Translate this page into:
Isolation and characterization of antioxidant and antimicrobial compounds from Anacardium occidentale L. (Anacardiaceae) leaf extract
*Corresponding author. Tel.: +234 803 581 5107 marogba@oauife.edu.ng (M.A. Aderogba) marogba@yahoo.co.uk (M.A. Aderogba)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 2 January 2015
Peer review under responsibility of King Saud University.
Abstract
The extracts obtained from Anacardium occidentale leaf were investigated for antioxidant and antimicrobial activities. Bioassay-guided fractionation using DPPH autobiographic analysis of the EtOAc soluble fraction of the crude extract resulted in the isolation of agathisflavone (1) and a mixture of quercetin 3-O-rutinoside (2) and quercetin 3-O-rhamnoside (3). Characterization of the isolated compounds was carried out using nuclear magnetic resonance (1D and 2D) and mass spectrometry. The antioxidant activities of the samples were determined using three complementary tests, namely; DPPH-radical scavenging, total antioxidant capacity (TAC) and ferric reducing antioxidant power (FRAP). The antimicrobial activity of the samples was evaluated using the disc diffusion and broth microdilution methods. The results indicated that the mixture (ratio 2:1) of compounds (2) and (3) was most active in its capacity to scavenge free radicals in the DPPH assay [IC50 = 0.96 ± 0.01 μg/mL] compared to the ethyl acetate (EtOAc) fraction, the ascorbic acid and quercetin standards. The mixture of compounds (2) and (3) exhibited the highest activities in the TAC assay (5332.00 ± 3.38 mg AAE/g, 8562.00 ± 5.43 mg QE/g) and FRAP assay (15,136.00 ± 7.14 mg AAE/g, 199,530.00 ± 94.12 mg QE/g) compared to the EtOAc fraction. The fractions of the extract showed comparatively better activities against gram-negative than the gram positive ones with the broadest spectrum of activities demonstrated for the ethyl acetate fraction. No inhibitions were obtained against the fungal strains investigated. The isolated compounds from the plant possess essentially inhibitory rather than cidal activities against these organisms with the Minimum Bactericidal Concentrations (MBCs) >2.0 mg/mL in all cases. The mixture of compounds 2 and 3 showed higher activity than compound 1 with the former having MIC as low as 0.25 mg/mL against Pseudomonas aeruginosa and Clostridium sporogens, 0.5 mg/mL against Staphylococcus aureus and 1.0 mg/mL against Escherichia coli and Klebsiella pneumonia strains investigated while the latter has an MIC of 1.0 mg/mL against most of the organisms.
This study concluded that the extracts and isolated compounds had strong antioxidant and moderate antibacterial activities and could be effective in the management of oxidative stress related diseases. These findings also justified the use of this plant’s extracts in folk medicine.
Keywords
Anacardium occidentale
Anacardiaceae
Antioxidant activity
Antimicrobial
1 Introduction
Anacardium occidentale L. is a tree in the family of the flowering plant Anacardiaceae. Extracts from roots, stems and fruits are extensively used in folk medicine for infectious, inflammatory and oxidative stress conditions (Sokeng et al., 2001; Chen and Chung 2000; Ojewole, 2004; Olajide et al., 2004, 2013). The leaf and/or the stem bark are used for eczema, genital problems, venereal diseases, impotence, bronchitis, cough and syphilis-related skin disorders (Franca et al., 1993). Traditional medicine in West Africa and South America uses the leaf infusion in the treatment of gastrointestinal disorders (acute gastritis, diarrhoea), mouth ulcers and throat problems (Kudi et al., 1999; Akinpelu, 2001; Goncalves et al., 2005; Taylor, 2005).
Analysis of the ethyl acetate fraction of a 70% ethanol leaf extract of A. occidentale using liquid chromatography coupled to mass spectrometry (LC/ESI-MS) indicated the presence of phenolic compounds such as: quercetin, myricetin, catechin, epicatechin, amentoflavone, tetramer of proanthocyanidin and unidentified quercetin glycosides (Konan and Bacchi, 2007). Flavonoids such as: quercetin, quercetin 3-O-rhamnoside, myricetin, and myricetin-3-O-rhamnoside were reported from the leaf extracts (Arya et al., 1989). Fractionation of the ethyl acetate fraction of the crude ethanol seed extract on Sephadex LH-20 column chromatograph resulted in the isolation of a mixture of catechin and epicatechin, while fractionation of the hexane fraction of the crude ethanol extract of the stem bark afforded sitosterol and estimasterol (Chaves et al., 2010).
Many of the phytochemical studies (De Brito et al., 2007; Paramashivappa et al., 2001; Shobba and Ravindranath, 1991) on the extracts of A. occidentale did not link specific activity to the identified constituents. In our continuing efforts for potent antioxidant and antimicrobial agents from natural sources, we have investigated the leaf extracts of A. occidentale for its bioactive constituents.
2 Materials and methods
2.1 General
All analyses of samples (fractions and isolated compounds) using thin layer chromatography (TLC) were performed at room temperature using pre-coated plates (MERCK, silica gel 60 F254 0.2 mm). Detection of spots was by ultraviolet lamp (254 and 366 nm). Silica gel and Sephadex LH-20 were used as stationary phases for column chromatography to fractionate active fractions. Nuclear magnetic resonance (NMR) spectra data were obtained from a Brucker Spectrometer. Chemical shifts are expressed in parts per million (ppm). Mass spectrum (TOF MS ES) was obtained from a Thermofinnigan Trace GC coupled to a Polaris Q Mass Spectrometer.
2.2 Plant material
The leaves of A. occidentale were collected (November 2010) at Obafemi Awolowo University Ile-Ife, Nigeria. Mr. Ibenesibor of the Herbarium Section, Department of Botany, OAU, Ile-Ife, authenticated the plant material collected. The voucher specimen (IFE Herbarium: 16834) was deposited at the University Herbarium. The collected leaves were air dried at room temperature for two weeks and ground to powder.
