch, xylose and trehalose (1%, w/v)), nitrogen sources (beef extract, yeast extract, peptone, glycine, ammonium sulphate, ammonium nitrate and urea (1%, w/v)), ions (Ca2+, Mg2+, Mn2+, Cu2+ and Hg2+ (1%, w/v)) was added (El-Batal et al., 2016).
2.6 Isolation of amylase from Streptomyces sp. Al-Dhabi-46
Amylase was purified from Streptomyces sp. by various steps including, ammonium sulphate precipitation, dialysis and chromatography. All purification steps were performed at 4 °C. The sample was precipitated with solid ammonium sulphate for overnight and separation of precipitated proteins at various percentages of saturation (20–30%, 30–40%, 40–50%, 50–60%, 60–70%, 70–80%). The precipitated sample was dialyzed against water and buffer for overnight. The purification process was performed as described previously. The purified enzyme was characterized by SDS–Polyacrylamide gel electrophoresis.
2.7 Properties of amylase from Streptomyces sp. Al-Dhabi-46
Purified enzyme was used for characterization studies. Amylase activity in response to pH value was carried out using various buffers ranging from 6.0 to 10.0. For the determination of pH stability, small quantity of the purified amylase sample was pre-incubated individually at specific buffer system for 10–60 min and enzyme assay was performed. Amylase stability was performed with 40 °C for 1 h. The effect of various metal ions (Mg2+, Mn2++, Cu2+++, Hg2+, Fe2++++, Zn2+, Na+, Co2+ and Ca2+) on enzyme activity was studied. About 0.2 ml of sample was incubated with ions for 30 min. The control test was assayed without any metal ions. Soluble starch was used for amylase determination.
2.8 Applications of amylases in the hydrolysis of cassava starch
The crude amylase from Streptomyces sp. (500 U) was added with cassava starch for 5 h and saccharification efficacy of amylolytic enzyme by Streptomyces sp. Al-Dhabi-46 was evaluated after 5 h of incubation.
3 Results
3.1 Screening and identification of actinomycetes for amylase production
Actinomycetes were isolated from the soil sample. Extracellular enzymes production by the actinomycetes isolates from the soil was assayed initially qualitatively. Initial screening revealed the ability of producing amylases by the isolated actinomycetes species on culture plates. Among the actinomycetes isolate, Al-Dhabi-46 which was isolated from the soil showed good activity on starch agar plates. Hence this organism was selected for optimization of enzymes production. The selected strain Al-Dhabi-46 was Gram-positive, coenocytic mycelic and branched, non-acid fast and filamentous type. It was catalase positive, reduced nitrate, hydrolyzed starch, Voges-Proskauer and indole negative. Based on microscopic observations and staining properties this organism was identified as, Streotomyces sp. Streptomyces sp. strain Al-Dhabi-46 showed 23 mm zone after flooding with Gram’s Iodine on starch agar plates. Based on 16S rDNA analysis this organism was identified as Streptomyces sp. Al-Dhabi-46 the sequence was accessed by KC292820.
3.2 Optimization of amylase production from Al-Dhabi-46
Amylase production was found to be maximum after 5 days of incubation (108 ± 4.1 U/ml) and decreased there after (93.4 ± 3.9 U/ml) (Fig. 1a). Amylase activity was maximum at pH 8.0 (118.1 ± 2.3 U/ml) and decreased at higher pH values (Fig. 1b). The production of amylase was very low at 20 °C (4.2 ± 0.5 U/ml) and was found to be maximum at 40 °C (124 ± 12.1 U/ml) (Fig. 1c). Enzyme production was found to be maximum in the culture medium containing starch as carbon source (208 ± 11.4 U/ml) and 1% starch was optimum to enhance amylase production (Fig. 2a and b). Amylase production was 241 ± 18.1 U/ml in the culture medium supplemented with beef extract. Enzyme production was significantly higher at 2.0% beef extract, when tested at various concentrations (Fig. 3a and b). Amylase production was optimum in the culture medium containing Mn2+ as ionic source (216.5 ± 3.1 U/ml). At 0.1% Mn2+ ions significantly enhanced amylase production (Fig. 4a and b).
