Translate this page into:
Isolation and characterization of deleterious Pseudomonas aeruginosa KC1 from rhizospheric soils and its interaction with weed seedlings
*Corresponding author. Tel.: +91 9470412424 vijayalakshminishant@gmail.com (Vijaya Lakshmi),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 9 May 2014
Peer review under responsibility of King Saud University.

Abstract
The bacterial isolate KC1 was screened from the rhizosphere of castor plants (Ricinus communis) indigenous to agricultural fields of Bihar. The isolate was Gram negative, non-spore forming, and exhibited fluorescence under UV light. Its molecular characterization is based on the sequencing of 16S rDNA (1450 bp) and alignment at GeneBank (NCBI, MaryLand). The strain has been validated as Pseudomonas aeruginosa (HM195190). The bacterium grew at 4–42 °C, with a temperature optima of 30 °C. The strain KC1 was found to produce cyanide (4.78 nmol l−1) over a period of 36 h. Data revealed enhanced cyanogenesis (6.98 nmol l−1), when glycine was provided in the King’s B medium. Seed bacterization exhibited reduction in root length, shoot length of weed seedlings (Amaranthus spinosus, Portulaca oleracea), which was significant (p < 0.05) in both laboratory and glasshouse experiments. Biomass was significantly reduced (p < 0.05) for the weed seedlings in glasshouse experiments. However, KC1 inoculated crop seedlings (Triticum aestivum) were found to be less inhibitory as compared to weed seedlings. The observations are significant to establish, that the secondary metabolites producing KC1 rhizobacterium, P. aeruginosa KC1 could be exploited as a weed biocontrol agent. The innate potential of KC1 could be further formulated and utilized in field applications for agricultural sustainability.
Keywords
Biocontrol agent
Hydrogen cyanide
Pseudomonas
Rhizobacteria
Rhizosphere
Weed
1 Introduction
The growth and development of plants are influenced directly by the complicated and diverse environment of the rhizosphere, a microecological zone established in direct proximity of plant roots. Its biology and chemistry are impacted greatly by plant root exudates and microorganisms utilizing these compounds, as carbon sources. Soil microorganisms and plants interact with each other, affecting not only individual plant species positively or negatively but also reducing, or enhancing native plant diversity (Callaway et al., 2004; Francis et al., 2010). Rhizosphere inhabiting bacteria (rhizobacteria) are aggressive root colonizers that may exert beneficial, neutral or deleterious effects on plant growth by altering rhizospheric environments, through the production of metabolites, incorporation of root exudates and competition with other soil microbes (Weller, 2007).
Deleterious rhizobacteria (DRB) are a group of saprophytic, non-parasitic pathogens, which excrete exopolysaccharides and alleochemicals in the form of cyanide, phytohormones, siderophores and phytotoxins that can affect the metabolism of plants negatively (Compant et al., 2005; Li and Kremer, 2006; Trivedi et al., 2008). Recently, this group of microorganisms has been realized to be used as a biological control agent of weeds (Li and Kremer, 2006; Kennedy and Stubbs, 2007; Mejri et al., 2010; Patil, 2013). Adam and Zdor (2001) demonstrated that certain bacteria isolated from Abutilon theophrasti Medik are potentially successful in reducing weed growth. Such organisms can specifically colonize weed roots and localize their metabolite production, thus minimizing potential deleterious effects on the growth of desirable plants (Kremer and Souissi, 2001). Several DRB have been isolated from the rhizosphere of different weed seedlings, adversely affecting seed germination, seedling vigor, plant growth and development (Flores-Vargas and O’Hara, 2006; Banowetz et al., 2008).
A major group of rhizobacteria with potential as biological control agents arepseudomonads like bacteria, which are common producers of cyanide (DeCoste et al., 2010; Lanteigne et al., 2012; Ramyasmruthi et al., 2012). Hydrogen cyanide is a toxic gas having the ability to form metal complexes, with functional groups of various enzymes; inhibiting processes like CO2 and nitrate assimilation; disruption of reduction of oxygen in the cytochrome respiratory chain and electron transport in photosynthesis. This possible phytotoxic mechanism that leads to a significant growth reduction in plants has been reported in Lactuca sativa and Echinochloa crus-galli (Kremer and Souissi, 2001; Zeller et al., 2007).
Pseudomonas spp. has gained major attention in the agricultural industry because of its widespread application in various biotechnological processes. An important ubiquitous member of this group, Pseudomonas aeruginosa is an opportunistic pathogen of plants and humans (Walker et al., 2004; de Bentzman and Plésiat, 2011). The conscious agricultural applications of P. aeruginosa not only pose a threat to human health and environment but also raise relevant ecological issues such as evolution of multi resistant bacteria and pathogenicity. Hence, deliberate application of strains of this organism, or any other microorganism with such pathogenicity should be carried out with immense care, following biosafety regulations.