2.2.1 Extraction procedure
Powdered material (1.8 kg) was extracted with 80% methanol (10 L) with occasional shaking for 24 h. The extract was filtered and the filtrate was concentrated to about ¼ of its original volume using a rotary evaporator at 40 °C. This afforded the crude extract of the plant (700 mL).
2.2.2 Solvent partitioning of the crude extracts
The crude extract obtained was suspended in distilled water in a separating funnel (5 L) and in turn partitioned with n-hexane (HEX), dichloromethane (DCM), ethyl acetate (EtOAc) and n-butanol (BuOH). This yielded four solvent fractions.
2.3 Qualitative screening for the antioxidant compounds
About 10 μL of each of the four fractions (1 mg/mL) obtained were spotted on silica gel TLC plate, the chromatogram was developed in DCM/MeOH (8.5:1.5) and the plates were sprayed with 0.2% 1,1-diphenyl-2-picrylhydrazyl (DPPH•) in MeOH for detection of antioxidant compounds in each of the fractions. From the chromatogram (results not shown), it was found that ethyl acetate and butanol fractions contained more antioxidant compounds than n-hexane and dichloromethane fractions. Ethyl acetate fraction which showed the highest number of antioxidant spots was selected for fractionation.
2.4 Isolation of antioxidant compounds from ethyl acetate fraction of A. occidentale
Accelerated Column Chromatography (AGC) using silica gel (230–400 mesh) as the stationary phase was used to fractionate ethyl acetate fraction (27.0 g). The column was eluted with n-hexane 100% followed by an increasing gradient of ethyl acetate starting with 10% up to 100% ethyl acetate. This was followed with an increasing gradient of methanol from 10% in ethyl acetate up to 100% methanol. Test tube fractions (15 mL each) collected were analysed on TLC plates using Hexane/EtOAc (3:7). This afforded five fractions (A1–E1).
From the chromatogram, fractions B1 and C1 had antioxidant compounds. Fractionation of B1 (9.8 g) on a silica gel column using hexane (100%) was followed by an increased gradient of ethyl acetate up to 100%. This was followed in turn by an increasing gradient of methanol up to 20%. Test tube fractions collected were analysed on TLC plate using Hexane/EtOAC (1:4) solvent system. Three fractions, A2–C2 were obtained. Open column chromatography using Sephadex LH-20 as stationary phase was used to fractionate B2 (5.8 g). The column was eluted using Hexane/EtOAc (1:1) followed by an increasing gradient of EtAOc up to 100%. Analysis of the fractions collected on TLC plate using 100% EtOAc yielded three fractions A3–C3. B3 was further purified on silica gel column using hexane (100%) followed by an increasing gradient of ethyl acetate up to 100%. This was followed in turn by an increasing gradient of methanol up to 10%. Analysis of the test tubes fractions collected on silica gel TLC plate using EtOAc:MeOH (9:1) showed that tubes 3–18 had single spots, this afforded compound 1 (19 mg).