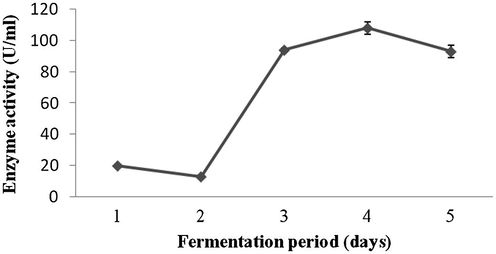
- Effect of fermentation period on enzymes production from Streptomyces sp. Al-Dhabi-46.

- Effect of pH on amylase production from Streptomyces sp. Al-Dhabi-46.
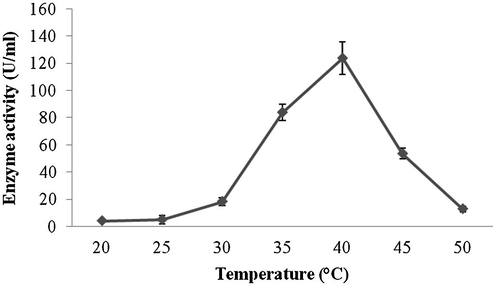
- Effect of temperature on amylase production from Streptomyces sp. Al-Dhabi-46.
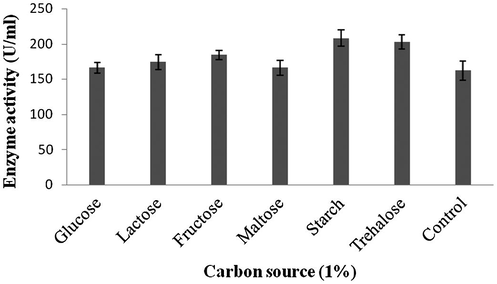
- Effect of carbon sources on enzymes production by Streptomyces sp. Al-Dhabi-46.
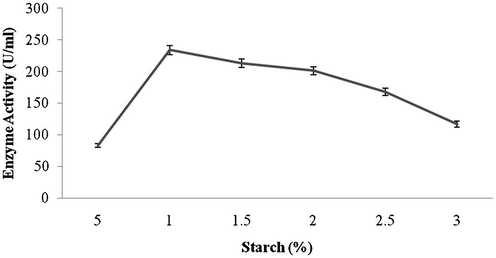
- Effect of various concentrations of starch on amylase production by Streptomyces sp. Al-Dhabi-46.
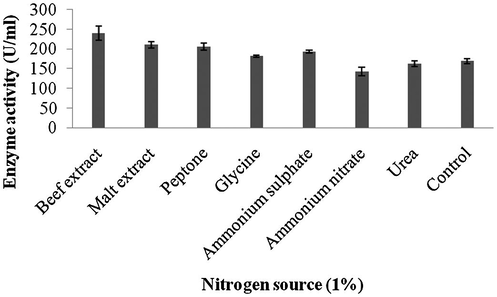
- Effect of nitrogen sources on enzyme production by Streptomyces sp. Al-Dhabi-46.
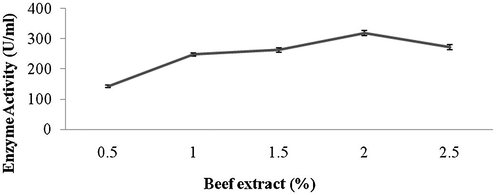
- Effect of various concentrations of beef extract on amylase production by Streptomyces sp. Al-Dhabi-46.
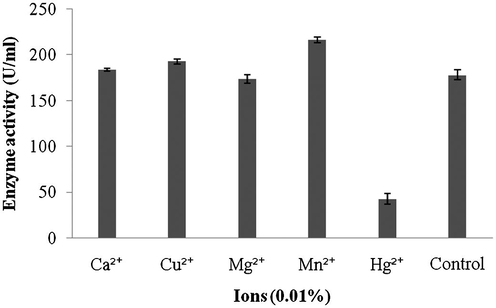
- Effect of ions on enzymes production by Streptomyces sp. Al-Dhabi-46.
