Translate this page into:
Isolation and characterization of anti-proliferative and anti-oxidative mannan from Saccharomyces cerevisiae
⁎Corresponding author. guraishi@yahoo.com (Saleh Al-Quraishy),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mannan oligosaccharide is one of the major components of the yeast cell wall. In the present study, the production, characterization, and bioactivity of yeast mannan were investigated. Four indigenous yeast isolates were obtained from various kinds of juice collected from local markets in Al-Ahsa, KSA, and analyzed using morphological and biochemical methods. Isolate RY1 showed the highest production of mannan. RY1 was identified as Saccharomyces cerevisiae based on morphological characteristics and sequencing of the 18S rRNA gene (GenBank accession number LC479088.1). Mannan-RY1 was characterized by polymer analytical methods, 13C and 1H nuclear magnetic resonance spectroscopy (NMR), and infrared spectroscopy (IR). Interestingly, the mannan extracted from RY1 showed a significant ability to scavenge hydroxyl radicals and superoxide anions. In addition, mannan was found to have pronounced anti-tumor activity against liver (HepG2) and breast cancer (MCF7) cell lines. The results presented in this study confirm the bio-therapeutic activity of S. cerevisiae mannan, which could be used as a potential drug for cancer treatment.
Keywords
Mannan
Saccharomyces cerevisiae
Cytotoxicity
Antioxidant
1 Introduction
Saccharomyces cerevisiae is well-characterized yeast that is a valuable model system for the study of yeast cell walls (Lee et al., 2001). It is well analyzed in terms of genetic studies and industrial applications (Botstein and Fink, 2011). In S. cerevisiae, the cell wall accounts for up to 50% of the cell volume and about 15 to 30% of the dry mass. In addition to its role in maintaining cell integrity, the cell wall also plays an important role in protecting yeast cells from adverse environmental conditions such as osmotic stress (Klis et al., 2002). The cell wall of S. cerevisiae is composed of three major groups of polysaccharides, including polymers of mannose (mannoprotein complexes, 35–40% of cell dry mass), polymers of glucose (1,3-β-D-glucan, 50–55%, and 1,6-β-D-glucan, 5–10%), and polymers of N-acetylglucosamine (chitin, 2%) (Kwiatkowski et al., 2009). However, the ratio of cell wall components may vary among yeast species. It has been demonstrated that members of the Saccharomyces genus may have probiotic and antibacterial properties (Nayak, 2011).
Mannan, a major component of yeast cell wall polysaccharides, consists of a highly branched complex carbohydrate, an α-1,6 backbone with α-1,2- and α-1,3-linked mannose side chains (Abbott et al., 2015). It has been reported that yeast-derived cell wall polysaccharides, e.g., mannan, possess some biological properties, such as antiproliferative (Salvador et al., 2008), antioxidant (Liu and Huang, 2018; Galinari et al., 2018), and immunomodulatory (Mehdi and Hasan, 2012). Moreover, strains of Candida albicans and Saccharomyces cerevisiae with defects in mannan synthesis showed hypersensitivity to some antibiotics (Striebeck et al., 2013).
Previous studies have shown the use of yeasts (S. boulardii and S. cerevisiae) as a probable bio-therapeutic agent for the treatment of diarrhea and colitis-associated microbiota (Kelesidis and Pothoulakis, 2011). The antibacterial efficacy of S. cerevisiae may be due to the production of extracellular protease (Roostita et al., 2011), inclusion and elimination of secreted toxins (Fooks and Gibson, 2002), stimulation of immunoglobulin A and secretion of inhibitory proteins (Qamar et al., 2001). In this study, we aimed to recover the polysaccharide mannan from S. cerevisiae isolated from local sources in Al-Ahsa, Saudi Arabia, and to evaluate its antioxidant and antiproliferative properties.
2 Materials and methods
2.1 Isolation and identification of yeast strains
Four yeast strains were isolated from apple and grape juice collected from the local market in Al-Ahsa, KSA. An amount of 10 ml of juice was homogenized in 90 ml of sterile saline at 150 rpm for 10 min, then decimally diluted and spread on the Sabouraud dextrose agar plates. After 72 hr of incubation at room temperature, the different colonies grown on the media were visually selected. Pure cultures were cultured on yeast extract peptone (sucrose, ribose, maltose, fructose, rhamnose, manetol, galactose, trehalose, lactose, dextrose, and xylose). The utilization ability of the yeast isolates was defined as shown by Forouhande et al. (2010).