Purification of C2 (1.9 g) was achieved by its fractionation on silica gel column eluted with dichloromethane followed by an increasing gradient of methanol up to 25%. The fractions collected in the test tubes were analysed on TLC plate using DCM/MeOH (4:1). Test tube 14–17 showed single spots, this yielded a mixture of compounds 2 and 3.
2.4.1 Structure elucidation of isolated compounds
Isolated compounds were characterized using spectroscopic techniques: mass spectrometry (ESI-TOF-MS), 1H (400 MHz) and 13C NMR (100 MHz), and DEPT together with 2D experiments (gCOSY, gHSQC and gHMBC).
2.4.1.1 Compound 1
Compound 1 (19 mg) was obtained as yellow powder. Its mass spectrum (TOF-MS-ES) showed the pseudo-molecular ion peak as the base peak at m/z = 537.0826, [M−H]−, consistent with the molecular formula C30H18O10. The 1H NMR (MeOD) of 1 showed signals between δ 7.91 and 6.38 mainly aromatic protons. The spectrum showed two separate doublets (ortho coupled related protons) at δ 7.90 (2H, d, J = 8.0 Hz, H-2′, H-6′) and 6.96 (2H, d, J = 8.0 Hz, H-3′, H-5′) for 1, 4-disubstituted phenyl ring (ring B) representing an A2B2 ring system, along with two separate singlet signals at 6.58 (1H, s, H-3′′) and 6.66 (1H, s, H-3). Another ortho coupled two separate doublets appeared at δ 7.54 (2H, d, J = 8.0 Hz, H-2′′′, H-6′′′) and 6.75 (2H, d, J = 8.0 Hz, H-3′′′, H-5′′′) for the second ring B. Two separate singlets also appeared at 6.70 (1H, s, H-8) and 6.38 (1H, s, H-6′′). The 13C NMR and DEPT spectra of this compound revealed the presence of 26 carbons. Eight of these carbons were classified as protonated from DEPT spectrum, out of which four were assigned to two units of 1,4-disubstituted phenyl and another two were characteristic of a flavone (C-3) signal. The remaining two protonated carbons were assigned to C-8 and C-6′′. The positions of attachment of the two flavone units were established by 2D NMR, Compound 1 was determined to be agathisflavone (Fig. 1). The 13C NMR (MeOD) – 166.4 (C-2), 104.1 (C-3), 184.4 (C-4), 161.5 (C-5), 104.9 (C-6), 164.5 (C-7), 94.8 (C-8), 159.2 (C-9), 105.4 (C-10), 123.4 (C-1′), 129.6 (C-2′), 117.0 (C-3′), 162.9 (C-4′), 117.0 (C-5′), 129.6 (C-6′), 166.2 (C-2′′), 103.6 (C-3′′), 184.1 (C-4′′), 162.6 (C-5′′), 100.0 (C-6′′), 164.2 (C-7′′), 100.4 (C-8′′), 157.1 (C-9′′), 105.7 (C-10′′), 123.5 (C-1′′′), 129.4 (C-2′′′), 117.2 (C-3′′′), 162.7 (C-4′′′), 117.2 (C-5′′′), 129.4 (C-6′′′). The spectroscopic data are in agreement with the literature (Svenningsen et al., 2006).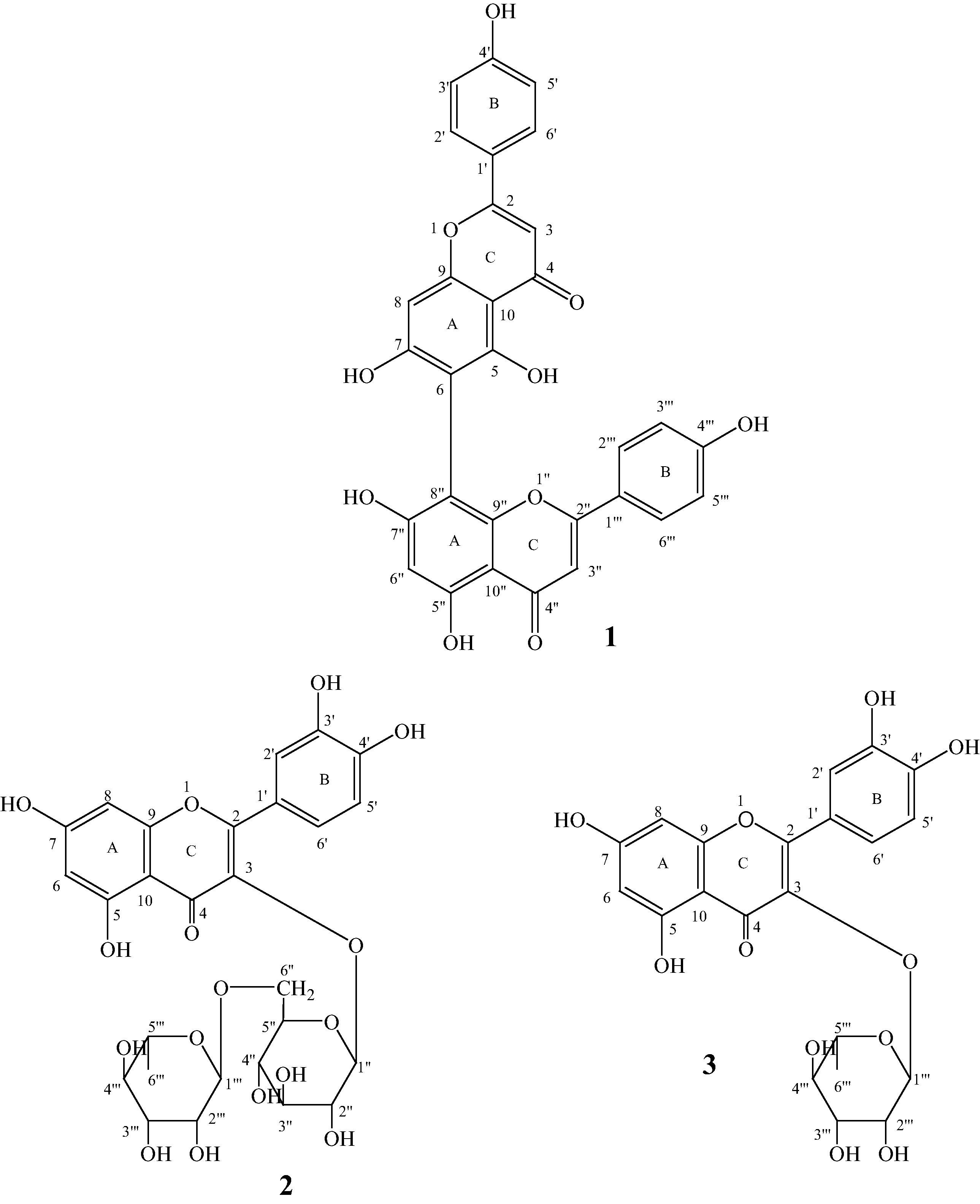
Antioxidant constituents of A. occidentale leaf extract.