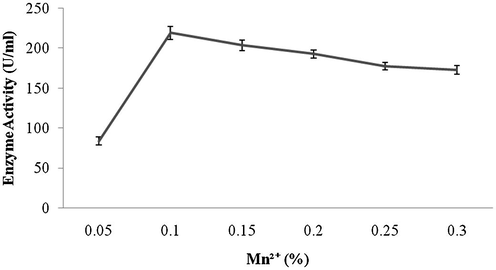
- Effect of various concentrations of Mn2+ ion on amylase production.
3.3 Purification of amylase from Streptomyces sp. Al-Dhabi-46
Amylase was purified from Streptomyces sp. Al-Dhabi-46 by salting out, dialysis and chromatography. The crude amylae showed 3.2-fold purification and the specific activity of amylase was 16.2 U/mg protein. Ammonium sulphate at 50–60% was optimum for the fractionation of amylase from Streptomyces sp. Al-Dhabi-46 and the other fractions did not show any significant amylase activity. The precipitated sample was dialyzed against water and buffer for overnight and enzyme activity was assayed. After purification using Sephadex G-100 chromatography, the specific activity of enzyme was 187 U/mg protein. The active amylase fractions obtained gel filtration chromatography was loaded on SDS – PAGE and the molecular weight of purified amylase was 44 kDa (Fig. 5).
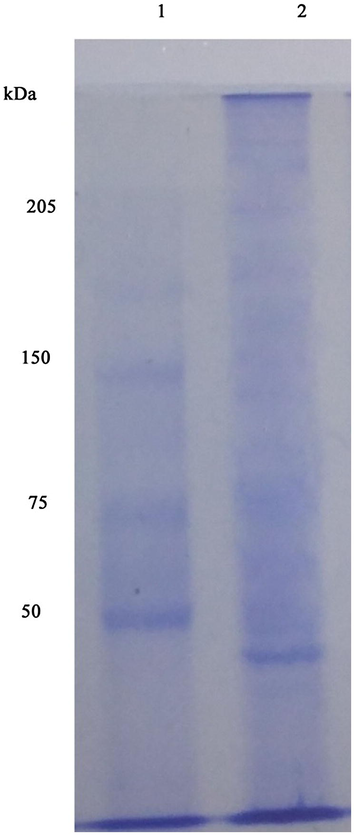
- SDS-PAGE analysis of amylase by Streptomyces sp. Al-Dhabi-46. The active fraction from gel filtration chromatography was loaded and the molecular weight of amylase was determined as 44 kDa (Lane 1: protein molecular weight marker; Lane 2: purified enzyme).
3.4 Characterization and saccharification efficiency of amylase from Streptomyces sp. Al-Dhabi-46
Amylase was highly active up to pH 8.0 and reduced activity was reported at higher pHs (pH 9.0 and 10.0). The optimum pH for amylase activity was pH 8.0, which was higher than most of amylases. The enzyme showed approximately 80% residual activity, when amylase was denatured for 30 min at pH 8.0, however, only 25% of enzyme activity was retained after 60 min of incubation at this pH value (Fig. 6a and b). Amylase was found to be highly active at 40 °C (100% activity), however, lost 45% of its original activity at 70 °C. Thermal stability of amylase showed increasing stability at higher temperatures (upto 50 °C and enzyme stability was just 35% after 60 min of incubation at 50 °C (Fig. 7a and b). Amylase activity was enhanced by the addition of only Mn2+++ at 10 mM concentrations. In this case, other ions inhibited enzyme activity (Fig. 8). The amylase from Streptomyces sp. Al-Dhabi-46 digested cassava starch at 25% and 55%, 78% and 95.4% level after 15 min, 30 min, 45 min and 60 min of enzymatic reaction. This amylase enzyme may be useful for the production of bioethanol.