Successful establishment of deleterious bacteria in weed rhizosphere could reduce growth parameters of weeds, with no or very less effect on desirable plants. This can prove to be an innovative, low cost and eco-friendly mode of weed biocontrol, than utilization of environmentally hazardous synthetic chemicals and herbicides (Asghar et al., 2004; Ali et al., 2010). In addition to environmental hazards, synthetic herbicides induce the development of herbicide resistance in many weed biotypes (Heap, 2012).The introduction of cyanogenic rhizobacteria in the vicinity of roots offers several advantages, like a shift in the balance of competition between the weed and crop (in favor of crop and against the weed), higher selectivity, lower resistance and introduction of practices that are agriculturally sustainable (Flores-Vargas and O’Hara, 2006). Therefore, the amalgamation of crop management practices with DRB can be devised as a modern day sustainable agricultural method. The main objectives associated with the study include (1) isolation of P. aeruginosa from the rhizosphere of castor plant and its characterization, (2) determination of its cyanogenic potential, (3) evaluation of its plant growth inhibiting properties, both in vitro and in glasshouse, and (4) exploitation of its growth inhibiting activities as a mode of biocontrol agent for sustainable agriculture.
2 Materials and methods
2.1 Sampling
The soil used for bacterial isolation was collected from the rhizosphere of castor plant (Ricinus communis) grown in agricultural fields of the Maliyabagh village (25°20′39″N latitude and 84°12′46″E longitude) in Rohtas district of Bihar. The sampling was carried when the prevailing atmospheric temperature was 26 °C. Randomly selected plants were uprooted carefully and the excess of soil was removed by gentle shaking and the soil adhering to the roots formed composite samples. The collected samples were placed in sterilized plastic bags and transferred to the laboratory under temperature controlled conditions.
2.2 Isolation
The soil adhering to the roots was removed by gentle agitation, serially diluted in physiological saline (0.85%, NaCl, w/v) containing quartz particles for 20 min, spread plated in triplicate on King’s B agar (KBA) medium (Atlas, 1995) and incubated at 30 °C for 48 h. Bacterial cultures were maintained on the respective slants. After incubation at 28–30 °C for 2–3 days, bacterial colonies were counted. Representative colonies were selected on the basis of distinct morphological characteristics, including pigments, colony form, elevation and margin; texture; and opacity. A predominant yellowish white colony that fluoresced under UV light was purified and maintained on nutrient agar slants at 4 °C. All the subsequent experiments were carried out after raising fresh cultures.
2.3 Phenotypic characterization
The isolate was characterized microscopically, along with different tests as described by Holt et al. (1994). All tests were performed using exponentially grown cultures, except for the spore test, which required cultures from the early stationary phase.
2.4 DNA extraction, 16S rRNA gene sequencing and phylogenetic analysis
Genomic DNA from the selected isolate was extracted from pure cultures (1.5–3 ml), previously grown in 10 ml of Luria Bertani broth (incubated at 37 °C for 24 h at 200 rpm) using Bacterial Genomic DNA Isolation Kit RKT09 (Chromous Biotech Pvt. Ltd., Bangalore, India) and visualized on 0.8% (w/v) agarose gel. The amplification of 16S rRNA gene was carried out by using a Thermal cycler (ABI 2720) in 100 μl reaction volume containing 2.5 mM each of four dNTP, 10× PCR buffer, 3U of Taq DNA polymerase, 10 ng template DNA and 400 ng each of primer (F) 5′-AGA GTR TGA TCM TYG CTW AC-3′ primer (R) 5′-CGY TAM CTT WTT ACG RCT-3′. The amplification program was set as an initial denaturation at 94 °C for 5 min., followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 2 min and a final extension at 72 °C for 5 min. The sequencing was done according to manufacturer’s protocol using Big Dye Terminator Cycle Sequencing Kit (v3.1, Applied Biosystems) and analyzed in an Applied Biosystems Analyzer. The sequence of 16S rDNA (1450 bp) was aligned by using the BLASTn program (Atschul et al., 1990) to identify the most similar sequence. The isolate showed 99% similar identity with the closest known species in the database. Henceforth, the isolate was identified as P. aeruginosa KC1 and has been submitted to the GeneBank under accession number HM195190. 16S rDNA sequences of different strains of P. aeruginosa and its phylogenetically related species and genera were downloaded from Genbank database (http://www.ncbi.nlm.nih.gov/entrez) and aligned to construct a neighbor-joining phylogenetic tree using Clustal W algorithm with the help of MEGA software version 4.1 (Tamura et al., 2007).
2.5 Estimation of HCN production
Qualitative estimation of HCN was done by using a modified method of Bakker and Schippers (1987) with and without glycine (4.4 g l−1) on KBA medium. The isolates changing the color of the filter paper (treated with picric acid and sodium carbonate) from deep yellow to orange and finally to orange brown to dark brown were selected for quantification of cyanide.
The quantification of cyanide was done as per the method of Lambert et al. (1975). Briefly, the selected isolate was cultured on KB agar with and without glycine in plates. A piece of filter paper (Whatman No.1), saturated in 1 M NaOH was placed in the lid and finally plates were sealed with parafilm. After 48 h incubation at 28 °C, the filter papers were removed, extracted with 5.0 ml of 1 M NaOH and titrated with acetic acid. Cyanide extracted into NaOH was further quantified by reacting with barbituric acid and pyridine reagent and the absorbance was read at 575 nm spectrophotometrically (Systronics 119). The amount of cyanide (nmol l−1 KCN) was calculated using a standard curve of KCN in NaOH.