2.4.1.2 Mixture of compounds 2 and 3
The mixture of compounds 2 and 3 (80 mg) was obtained as brown powder. The mixture components were identified by NMR spectra (mainly 2D) peak intensities. The higher intensity signals were allocated to compound 2 while the lower intensity signals were assigned to compound 3. The ratio of the two components in the mixture was also determined by comparison of the signal intensities as 2:1 for compounds 2 and 3 in the mixture. The 1H NMR of 2 in the mixture showed aromatic signals indicating the presence of 1,3,4-trisubstituted ring B representing an ABX ring system. The spectrum peaks appeared at δ 7.62 (1H, d, J = 2.1 Hz, H-2′), 7.59 (1H, dd, J = 2.2 and 8.4 Hz, H-6′) and 6.87 (1H, d, J = 8.4 Hz, H-5′). Two separate meta coupled doublets appeared at δ 6.38 (1H, d, J = 2.1 Hz, H-8) and 6.20 (1H, d, J = 2.1 Hz, H-6) for ring A. The 1H NMR (MeOD) of 3 represented by lower intensity signals showed a meta related doublet at δ 7.34 (1H, d, J = 2.1 Hz, H-2′), ortho and meta related doublet of doublet at δ 7.31 (1H, dd, J = 2.1 and 8.3 Hz, H-6′) and ortho related doublet at δ 6.92 (1H, d, J = 8.3 Hz, H-5′) for ring B indicating second ABX ring unit. Another two separate meta related doublets also appeared at δ 6.36 (1H, d, J = 2.1 Hz, H-8) and 6.20 (1H, d, J = 2.1 Hz, H-6) for the ring A of the second flavonoid ring. The 13C NMR and DEPT spectra of these compounds revealed the presence of two quercetin derivatives. There was the presence of sugar signals indicating rhamnosyl and glucosyl moieties. Anomeric proton of rhamnosyl appeared at δ 5.36 (1H, d, J = 1.56 Hz) while that of glucosyl appeared at δ 5.17 (1H, d, J = 7.2 Hz). In the 13C NMR (100 MHz, MeOD) spectrum, there were thirty carbon signals for the two flavonoid units in addition to the sugar signals. The anomeric carbon of the rhamnosyl appeared at δ 103.4 and C-6 at δ 17.7. Glucosyl anomeric carbon appeared at δ 104.4 and C-6 at δ 67.3. The 2D NMR (HMBC) of this compound mixture revealed that the anomeric of the rhamnosyl attached to the C-6 of the glucosyl making the sugar a disaccharide (rutinosyl). However, the HMBC spectrum further showed that there are two points of attachment for rhamnosyl indicating the presence of two units of rhamnosyl in the mixture. This is indicated by the intensities of the rhamnosyl signals in the 13C NMR. The point of attachment of the rutinosyl correlated to C-3 of aglycone of compound 2 at δ 135.5 while the second point of attachment of the second unit of rhamnosyl appeared at C-3 of compound 3 at δ 136.3.
Compound 2: 13C NMR (MeOD): 159.0 (C-2), 135.5 (C-3), 179.5 (C-4), 163.1 (C-5), 100.0 (C-6), 166.1 (C-7), 94.8 (C-8), 158.5 (C-9), 105.7 (C-10), 123.4 (C-1′), 117.3 (C-2′), 146.1 (C-3′), 150.0 (C-4′), 116.1 (C-5′), 123.0 (C-6′), 104.4(C-1′′), 73.4 (C-2′′), 77.6 (C-3′′), 72.1 (C-4′′), 75.3 (C-5′′), 67.3 (C-6′′), 103.4 (C-1′′′), 72.0 (C-2′′′), 72.1 (C-3′′′), 72.3 (C-4′′′), 71.1 (C-5′′′), 17.7 (C-6′′′).
Compound 3: 13C NMR (MeOD): 159.4 (C-2), 135.3 (C-3), 179.7 (C-4), 163.2 (C-5), 99.9 (C-6), 165.9 (C-7), 94.8 (C-8), 158.6 (C-9), 106.0 (C-10), 123.1 (C-1′), 117.1 (C-2′), 146.4 (C-3′), 149.8 (C-4′), 116.5 (C-5′), 123.1 (C-6′), 103.4 (C-1′′), 72.0 (C-2′′), 72.1 (C-3′′), 72.3 (C-4′′), 71.1 (C-5′′), 17.7 (C-6′′). The spectra data are in good agreement with the literature (Aderogba et al., 2006 and Aderogba et al., 2012) for compounds 2 and 3 respectively.
2.5 Antioxidant assays
2.5.1 DPPH-radical scavenging assay
DPPH radical scavenging activities of the test samples were measured according to the method of Brand-Williams et al. (1997). An aliquot of 1 mL of 0.004% DPPH solution in ethanol and 1 mL of test samples at various concentrations by serial dilution (starting with 100 μg/mL for crude, hexane and dichloromethane extracts, 10 μg/mL for ethyl acetate and butanol extracts, 200 μg/mL for compound 1 and 10 μg/mL for mixture of compounds 2 and 3) were mixed and incubated at 37 °C for 30 min. The absorbance of test samples was read at 517 nm. The percentage inhibition of DPPH radical by the test samples was calculated by comparing the results obtained with those of the control using the formula: ABSControl is the absorbance of the negative control; ABSTest is the absorbance of test samples.
2.5.2 Determination of total antioxidant capacity
The total antioxidant capacity (TAC) of the test sample was performed by the phosphomolybdenum method of Prieto et al. (1999) with slight modifications. Briefly 1 mL of the reagent solution consisting of 28 mM sodium phosphate monobasic, and 4 mM ammonium molybdate and 0.6 M sulphuric acid was mixed with 100 μL of the test sample (1 mg/mL for crude and fractions, and 200 μg/mL for compounds). The reaction mixture was then incubated at 95 °C for 90 min. The reaction mixture was then cooled under running water and the absorbance measured at 695 nm. Total antioxidant capacity was calculated as mg ascorbic acid equivalent/g (mg AAE/g) and mg quercetin equivalent/g (mg QE/g) of test sample.
2.5.3 Determination of ferric reducing antioxidant power (FRAP)
The ferric reducing antioxidant power (FRAP) assay was carried out as previously described by Benzie and Strain (1999). A 300 mmol/L of acetate buffer (pH 3.6), 10 mmol/L of 2,4,6-tris (2-pyridyl)-1,3,5-triazine, and 20 mmol of ferric chloride were mixed together in the ratio 10:1:1 respectively to give the FRAP working reagent. To 50 μL solution of the different test samples (1 mg/mL for crude and solvent fractions, and 200 μg/mL for compounds) 1 mL of the working FRAP reagent was added. The reaction mixtures were then incubated at 37 °C for 10 min and the absorbance then read at 593 nm in a spectrophotometer. The test was carried out in triplicate and ascorbic acid and quercetin were used as reference standards. The ferric reducing antioxidant power was calculated as equivalence of ascorbic acid and quercetin (mg AAE/g) and (mg QE/g).