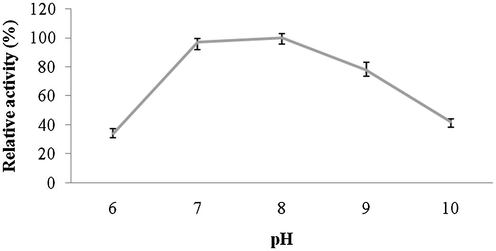
- Effect of pH on enzyme activity from Streptomyces sp. Al-Dhabi-46. Purified enzyme was individually assayed to reveal optimum pH value.
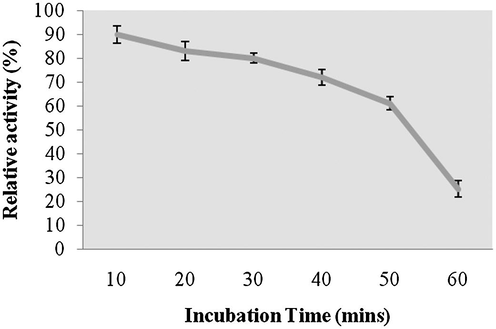
- Effect of pH on enzyme stability from Streptomyces sp. Purified enzyme was individually assayed to reveal optimum pH value.
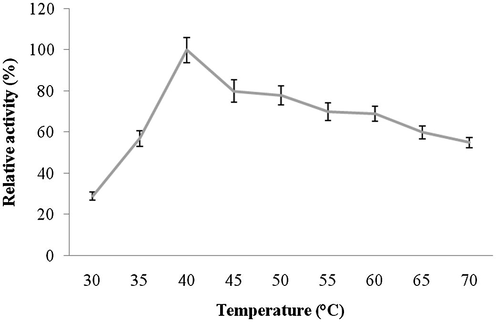
- Effect of temperature on enzyme activity. Enzyme assay was performed at various temperatures (30–70 °C) individually, and relative activity was measured.
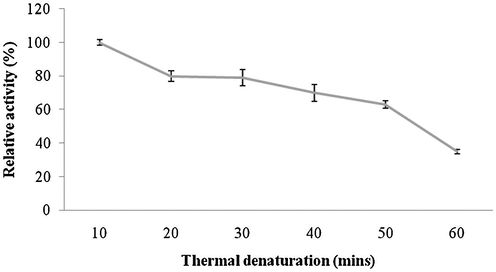
- Effect of temperature on enzyme stability. The purified enzyme was individually pre-incubated at for 60 min and enzyme assay was performed by standard method.
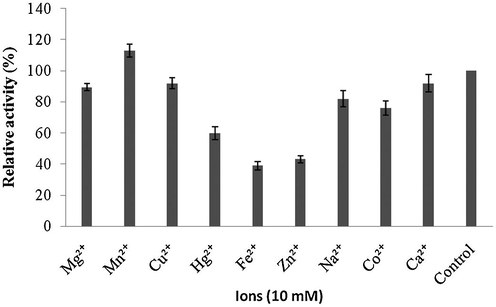
- Effect of ions on enzyme activity.