Bacterial cultures on each plate were suspended in sterile water, extracted with trichloroacetic acid, resuspended in 0.1 M NaOH in 2% Na2CO3, and analyzed for protein by a modified Lowry procedure (Ohnishi and Barr, 1978).
2.6 Laboratory experiments
Pre-germinated seeds of wheat (Triticum aestivum), spiny amaranth (Amaranthus spinosus) and pigweed (Portulaca oleracea) were used for laboratory experiments. Seeds were surface sterilized by immersing them in 95% ethanol for a few seconds (3–4 s) and then in 0.2% solution of HgCl2 for three min, followed by several rinses with sterilized distilled water to remove sterilant, as documented in other investigations as well (Russel et al., 1982). Only sterile and viable seeds were used for further experiments.
In order to study the effect of bacteria on root and shoot growth of wheat, spiny amaranth and pigweed seedlings, two sets of experiments were carried out for seed bacterization, viz. (i) Contact method and (ii) Paired plate assembly method. In the contact method, pre-germinated seeds of each species with uniform radicles were inoculated with approximately 106–108 cfu of a 48 h old bacterial suspension. Control seeds were without the inoculum. The treated seeds were placed on 1% agar plates, sealed with parafilm, incubated at 28 °C and after 5 days root and shoot lengths were measured.
In the paired plate assembly method, pre-germinated surface-sterilized seeds (five per plate) of each species with uniform radicle length were evenly distributed on the surface of plates containing 1% water agar. In the other plate, bacterial isolates were streaked on the KB agar. Both the plates were paired in such a way that the plate containing weed seeds was in the lower position and the bacterium in the upper one. The paired plate assemblies were sealed with parafilm and incubated at 28 °C in triplicate. After 5 days, the root and shoot lengths were measured. Results were compared with root and shoot lengths in paired plate assemblies containing a non-inoculated KB agar plate, treated as control.
2.7 Glasshouse experiments
Five surface sterilized seeds of wheat (T. aestivum), spiny amaranth (A. spinosus) and pigweed (P. oleracea) were planted into 120 mm pot containing sieved and sterilised agricultural soil (pH = 7.7 ± 0.3% carbon = 0.56 ± 0.14% nitrogen = 0.08 ± 0.4% phosphate = 0.09 ± 0.3; steam heated at 110 °C for 2.5 h for three consecutive days). Bacterial cultures were grown as described for the laboratory experiments. The five seedlings in each pot were inoculated with a 0.5 ml of suspension (108 cfu) of strain. After inoculation a 1 cm layer of sterilised sand was placed on the surface of each pot to reduce evaporation and air borne contamination. The plants were grown in a temperature controlled glasshouse at 25 °C, and watered with sterile distilled water every second day. There were four replicates for each seedling treatment. After 6 weeks plants were harvested, roots were washed free of sand and soil, and shoot and root lengths were measured. The entire plant was dried in an oven at 72 °C for 72 h and dry weight was recorded. The experiment was repeated thrice.
2.8 Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (SPSS 16.0) software, and treatment means were compared at 5% level of significance.
3 Results
3.1 Phenotypic characterizations
The bacterial isolate formed yellowish white, mucoid colonies of 1–2.5 mm diameter with smooth, regular margins and fruity odor on KBA plates at 28 °C for 1–2 days. The colonies fluoresced under UV light. Phenotypic characterization revealed that the isolate was motile, small rod, (1.9 μm × 0.5 μm) and stained Gram negative. It grew at the temperatures ranging from 4 to 42 °C but maximum growth was recorded at 30 °C. Positive reactions were recorded for, citrate utilization, catalase activity, oxidase activity, gelatine hydrolysis and lysine decarboxylase enzyme. The bacterial isolate was also able to utilize glucose, galactose, fructose, mannitol, trehalose and glycerol as sole carbon sources. Negative reactions were recorded for indole test, Voges–Proskauer, casein hydrolysis, amylase and H2S production and urease activity.