2.6 Antimicrobial assay
2.6.1 Test organisms
The strains used were Escherichia coli, American Type Culture Collection (ATCC) 25922, Pseudomonas aeruginosa, American Type Culture Collection (ATCC) 27853, Staphylococcus aureus, American Type Culture Collection (ATCC) 29213, Proteus mirabilis (clinical strain), Bacillus subtilis, National Culture of Industrial Bacteria (NCIB) 3610, Klebsiella pneumonia (clinical strain) and Clostridium sporogens, National Culture of Industrial Bacteria (NCIB) 532 for bacteria and Candida albicans National Collection of Yeast Culture (NCYC) 6 and Candida pseudotropicalis (clinical strain) for fungi. The strains were from stocks of culture collections maintained in the Pharmaceutical Microbiology laboratory of the Department of Pharmaceutics, Faculty of Pharmacy, Obafemi Awolowo University where the experiments were performed.
2.6.2 Agar diffusion test
Bacteria were maintained both in cryopreservation medium and on nutrient agar slants while fungi were maintained on Sabouraud Dextrose Agar slants at 4 °C and subcultured monthly. The crude extract and the fractions were each dissolved in MeOH/H2O or DMSO as appropriate to give varying concentrations ranging from 0.5 to 20 mg/mL, (Table 4). The agar diffusion test followed the procedure described in Aiyelabola et al. (2012) and antimicrobial activity was evaluated by noting the zone of inhibition against the test organisms. Diameter of zone of inhibition of streptomycin (1 mg/mL) for each organism was: E. coli ATCC 25922, 14.0 mm; P. aeruginosa ATCC 27853, 14.0 mm; S. aureus ATCC 29213, 14.0 mm; P. mirabilis (clinical), 13.0 mm; B. subtilis NCIB 3610, 14.0 mm; K. pneumonia (clinical), 13.0 mm; Cl. sporogens NCIB 532, 10.0 mm.
Agent
Organisms
Concentration (mg/mL)
Diameter of zone of inhibition (mm)**
Crude extract
Escherichia coli ATCC 25922
10.0
4.0
20.0
7.0
Bacillus subtilis NCIB 3610
10.0
5.0
20.0
7.0
Butanol fraction
Escherichia coli ATCC 25922
5.0
3.0
10.0
4.0
20.0
6.0
Pseudomonas aeruginosa ATCC 27853
20.0
3.0
Bacillus subtilis NCIB 3610
10.0
1.0
20.0
5.0
DCM fraction
Escherichia coli ATCC 25922
0.5–10.0
8.0
20.0
13.0
Pseudomonas aeruginosa ATCC 27853
10.0–20.0
3.0
Ethyl acetate fraction
Escherichia coli ATCC 25922
1.0–2.0
4.0
5.0–10.0
5.0
20.0
9.0
Pseudomonas aeruginosa ATCC 27853
1.0–2.0
4.0
5.0
6.0
10.0
7.0
20.0
8.0
S. aureus ATCC 29213
2.0
5.0
5.0–20.0
7.0
Proteus mirabilis (clinical)
2.0–5.0
4.0
10.0
6.0
20.0
8.0
Bacillus subtilis NCIB 3610
1.0–5.0
2.0
10.0
5.0
20.0
10.0
K. pneumonia (clinical)
5.0
5.0
10.0
10.0
20.0
15.0
Cl. sporogens NCIB 532
2.0
4.0
5.0–10.0
6.0
20.0
8.0
Hexane fraction
Escherichia coli ATCC 25922
0.5–20.0
8.0
S. aureus ATCC 29213
20.0
5.0
Bacillus subtilis NCIB 3610
20.0
4.0
Cl. sporogens NCIB 532
20.0
4.0
2.6.3 The antimicrobial screening of pure compounds
The MIC values of the isolated compounds against the bacteria strains were determined using the micro-well dilution method as described by Zgoda and Porter (2001) and modified by Taiwo et al. (2013). The bacteria strains used were from 24 h nutrient agar plate cultures and the colonies were suspended in sterile distilled water in order to obtain bacteria load of approximately 106 CFU/mL, by comparison with the McFarland tube No. 0.5. These were then used in the microwells. The compounds were tested at concentrations ranging from 2 to 0.0078125 mg/mL. Streptomycin (Shijiazhuang, China) was used at the concentration range of 1000–3.90625 μg/mL as positive control. To prevent drying, prepared microtitre plates were sealed and thereafter incubated overnight at 37 °C.
Microbial growth was determined by careful observation for growth in bright light and confirmed by determining the absorbance at 630 nm using the DNM-9602 universal microplate reader as described by Aderogba et al. (2011) following the protocol of Jorgensen and Turnidge (2007). The MIC was defined as the lowest concentration of each of the compounds that inhibits the growth of bacteria. MBC was determined by plating drops from concentrations that showed inhibition of growth on freshly prepared Nutrient Agar plates. After 24-hr incubation at 37 °C the lowest concentration at which growth could not be recovered was taken as the MBC.
2.6.4 Statistical analysis
Concentration–response curves were plotted using both linear regression plot and non-linear plot as appropriate on a Prism Software package 5.00 for windows (Graphpad® San Diego California, USA). Sample’s data were obtained from three independent experiments with each performed in triplicate (n = 9) and represented as mean ± SD.
3 Results and discussion
3.1 Structure elucidation
A bioactivity directed fractionation was used to target the antioxidative constituents in the EtOAc fraction of the 80% MeOH extract of leaves of A. occidentale. Repeated column chromatography and further purification by Sephadex LH-20 yielded a bioflavonoid, agathisflavone (1), and a mixture of quercetin 3-O-rutinoside (2) and quercetin 3-O-rhamnoside (3), (ratio 2:1). The isolated compounds were characterized using spectroscopic techniques: NMR (1D and 2D) and mass spectrometry (ESI-TOF-MS).