4 Discussion
In this study, seventeen actinomycetes were isolated and screened for amylase production. Among the 17 isolates, nine actinomycetes showed activity on starch agar plates. The strain Al-Dhabi-46 showed more hydrolytic zone (19 mm) on starch agar plates. This strain was identified by biochemical characters and 16S rDNA sequencing and identified as Streptomyces sp. Al-Dhabi-46. Amylase activity was reported from Bacillus subtilis B19 by Dash et al. (2015). Recently, Gopinath et al. (2017) used agar plates for the determination of amylase activity from Penicillium sp. and Aspergillus versicolor (Al-Dhabi and Ghilan, 2019; Arasu et al., 2019). In Streptomyces, amylase assay has been carried out in S. avermitilis and S. rochei BTSS 1001 (Hwang et al., 2013; Syed et al., 2009). Syed et al. (2009) isolated Streptomyces gulbargensis DAS 131 for the production of extracellular amylase and this was thermotolerant in nature. Kafilzadeh and Dehdari (2015), isolated actinomycetes from the aquatic environment and showed 62.97 U/ml in submerged fermentation. Box-Behnken design has been employed to optimize the culture condition for the production of amylase and achieved 145.32 U/ml amylase (Khusro et al., 2017). Amylase production was found to be high after 5th day of incubation at 37 °C (108 ± 4.1 U/ml) and attained maximum amylase activity at pH 8.0 (118.1 ± 2.3). Optimum pH and temperature for amylase activity differ from species to species (Gupta et al., 2003). In our study, amylase production was maximum at 40 °C (123.12.1 U/ml) in submerged fermentation. Among the carbon sources, starch enhanced on amylase production. The selected other carbon sources showed less amount of amylase activity. Although starch is the most preferred carbon source for the production of amylase in most of the bacterial species the sugar such as, lactose, maltose, fructose and glucose have been observed to produce maximum amount of amylase (Aqeel and Umar, 2010; Ashwini and Gaurav, 2011; Salva and Moraes, 1995; Suman and Ramesh, 2010). Lin et al. (1998) reported catabolite repression of fructose and glucose in amylase production. In our study, amylase production was effectively stimulated by beef extract and amylase production was 241 ± 18.1 U/ml. However, requirement of these sources may differ from species to species. Previously, supplementation of yeast extract, peptone and tryptone enhanced amylase production (van Hille et al., 2009). Also the influence of various inorganic nitrogen source on enzyme production has been reported. The inorganic nitrogen sources such as nitrate ions and ammonium ions positively influenced on amylase production (Aqeel and Umar, 2010). In this study, 1% inoculums were inoculated throughout the experiments. Previously, 2% inoculums was recommended for α-amylase production (Dash et al., 2015). In a study, Abdel-Fattah et al., 2012; screened B. licheniformis for the production of amylase and optimum pH ranged between 6.0 and 7.5, temperature ranged between 60 and 80 °C. In a study, (Kaur et al., 2003) isolated amylase producing bacteria from the agricultural field. In a study, Kafilzadeh et al. (2012) screened marine actinomycete, Streptomyces from aquatic sediments for the production of amylase (Arasu et al., 2013; Arokiyaraj et al., 2015; Valsalam et al., 2019a). The purified amylase of Streptomyces sp. Al-Dhabi-46 was found to be 44 kDa. The purified amylase was highly active at pH 8.0 and it was stable for 30 min at this temperature. Among the tested ions, 10 mM Mn2+ enhanced amylase production. It was highly active at 40 °C and showed stability for 50 min at this temperature. In the case of amylase, enzyme stability and activity is also dependent on time and temperature. Amylases also stable between pH value 4.0 and 11.0 (Omemu et al., 2005).
5 Conclusion
In this study, the soil Streptomyces isolate Al-Dhabi-46 showed potent activity to produce industrially useful amylase. This group is considered as a significant source for amylases. These Streptomyces spp. have enormous potential as secrete novel extracellular amylases. The isolated amylase showed pH and temperature stability and has great potential in various industrial processes including starch saccharification.
Acknowledgement
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (11-BIO1873-02).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Production, purification, and characterization of thermostable α-amylase produced by Bacillus licheniformis isolate AI20. J. Chem.. 2012;2013
- [Google Scholar]
- Taxonomy and polyphasic characterization of alkaline amylase producing marine actinomycete Streptomyces rochei BTSS 1001. Int. J. Microbiol.. 2013;2013
- [Google Scholar]
- Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles. 2016;20:79-90.
- [Google Scholar]
- Chemical profiling of Streptomyces sp. Al-Dhabi-2 recovered from an extreme environment in Saudi Arabia as a novel drug source for medical and industrial applications. Saudi J Biol Sci. 2019;26:758-766.
- [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol. B. 2018;189:176-184.