3.2 Genotypic characterizations
The 16S rRNA gene sequence of the isolate had 99% identity with the type strain of P. aeruginosa (Accession No. EU714901), available in the NCBI GeneBank. The phylogenetic analysis of strain KC1, showing sequence homology with P. aeruginosa, is represented as a phylogenetic tree (Fig. 1).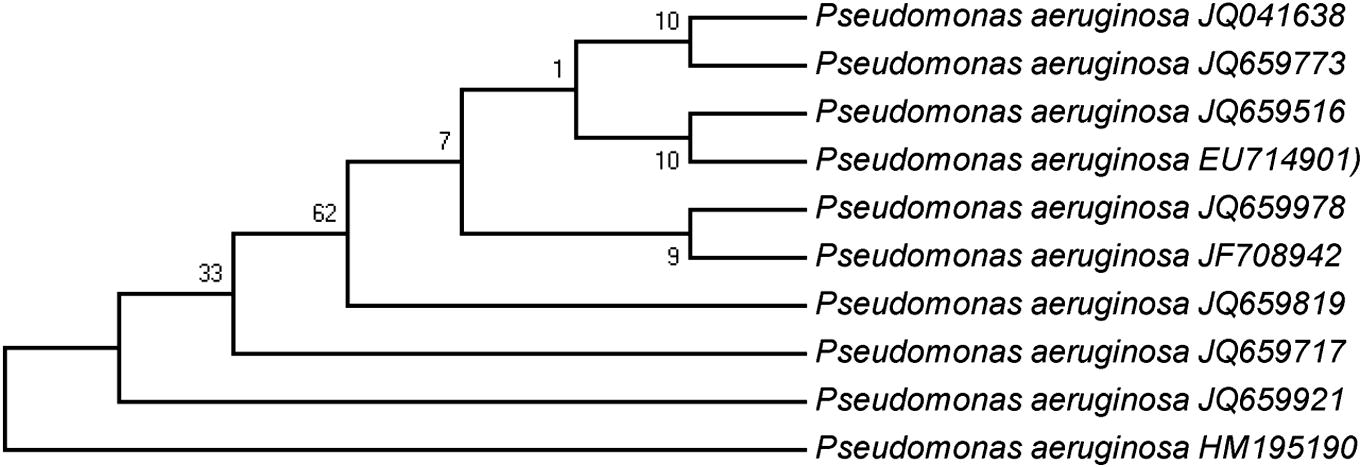
Phylogenetic tree showing the genetic relationship of Pseudomonas aeruginosa with taxonomically similar strains, species, and genus based on 16S rDNA sequences. Genbank accession number of each isolate is given in parentheses. The bootstrap values (n = 1000) are displayed at nodes.
3.3 HCN production
During qualitative analysis, the isolate KC1 changed the color of impregnated filter paper from yellow to reddish brown after 48 h of incubation, indicating its potent cyanogenic property. Results of indicator assay for cyanogenesis were further confirmed by quantitative estimation and it was found that the isolate produced a significant level of HCN (3.80–4.78 nmol l−1) in broth cultures containing 1 × 10−7 ml−1 bacterial cells, over a period of 36 h (Fig. 2). The production of HCN was increased up to 6.98 nmol l−1 when glycine was added in the culture media over a period of 36 h. It is evident from Fig. 2 that cyanide production in the presence of glycine mediated medium is high and cyanide accumulation in the culture was highest in the early exponential phase followed by early stationary phase with gradual decline in late stationary phase.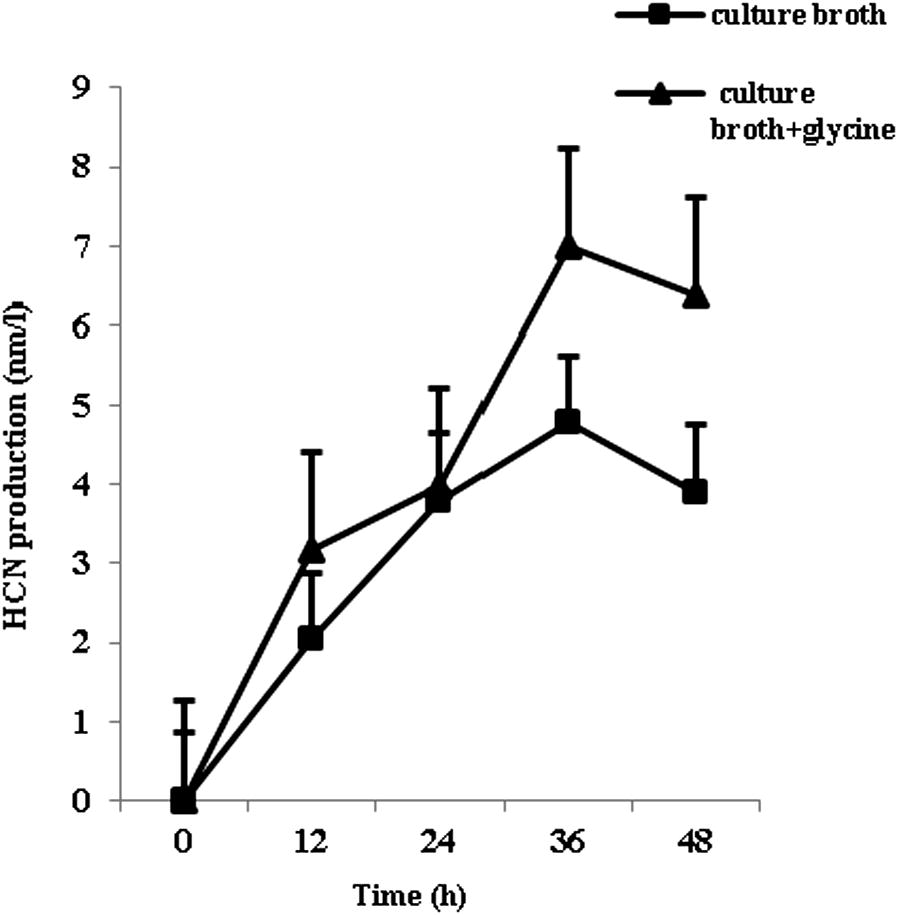
Time course of cyanide production by Pseudomonas aeruginosa strain KC1 (HM195190). Data are the mean ±SD of 3 replicates and the experiment was repeated on two independent occasions. The bars indicate standard error of mean.