3.2 Antioxidant activities
Quantitative antioxidant activities of the crude extract, solvent fractions and isolated compounds were determined using three complementary antioxidant assays DPPH-radical scavenging, Total Antioxidant Capacity (TAC) and Ferric Reducing Antioxidant Power (FRAP) assays. The results of assays are shown in Tables 1–3. Of all the extracts, ethyl acetate fraction was the most active in the DPPH• assay (IC50 = 5.66 ± 0.16 μg/mL) and the mixture of quercetin 3-O-rutinoside (2) and quercetin 3-O-rhamnoside (3), (ratio 2:1) elicited the highest activity (IC50 = 0.966 ± 0.01 μg/mL) amongst the test samples including the two standards (ascorbic acid and quercetin) used in the assay, (Table 1). Similarly, in the TAC and FRAP assays the mixture of compounds (2) and (3) ratio (2:1), exhibited a higher activity than the other test samples with values of 5332.00 ± 3.38 mg AAE/g, and 8562.00 ± 5.43 mg QE/g in the TAC assay and (15136.00 ± 7.14 mg AAE/g, and 199530.00 ± 94.12 mg QE/g) in the FRAP assay respectively(Tables 2 and 3). Data represents mean ± S.E.M. of triplicate determinations. DPPH free radical scavenging activity is expressed as IC50 (μg/mL) values. Data represents mean ± S.E.M. of triplicate determinations. Total antioxidant capacity is expressed as mg Ascorbic acid equivalent per g (mg AAE/g) and mg quercetin equivalent per g (mg QE/g) of the test samples. Data represents mean ± S.E.M. of triplicate determinations. FRAP Antioxidant activity is expressed in Ascorbic acid equivalent mg per g (mg AAE/g) and quercetin equivalent mg per g (mg QE/g) of samples.
Sample
Mean IC50 (μg/mL)
Ascorbic acid
4.57 ± 0.07
Quercetin
3.01 ± 0.14
Crude
9.94 ± 0.14
Hexane
157.49 ± 1.00
Dichloromethane
85.13 ± 1.50
Ethyl acetate
5.66 ± 0.16
Butanol
7.77 ± 0.34
1
366.37 ± 32.59
2 and 3
0.96 ± 0.01
Sample
mg AAE/g
mg QE/g
Crude
1997.00 ± 5.63
3206.15 ± 9.04
Hexane
1611.00 ± 7.90
2587.71 ± 12.66
Dichloromethane
1865.00 ± 15.77
2994.58 ± 25.32
Ethyl acetate
2080.00 ± 5.64
3339.97 ± 9.05
Butanol
2087.00 ± 7.89
3350.82 ± 12.66
1
2782.00 ± 15.77
4467.00 ± 25.32
2 and 3
5332.00 ± 3.38
8562.00 ± 5.43
Sample
mg AAE/g
mg QE/g
Crude
3038.00 ± 3.57
40,047.00 ± 47.06
Hexane
1639.00 ± 28.56
21,600.00 ± 376.47
Dichloromethane
2813.00 ± 103.53
37,082.00 ± 1364.7
Ethyl acetate
3051.00 ± 1.65
40,212.00 ± 23.53
Butanol
3056.00 ± 3.57
40,282.00 ± 47.06
1
1714.00 ± 28.56
22,590.00 ± 376.47
2 and 3
15,136.00 ± 7.14
199,530.00 ± 94.12
The antioxidant activity reported in this study for the isolated compounds in the DPPH assay is in agreement with similar studies undertaken on the compounds. For instance Bansala et al. (2011) reported quercetin 3-O-rutinoside from Pilea microphylla with an IC50 value 20.40 ± 0.3 μmol/L. In another study, Bonacorsi et al. (2011) reported quercetin 3-O-beta-d-galactopyranoside from Anacardium humile with an EC50 value 9.9 μg/mL. Recently, Li et al. (2014) reported amentoflavone with IC50 value 232.55 ± 45.22 μg/mL. In this study however, the difference in activity recorded for agathisflavone in the DPPH assay (IC50 value 336.37 ± 32.59 μg/mL) compared to amentoflavone could be ascribed to differences in substitution pattern of the two biflavonoids. In considering the activity given by the mixture of compounds 2 and 3 in the DPPH assay the potent activity (IC50 value 0.96 ± 0.01 μg/mL) observed can be adduced to the additive combination of the two compounds.
3.3 Antimicrobial activities
The compounds were screened for possible antibacterial and antifungal activities using two fungi strains as well as three Gram-positive and four Gram-negative bacteria. The Gram-positive bacteria were B. subtilis, NCIB 3610; S. aureus ATCC 29213, and Cl. sporogens NCIB 532. The Gram-negative bacteria were E. coli ATCC 25922, P. aeruginosa ATCC 27853, P. mirabilis (clinical strain) and K. pneumonia (clinical strain), while C. albicans NCYC 6 and C. pseudotropicalis (clinical strain) were the fungi. The standard antimicrobial agents used were acriflavine and streptomycin.
The plant investigated is used in ethnomedicine to treat a wide variety of conditions including infectious diseases. The crude extract of A. occidentale has little activity against the selected bacteria and no activity against the fungi at the concentrations tested. The fractions of the extract showed comparatively better activities against gram-negative bacteria than the gram positive ones with the broadest spectrum of activities demonstrated for the ethyl acetate fraction (Table 4). No inhibitions were obtained against the fungal strains investigated.
The isolated compounds from the plant were further screened against these bacteria strains to determine their contribution to the antimicrobial activities. The results obtained indicated that the identified compounds possess essentially inhibitory rather than cidal activities against these organisms. The mixture of compounds 2 and 3 (ratio 2:1), showed higher activity than compound 1 with the former having MIC as low as 0.25 mg/mL against P. aeruginosa and Cl. sporogens, 0.5 mg/mL against S. aureus and 1.0 mg/mL against E. coli, K. pneumonia and P. sporogens. The MIC of compound 1 was observed to be 1.0 mg/mL against most of the bacteria tested except Cl. sporogens where an MIC of 0.5 mg/mL was obtained. The MBC of the compounds was also greater than 2.0 mg/mL in all cases (Table 5).