- [Google Scholar]
- Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol., B 2019111529
- [Google Scholar]
- Characterization of silver nanomaterials derived from marine Streptomyces sp. Al-Dhabi-87 and its in vitro application against multidrug resistant and extended-spectrum beta-lactamase clinical pathogens. Nanomaterials. 2018;8(5)
- [Google Scholar]
- Bioactivity assessment of the Saudi Arabian Marine Streptomyces sp. Al-Dhabi-90, metabolic profiling and its in vitro inhibitory property against multidrug resistant and extended-spectrum beta-lactamase clinical bacterial pathogens. J Infect Public Health. 2019;12:549-556.
- [Google Scholar]
- Effect of alternative carbon and nitrogen sources on production of alpha-amylase by Bacillus megaterium. World Appl Sci J. 2010;8:85-90.
- [Google Scholar]
- Green chemical approach towards the synthesis of CeO2 doped with seashell and its bacterial applications intermediated with fruit extracts. J. Photochem. Photobiol., B. 2017;172:50-60.
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol., B. 2019;190:154-162.
- [Google Scholar]
- Antibacterial and antifungal activities of polyketide metabolite from marine Streptomyces sp. AP-123 and its cytotoxic effect. Chemosphere. 2013;90(2):479-487.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J Biol Sci. 2015;2:115-118.
- [Google Scholar]
- Optimization, production and partial purification of extracellular α-amylase from Bacillus sp. marini. Arch. Appl. Sci. Res.. 2011;3:33-42.
- [Google Scholar]
- Clinically important microbial diversity and its antibiotic resistance pattern towards various drugs. J. Infection Public Health. 2019;19:1876-10341.
- [Google Scholar]
- Chakraborty S, Raut G, Khopade A, Mahadik K, Kokare C. Study on calcium ion independent α-amylase from haloalkaliphilic marine Streptomyces strain A3. 2012.
- Molecular identification of a newly isolated Bacillus subtilis BI19 and optimization of production conditions for enhanced production of extracellular amylase. BioMed Res. Int.. 2015;2015
- [Google Scholar]
- Effect of environmental and nutritional parameters on the extracellular lipase production by Aspergillus niger. Int. Lett. Nat. Sci.. 2016;60:18-29.
- [Google Scholar]
- Biotechnological processes in microbial amylase production. BioMed Res. Int.. 2017;2017
- [Google Scholar]
- Microbial α-amylases: a biotechnological perspective. Process Biochem.. 2003;38:1599-1616.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol., B. 2019;193:118-130.
- [Google Scholar]
- Thermal stability and starch degradation profile of α-amylase from Streptomyces avermitilis. Biosci. Biotechnol. Biochem.. 2013;77:2449-2453.
- [Google Scholar]
- Growth and metabolite profile of Pediococcus pentosaceus and Lactobacillus plantarum in different juice. South Indian J. Biol. Sci.. 2015;1:1-6.
- [Google Scholar]
- Isolation of amylase producing aquatic Actinomycetes from the sediments of mangrove forests in south of Iran. African J. Microbiol. Res.. 2012;6:6281-6285.
- [Google Scholar]
- Amylase activity of aquatic actinomycetes isolated from the sediments of mangrove forests in south of Iran. Egypt. J. Aquatic Res.. 2015;41:197-201.
- [Google Scholar]
- Statistical optimization of á-amylase production by Streptomyces erumpens MTCC 7317 cells in calcium alginate beads using response surface methodology. Pol. J. Microbiol.. 2008;57:49-57.
- [Google Scholar]
- Production of α-amylase by Aspergillus niger using wheat bran in submerged and solid state fermentations. Indian J. Microbiol.. 2003;43:143-145.
- [Google Scholar]
- Optimization of thermo-alkali stable amylase production and biomass yield from Bacillus sp. under submerged cultivation. Fermentation. 2017;3:7.
- [Google Scholar]
- The family Streptomycetaceae. The Prokaryotes. A handbook on the biology of bacteria: ecophysiology, isolation, identification, applications, Chap. 41. Berlin: Springer; 1991.