3.4 Laboratory experiments
In laboratory experiments, the growth reduction in root length (19.26–89.3%) and in shoot length (31.17–75.85%) was more in all the three seedlings in the paired plate assemblies as compared to the contact method (Figs. 3 and 4). The maximum growth inhibition was seen in pigweed followed by spiny amaranth and wheat. Results of in vitro tests marked greater growth inhibition by gaseous metabolites released in paired plate assemblies than by other metabolites on weeds. The remarkable feature of the in vitro test was that wheat seedlings were found to be less influenced than the weed seedlings.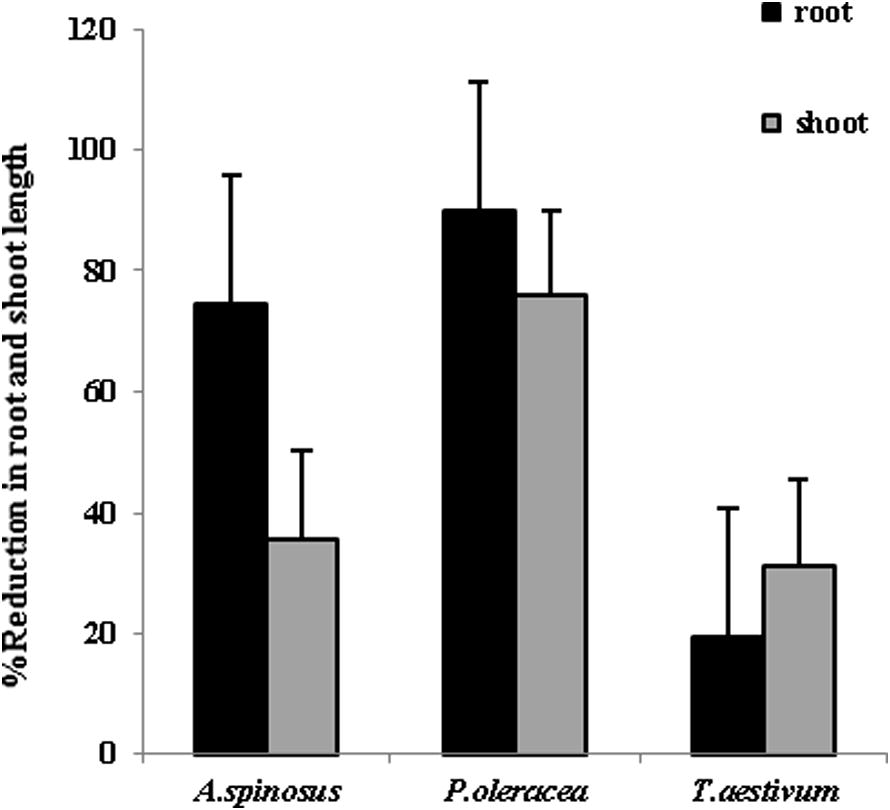
Percentage reduction in root length and shoot length of spiny amaranth, pigweed and wheat seedlings in paired plate assemblies (n = 3).
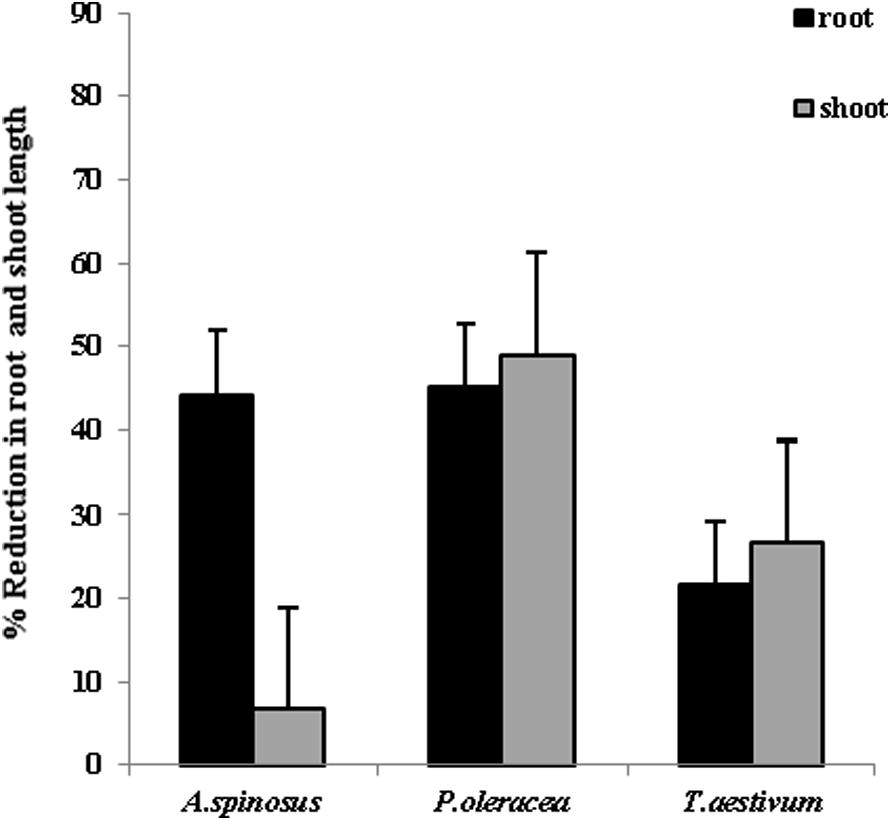
Percentage reduction in root length and shoot length of spiny amaranth, pigweed and wheat seedlings in contact seedling assay (n = 3).