Organisms
Streptomycin
Compound 1
Compound 2 and 3
MIC
MBC
MIC
MBC
MIC
MBC
B. subtilis NCIB 3610
0.50
0.500
1.00
>2.00
0.500
>2.00
Cl. sporogens NCIB 532
0.125
0.500
0.500
>2.00
0.250
>2.00
E. coli ATCC 25922
0.125
0.500
1.00
>2.00
1.00
>2.00
K. pneumonia (clinical)
0.125
0.500
1.00
>2.00
1.00
>2.00
P. fluorescence NCIB 3756
0.50
0.500
1.00
>2.00
1.00
>2.00
P. aeruginosa ATCC 27853
0.50
0.500
1.00
>2.00
0.250
>2.00
S. aureus ATCC 29213
0.125
0.500
1.00
>2.00
0.500
>2.00
Compound 1 is agathisflavone which is a biflavonoid. A number of biological activities have been reported for this group of flavonoid, for instance Cushnie and Lamb (2005) and Pegnyemb et al. (2005) reported antibacterial activity, while Njock et al. (2012) reported anti-inflammatory activity. For agathisflavone (1), activities previously reported are antineoplastic, hepatoprotective and antiviral properties (Konan et al., 2012; Njock et al., 2012). In this study, it is demonstrated that this compound also possesses inhibitory antibacterial activities against a wide spectrum of organisms.
Quercetin and its glycosides have also been reported to demonstrate antiviral, antibacterial, anti-inflammatory, anticancer and other biological activities (Yu et al., 2007; Davis et al., 2009). A mixture of quercetin 3-O-rutinoside (2) and quercetin 3-O-rhamnoside (3) isolated in this study are glycosides of quercetin with rutinosyl and rhamnosyl respectively. Quercetin 3-O-rutinoside (2) has been reported to contribute to the antibacterial and antioxidant properties of some plant species (van der Watt and Pretorius, 2001; Ibtissem et al., 2012). It can therefore be inferred that the antibacterial activities reported for the mixture of compounds 2 and compound 3 are mainly due to the synergistic effects since the two compounds have the same flavonoid nucleus.
The good activities demonstrated by the mixture of 2 and 3 against P. aeruginosa, a pathogen characterized by a low intrinsic susceptibility to most antimicrobial agents and Cl. sporogens, a sporulating organism, also with intrinsically high resistance properties is highly instructive. It is an indication that this mixture could be utilized as effective agents against pseudomonal and clostridial infections that are often reported and known to be highly recalcitrant to therapy. The specificity in activities may be employed maximally in targeted therapy. The ethyl acetate fraction of the plant extract and identified chemical isolates may therefore be a readily available source of cheap and potent antimicrobial agents to be used in the therapy of infections caused by these often multi resistant organisms.
It has been shown also that the degree of antimicrobial activities of flavonoids depends largely on the freedom of the attached phenolic groups (Alcaraz et al., 2000). Those with free phenolic groups have been reported to demonstrate better antimicrobial activity than their substituted counterparts (Alcaraz et al., 2000; Aderogba et al., 2011). This observation is also demonstrated by the results of this study as slightly higher antibacterial activities are demonstrated for mixture of 2 and 3 compared with compound 1. Further to these, the lack of inhibitory effects by the extracts and fractions of this plant against the fungal strains is not out of place because these compounds have not been reported to express significant antifungal activities.
This study has established that the extracts and isolated compounds have strong antioxidant and moderate antimicrobial activities and could be effective in the management of oxidative stress and infectious related diseases. The findings justified the use of this plant in folk medicine.
In conclusion, the use of A. occidentale L. in the management of oxidative stress and infectious related diseases prompted us to investigate the antioxidant and antimicrobial constituents of this plant extracts. Based on the qualitative antioxidant screening using DPPH TLC assay, all the four solvent fractions (hexane, dichloromethane, ethyl acetate and butanol) contained antioxidant compounds. However, ethyl acetate fraction contained the highest number of antioxidant compounds and showed the broadest antibacterial spectrum of activities. A DPPH directed fractionation of the ethyl acetate fraction afforded agathisflavone (1) and a mixture of quercetin 3-O-rutinoside (2) and quercetin 3-O-rhamnoside (3). The plant extracts and identified constituents demonstrated varying degree of activities in the three antioxidant assays. The compounds also exhibited a broad spectrum of antimicrobial activities. These findings provided a rationale for the ethnomedicinal use of the plant in traditional medicine.
References
- Isolation of antioxidant constituents from Combretum apiculatum subsp apiculatum. S. Afr. J. Bot.. 2012;79:125-131.
- [Google Scholar]
- Antioxidant and antimicrobial activities of flavonoids glycosides from Dennettia tripetala G. Baker leaf extract Nig. J. Nat. Prod. Med.. 2011;15:49-52.
- [Google Scholar]
- Isolation of two flavonoids from Bauhinia monandra (kurz) leaves and their antioxidative effects. Afr. J. Tradit. Complement. Altern. Med.. 2006;3:59-65.
- [Google Scholar]
- Antimicrobial activity of Anacardium occidentale, bark. Fitoterapia. 2001;72:286-287.
- [Google Scholar]
- Structural and antimicrobial studies of coordination compounds of phenylalanine and glycine. Int. J. Chem.. 2012;4:49-59.
- [Google Scholar]
- Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J. Theor. Biol.. 2000;205:231-240.