- Krishnakumar, S., Bai, V., Premkumar, J., 2015. Production of alpha amylase by salt–Tolerant Actinomycete Streptomyces sp.–SBU3 isolated from marine sponge.
- Optimization of alpha amylase extracted from marine actinomycetes-Streptomyces gancidicus ASD-KT852565. Int. Res. J. Pharm.. 2015;6:729-735.
- [Google Scholar]
- Improved thermostable α-amylase activity of Bacillus amyloliquefaciens by low-energy ion implantation. Genet. Mol. Res.. 2011;10:2181-2189.
- [Google Scholar]
- Production and properties of a raw-starch-degrading amylase from the thermophilic and alkaliphilic Bacillus sp. TS-23. Biotechnol. Appl. Biochem.. 1998;28:61-68.
- [Google Scholar]
- Protein engineering of bacterial α-amylases. Biochim. Biophys. Acta. 2000;1543:253-274.
- [Google Scholar]
- Diversity and antimicrobial potential of culturable actinobacteria from desert soils of Saudi Arabia. J. Pharm. Sci. and Res.. 2015;7:117.
- [Google Scholar]
- Hydrolysis of raw tuber starches by amylase of Aspergillus niger AM07 isolated from the soil. Afr. J. Biotechnol.. 2005;4:19-25.
- [Google Scholar]
- Main characteristics and applications of solid substrate fermentation. Electron. J. Environ. Agric. Food Chem. (2)
- [Google Scholar]
- Bioprocessing of banana peel for production of amylase by Penicilliun sp. Asian J. Exp. Biol. Sci.. 2011;2:257-264.
- [Google Scholar]
- Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill andevaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol., B 2019
- [CrossRef] [Google Scholar]
- CuO/C nanocomposite: synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng., C. 2019;101:404-414.
- [Google Scholar]
- Effect of the carbon source on alpha-amylase production by Bacillus subtilis BA-04. Rev. Microbiol.. 1995;26:46-51.
- [Google Scholar]
- Isolation, optimization, and partial purification of amylase from Chrysosporium asperatum by submerged fermentation. J. Microbiol. Biotechnol.. 2011;21:470-476.
- [Google Scholar]
- Purification and characterization of an organic-solvent-tolerant halophilic α-amylase from the moderately halophilic Nesterenkonia sp. strain F. J. Indus. Microbiol. Biotechnol.. 2011;38:275-281.
- [Google Scholar]
- Bioprocessing of food industrial waste for α-amylase production by solid state fermentation. Int. J. Adv. Biotechnol. Res.. 2011;2:473-480.
- [Google Scholar]
- Influence of carbon and nitrogen sources on the a-amylase production by a newly isolated Thermophilic Streptomyces Sp. Asian J. Biotechnol.. 2011;3:540-553.
- [Google Scholar]
- a-Amylases from microbial sources–an overview on recent developments. Food Technol. Biotechnol.. 2006;44:173-184.
- [Google Scholar]
- Production of a thermostable extracellular amylase from thermophilic Bacillus species. J. Pharm. Sci. Res.. 2010;2:149.
- [Google Scholar]
- Production and partial purification of α-amylase from a novel isolate Streptomyces gulbargensis. J. Indus. Microbiol. Biotechnol.. 2009;36:189-194.
- [Google Scholar]
- Microbial starch converting enzymes of the α-amylase family. Microb. Biotechnol. Horticult.. 2006;1:421-472.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol. B: Biol. 2019
- [CrossRef] [Google Scholar]
- Properties and applications of starch-converting enzymes of the α-amylase family. J. Biotechnol.. 2002;94:137-155.
- [Google Scholar]
- The effect of nutrient supplementation on growth and leaching performance of bioleaching bacteria. Trans Tech Publ Advanced Materials Research; 2009. p. :413-416.
- Amylase production on submerged fermentation by Bacillus spp. World J. Chem.. 2009;4:89-91.
- [Google Scholar]