3.5 Glasshouse experiments
The glass house experiments witnessed root growth reduction up to 51.6% for pigweed seedlings, followed by spiny amaranth (42.5%) and wheat seedlings (12.72%). KC1 inoculated spiny amaranth seedlings showed the highest reduction in shoot lengths (43.5%) as compared to control (Fig. 5). Highest biomass reduction was recorded for pigweed seedlings (77.4%), while wheat seedlings recorded lowest biomass reduction (58.8%) as compared to untreated seedlings. Glasshouse experiment results were in accordance with laboratory experiment results, where weed seedlings growth parameters were reduced to a higher extent than crop seedlings.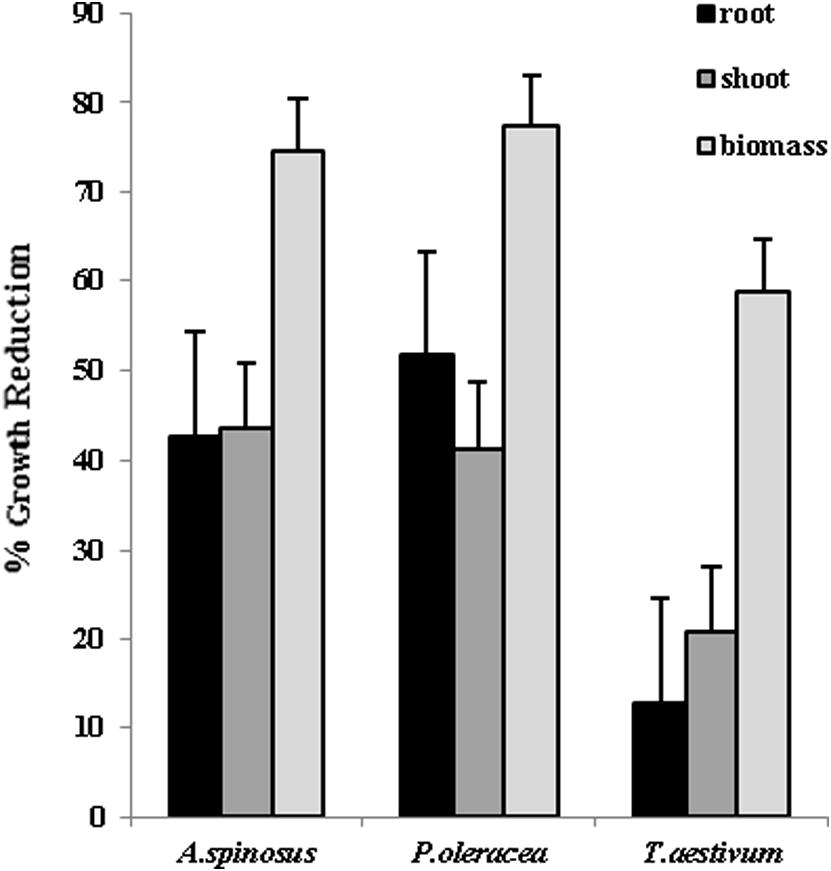
Percentage reduction in growth parameters of spiny amaranth, pigweed and wheat seedlings in glasshouse experiments (n = 3).
4 Discussion
The ability of DRB to inhibit the growth of various weed plants in different cropping systems is well documented (Gealy et al., 1996; Kremer and Souissi, 2001; Patil, 2013). In the present study, the production of a secondary metabolite, HCN, has been assessed to elucidate the agronomic significance of the isolate KC1, from the rhizosphere of R. communis. The isolate was thoroughly investigated for its morphology, Gram staining and biochemical parameters, suggesting its closeness to the species P. aeruginosa, but the final confirmation was based on 16s rDNA sequencing and alignment at NCBI database. To understand the role of cyanogenesis in plant infection model system, a systematic bioassay driven approach was followed. The effect of strain KC1 was tested on wheat seedlings as crop model plant and two weed seedlings viz. spiny amaranth and pigweed. The paired plate assembly revealed the involvement of diffusible volatile metabolites resulting in enhanced growth inhibition of root and shoot lengths of seedlings (Fig. 3). However, the growth inhibition was also exhibited when the seedlings were treated with metabolites in the contact plate method (Fig. 4). Similar results were obtained by Kremer and Souissi (2001), when cyanide was exogenously supplied in the form of KCN and HCN causing inhibitory effects on root growth. The inhibitory effect of KC1 on growth was more pronounced in spiny amaranth and pigweed than wheat as observed in glasshouse experiments (Fig. 5). HCN as a major factor in plant growth inhibition has been reported in various studies (Alstrom and Burns, 1989). Albert and Anderson (1987) reported that cyanide producing rhizobacteria are involved in the reduction of plant development. A significant reduction in the seedling growth of lettuce, barnyard grass and green foxtail was observed when treated with pseudomonad released metabolites, which was further correlated to HCN production (Kremer and Souissi, 2001).