- [Google Scholar]
- Phytochemical examination of the leaves of Anacardium occidentale. J. Indian Chem. Soc.. 1989;66:67-68.
- [Google Scholar]
- Phenolic compounds isolated from Pilea microphylla prevent radiation-induced cellular DNA damage. Acta Pharm. Sin. B. 2011;1(4):226-235.
- [Google Scholar]
- Ferric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol.. 1999;299:15-27.
- [Google Scholar]
- Relative antioxidant activity of Brazilian medicinal plants for gastrointestinal diseases. J. Med. Plants Res.. 2011;5:4511-4518.
- [Google Scholar]
- Kinetics and mechanisms of antioxidant activity using the DPPH• free radical method. Lebensm. Wiss. Technol.. 1997;30:609-615.
- [Google Scholar]
- Total phenolics, antioxidant activity and chemical constituents of extracts of Anacardium occidentale L. Anacardiaceae. Braz. J. Pharmacogn.. 2010;20:1-10.
- [Google Scholar]
- Mutagenicity and antimutagenicity studies of tannic acid and its related compounds. Food Chem. Toxicol.. 2000;38:1-5.
- [Google Scholar]
- Quercetin increases brain and muscle mitochondrial biogenesis and exercise tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol.. 2009;296:R1071-R1077.
- [Google Scholar]
- Determination of the flavonoid components of cashew apple (Anarcardium occidentale) by LC-DAD-ESI/MS. Food Chem.. 2007;105:1112-1118.
- [Google Scholar]
- An evaluation of the Effect of a bark extracts from the cashew (Anacardium occidentale L.) on infection by Leishmania (Viannnia) brasiliensis [in Portuguese] Rev. Soc. Bras. Med. Trop.. 1993;26:151-155.
- [Google Scholar]
- In vitro anti-rotavirus activity of some medicinal plants used in Brazil against diarrhoea. J. Ethnopharmacol.. 2005;99:403-407.
- [Google Scholar]
- Antioxidant and antibacterial properties of Mesembryanthemum crystallinum and Carpobrotus edulis extracts. Adv. Chem. Engineer. Sci.. 2012;2 359-36
- [Google Scholar]
- Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P.R., Baron E.J., Jorgensen J.H., Landry M.L., Pfaller M.A., eds. Manual of Clinical Microbiology (9th). Washington, DC: American Society for Microbiology; 2007. p. :1152-1172.
- [Google Scholar]
- Antiulcerogenic effect and acute toxicity of a Hydroethanolic extract from the cashew (Anacardium occidentale L.) leaves. J. Ethnopharmacol.. 2007;112:237-242.
- [Google Scholar]
- Cytotoxicity of cashew flavonoids towards malignant cell lines. Exp. Toxicol. Pathol.. 2012;64:435-440.
- [Google Scholar]
- Screening of some Nigerian medicinal plants for antibacterial activity. J. Ethnopharmacol.. 1999;67:225-228.
- [Google Scholar]
- Amentoflavone protects against hydroxyl radical-induced DNA damage via antioxidant mechanism. Turk. J. Biochem.. 2014;39:30-36.
- [Google Scholar]
- NASCA-HMBC, a new NMR methodology for the resolution of severely overlapping signals: application to the study of agathisflavone. Phytochem. Anal.. 2012;23:126-130.
- [Google Scholar]
- Potentiation of the anti-inflammatory effect of Anacardium occidentale L. stem-bark extract by grape fruit juice. J. Clin. Pharmacol.. 2004;26:183-188.
- [Google Scholar]
- Effects of Anacardium occidentale stem bark extract on in vivo inflammatory models. J. Ethnopharmacol.. 2004;95:139-142.
- [Google Scholar]
- Mechanisms of anti-inflammatory property of Anacardium occidentale stem bark: inhibition of NF-κB and MAPK signaling in the microglia. J. Ethnopharmacol.. 2013;145:42-49.
- [Google Scholar]
- Novel method for isolation of major phenolic constituents from cashew (Anacardium occidentale) nut shell liquid. J. Agric. Food Chem.. 2001;49:2548-2552.
- [Google Scholar]
- Antimicrobial biflavonoids from the aerial parts of Ouratea sulcata. Phytochemistry. 2005;66:1922-1926.
- [Google Scholar]
- Spectrophotometric quantification of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application of vitamin E. Anal. Biochem.. 1999;269:337-341.
- [Google Scholar]
- Supercritical carbon dioxide and solvent extraction of the phenolic lipids of cashew nut (Anacardium occidentale) shells. J. Agric. Food Chem.. 1991;39:2214-2217.
- [Google Scholar]
- Biflavones from Rhus species with affinity for the GABAA/benzodiazepine receptor. J. Ethnopharmacol.. 2006;103:276-280.
- [Google Scholar]
- Hypoglycemic activity of Anacardium occidentale L. aqueous extract in normal and streptozotocin-induced diabetic rats. J. Diabetes Res.. 2001;36:1-9.
- [Google Scholar]
- Antimicrobial and antiplasmodial activities of a Quaternary compound from Ritchiea capparoides var. Longipedicellata. Afr. J. Tradit. Complement. Altern. Med.. 2013;10:528-531.
- [Google Scholar]
- The Healing Power of Rainforest Herbs: A Guide to Understanding and Using Herbal Medicinals. Garden City Park: Square One Publishers; 2005. p. 535
- Purification and identification of active antibacterial components in Carpobrotus edulis. J. Ethnopharmacol.. 2001;76:87-91.
- [Google Scholar]
- Effects of triterpenoids and flavonoids isolated from Alnus firma on HIV-1 viral enzymes. Arch. Pharm. Res.. 2007;30:820-826.
- [Google Scholar]
- A convenient microdilution method for screening natural products against bacteria and fungi. Pharm. Biol.. 2001;39:221-225.
- [Google Scholar]