The reproducibility of in vitro and glass house results was verified on three separate occasions with similar results each time. This study demonstrates the potential of exploiting indigenous fluorescent Pseudomonas as a biocontrol agent of weeds, which could reduce the growth and vigor of weed seedlings, but do not affect the desired crop plants adversely. Cyanide producing P. aeruginosa has been demonstrated as a mode of biocontrol against various pathogenic fungi causing plant diseases (Minaxi and Saxena, 2010; Maiti et al., 2012). Cyanogenic DRB selected for specific weeds could be applied in fields to inhibit weed seedling emergence and growth, leading to reduced competition with desired crop plants as well as limiting the use of synthetic herbicide, thus reducing their negative environmental impacts. Moreover, the cyanogenic strain P. aeruginosa KC1 exhibited innate potential of inhibiting the growth of seedlings in vitro. The in vitro results inflict the future prospect of the strain for agricultural purposes. Data from this study revealed that gaseous metabolites were more effective in causing growth inhibition than liquid metabolites. Kremer and Souissi (2001) and Adam and Zdor (2001) have reported similar results. As per data, inhibitory effect of volatile metabolites on wheat seedlings was much less as compared to weed seedlings which suggests that rhizobacteria inhibiting weed seedlings could be used as a selective biocontrol agent against the specified weeds. The glasshouse studies clearly indicated the effectiveness of P. aeruginosa KC1 against growth parameters of weed seedlings. The strain had significant non-inhibitory effects on the root and shoot lengths of wheat seedlings, however, when the biomass reduction for wheat and weed seedlings was compared, it was not translated into a non-significant form. (Fig. 5). We hypothesized that this could have been due to the deleterious effects caused by the rhizobacterial inoculum on carbon assimilation and biomass accumulation. In this respect, the application of the strain on crop seedlings is not striking enough, though the effects on weed seedlings were promising. In vitro studies have certain limitations, where the biocontrol effectiveness may not be expressed in planta conditions (Inam-ul-Haq et al., 2003). The lack of such correlation has been documented in other investigations as well (Ran et al., 2005). Rajkumar et al. (2005), suggested in vitro seedling assay as a rapid method for the selection of effective biocontrol agents. Mechanisms involved in the deleterious effects of rhizobacteria are still not understood and demand extensive research of the edaphic factors before applying in the field.
Weed management with DRB does not depend on the development of endemic disease on established weeds. Rather the control strategy using rhizobacteria is to regulate the development of weeds before or coincident with the emergence of crop plants. So, DRB do not necessarily eradicate the problem weed, but significantly suppress the early growth of weeds and allow the development of crop plants to effectively compete with weakened weed seedlings (Schroth and Hancock, 1982; Kremer and Kennedy, 1996). Our investigations indicate that the cyanogenic isolate KC1, characterized as P. aeruginosa, has the potential for the possible inhibition of weed seedlings through rhizosphere introduction.
5 Conclusion
The cyanogenic strain P. aeruginosa KC1 probably possesses the detrimental capacity of inhibiting weed seedling growth parameters both in vitro and in glasshouse studies, with reduced inhibitory effects on crop seedlings. This has the potential for the development of effective bacteria based systems for integration with biological weed control management. Their application in the field in correlation with crop plants requires further work, so that crops are not affected adversely. Extensive investigations are needed to gain an understanding of inoculum response in diverse cropping systems and edaphic factors. Additionally, it is necessary to develop appropriate inoculum technology to procure targeted weed control through the introduction of desired bacteria (DRB) as biocontrol agents.
Acknowledgments
We are thankful to Chromous Biotech Pvt. Ltd. for their technical assistance and the Department of Botany, Patna University for providing laboratory facilities.
References
- Effect of cyanogenic rhizobacteria on the growth of velvetleaf (Abutilon theophrasti) and corn (Zea mays) in autoclaved soil and the influence of supplemented glycine. Soil Biol. Biochem.. 2001;33:801-809.
- [Google Scholar]
- The effect of Pseudomonas putida colonization on root surface peroxidases. Plant Physiol.. 1987;85:535-541.
- [Google Scholar]
- Rhizobacterial potential to alter auxin content and growth of Vigna radiate (L.) World J. Microbiol. Biotechnol.. 2010;26:1379-1384.
- [Google Scholar]
- Cyanide production by rhizobacteria as a possible mechanism of plant growth inhibition. Biol. Fertil. Soils. 1989;7:232-238.
- [Google Scholar]
- Screening rhizobacteria for improving the growth, yield, and oil content of canola (Brassica napus L.) Aust. J. Agric. Res.. 2004;55:187-194.
- [Google Scholar]
- The Handbook of Microbiological Media for the Examination of Food. Boca Raton: CRC Press; 1995. p. 197
- Microbial cyanide production in the rhizosphere in relation to potato yield reduction and Pseudomonas spp. mediated plant growth reduction. Soil Biol. Biochem.. 1987;19:452-458.
- [Google Scholar]
- Germination-arrest factor (GAF): biological properties of a novel, naturally occurring herbicide produced by selected isolates of rhizosphere bacteria. BioControl. 2008;46:380-390.
- [Google Scholar]
- Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl. Environ. Microbiol.. 2005;71:4951-4959.
- [Google Scholar]
- The Pseudomonas aeruginosa opportunistic pathogen and human infections. Environ. Microbiol.. 2011;13:1655-1665.
- [CrossRef] [Google Scholar]
- Verticillium dahliae alters Pseudomonas spp. populations and HCN gene expression in the rhizosphere of strawberry. Can. J. Microbiol.. 2010;56:906-915.
- [Google Scholar]
- Isolation and characterization of rhizosphere bacteria with potential for biological control of weeds in vineyards. J. Appl. Microbiol.. 2006;100:946-954.
- [Google Scholar]
- The Gram positive side of plant–microbe interactions. Environ. Microbiol.. 2010;12:1-12.
- [Google Scholar]
- Isolation and characterization of metabolites from Pseudomonas syringae strain 3366 and their phytotoxicity against certain weed and crop species. Weed Sci.. 1996;44:383-392.
- [Google Scholar]
- Heap, I.M., 2012. International survey of herbicide resistant weeds. <http://www.weedscience.org> (accessed 06.06.10).
- Bergey’s Manual of Determinative Bacteriology (ninth ed.). Baltimore, Maryland, USA: Williams & Wilkins; 1994.
- Evaluation of different strains of Pseudomonas fluorescens for the biocontrol of fusarium wilt of chick pea. Pak. J. Plant Pathol.. 2003;2:65-74.
- [Google Scholar]
- Management effects on the incidence of jointed goat grass inhibitory rhizobacteria. Biol. Control. 2007;40:213-221.
- [Google Scholar]
- Cyanide production by rhizobacteria and potential for suppression of weed seedling growth. Curr. Microbiol.. 2001;43:182-186.
- [Google Scholar]
- Stable reagents for the colorimetric determination of cyanide by modified Konig reactions. Anal. Chem.. 1975;47:916-918.
- [Google Scholar]
- Production of DAPG and HCN by Pseudomonas sp. LBUM3s00 contributes to the biological control of bacterial canker of tomato. Phytopathology. 2012;102:967-973.
- [Google Scholar]
- Growth response of weed and crop seedlings to deleterious rhizobacteria. Biol. Control. 2006;39:58-65.
- [Google Scholar]
- Pseudomonas aeruginosa WS-1 for biological control of leaf blight disease of Withania Somnifera. Arch. Phytopathol. Plant Protect.. 2012;45:796-805.
- [Google Scholar]
- Biological control of great brome (Bromus diandrus) in durum wheat (Triticum durum): specificity, physiological traits and impact on plant growth and root architecture of the fluorescent pseudomonas strain X33d. BioControl. 2010;55:561-572.
- [Google Scholar]
- Characterization of Pseudomonas aeruginosa RM-3 as a potential biocontrol agent. Mycopathology. 2010;170:181-193.
- [Google Scholar]
- A simplified method of quantitating proteins using biuret and phenol reagents. Anal. Biochem.. 1978;86:193-195.
- [Google Scholar]
- Rhizospheric bacteria with the potential for biological control of Parthenium hysterophorus. J. Chem. Biol. Phys. Sci. Sec. B. 2013;3:2679-2686.
- [Google Scholar]
- Screening of bacterial antagonists for biological control of phytopthora blight of pepper. J. Basic Microbiol.. 2005;45:55-63.
- [Google Scholar]
- Chitinolytic and secondary metabolite producing Pseudomonas fluorescens isolated from Solanaceae rhizosphere effective against broad spectrum fungal phytopathogens. Asian J. Plant Sci. Res.. 2012;2:16-24.
- [Google Scholar]
- Suppression of bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. in China. BioControl. 2005;32:111-120.
- [Google Scholar]
- Russel, A.D., Hugo, W.B., Aylif, G.A.J., 1982. Principles and Practices of Disinfection, Preservation and Sterilization, Blackwell Scientific, London.
- Disease-suppressive soil and root-colonizing bacteria. Science. 1982;216:1376-1381.
- [Google Scholar]
- MEGA 4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol.. 2007;24:1596-1599.
- [Google Scholar]
- In vitro evaluation of antagonistic properties of Pseudomonas corrugata. Microbiol. Res.. 2008;163:329-336.
- [Google Scholar]
- Pseudomonas aeruginosa-plant root interactions. Pathogenicity, biofilm formation and root exudation. Plant Physiol.. 2004;134:320-331.
- [CrossRef] [Google Scholar]
- Pseudomonas biocontrol agents of soil borne pathogens: looking back over 30 years. Phytopathology. 2007;97:250-256.
- [Google Scholar]
- Host-plant selectivity of rhizobacteria in a crop/weed model system. PLoS One. 2007;2:846.
- [CrossRef] [Google Scholar]