For molecular identification, the DNA of the yeast strains was isolated using the SDS -proteinase K-CTAB method (Sambrook and Russell, 2001). PCR was used to amplify a subset of the rDNA using universal fungal primers for 18S rRNA, NS1 (5′- GTA GTC ATA TGC TTG TCT C-3′) and NS4 (5′- CTT CCG TCA ATT CCT TTA AGC TTC CGT CAA TTC CTT TAA G-3′) as described by White et al. (1990). The PCR products were purified using a gel extraction kit (GeNei, India) and sequenced using the Big Dye Terminator Cycle Sequencing Kit (Applied Biosystems, USA). They were then purified using a Big Dye X-Terminator purification kit (Applied Biosystems, USA) and analyzed in 3730 DNA Analyzer (Applied Biosystems, USA). The sequence obtained was aligned using the algorithm BLAST to find matches in the NCBI database. In addition, the sequence data were submitted to GenBank.
2.2 Nuclear magnetic resonance (NMR) spectroscopy and infrared (IR) spectroscopy
For (NMR) spectroscopy, S. cerevisiae mannoprotein was freeze-dried three times from D2O (99.96%, Sigma Chemical Co. Ltd., UK) and finally dissolved in 1 ml D2O. Quantitatively valuable 1H NMR spectra and 13C NMR spectra were obtained using an AVANCE -DPX400 NMR spectrometer (Bruker, Switzerland) at 25 °C (Ovchinnikova et al. 2011). FTIR spectra were recorded using a Fourier transform infrared spectrometer FTS 7000 with a DTGS detector (DIGILAB, Randolph, MA, USA). The spectra were recorded in absorption mode from 500 to 4000 cm−1 (mid-infrared region). To avoid the interference of water, all samples were first dried in a vacuum oven at 80 °C for 3 hr and then placed in a vacuum desiccator with a desiccant and kept for 24 hr before measurement, according to Wang et al. (2012).
2.3 Extraction and production of crude mannan oligosaccharides
Water-soluble mannan oligosaccharides were isolated from 5 g dry yeast grown in YPDA medium (Hi-media, India) by extraction three times in 1% NaOH for 3 hr at 100 °C, chilled and neutralized with 4 M HCl. The neutralized extract was centrifuged at 3000 rpm for 10 min and the supernatant was collected. To the supernatant, 200 ml (4 volumes) of ethanol absolute was added to precipitate the mannan oligosaccharides. The precipitated mannan was then washed with absolute ethanol and diethyl ether according to Huang et al. (2010).
2.4 Amino acid analysis
The polysaccharide was hydrolyzed with 2 N HCl for 24 hr at 110 °C. After cooling and filtration, the filtrate was dried in a vacuum, and 0.02 N HCl was added and reacted in the atmosphere for 30 min. Then, 50 μl of the hydrolyzed polysaccharide was measured at 570 and 440 nm (to study Pro and Pro- OH) using a program with specific conditions of a temperature of 53 °C, the pressure of 80–130 kg/cm2 and flow rate of 0.225 ml/min (Nakanishi-Shindo et al., 1994).
2.5 Antioxidant capacity of the extracted mannan
The determination of scavenging activity for superoxide anions was performed according to Liu and Huang (2018). Basically, 3 ml of 0.05 mol/l Tris-HCl buffer solution (pH 8.2), 0.2 ml of different concentrations (0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 mg/ml) of the sample solution (as opposed to distilled water) were added to the test tubes. The tubes were incubated at 25 °C for 10 min in a water bath. Then 12 μl of the pre-warmed pyrogallic acid solution (0.03 mol/l) was added and reacted for 4 min. The absorbance at 320 nm was measured using vitamin C (Vc) as a positive control and Tris-HCl buffer solution as a blank. Measurements were repeated three times for each group. The clearance rate (E) was calculated as mentioned in Liu and Huang (2018) using the following formula: E = (Ac-As/Ac-Ab) × 100%, [where Ac: the absorbance value of the Tris-HCl buffer solution and pyrogallic acid solution (the system not studied); As: the absorbance value of the sample; and Ab: the absorbance value of the Tris-HCl buffer].
The scavenging activity for hydroxyl radicals was performed according to Liu and Huang (2018). 1 ml of the mannan sample solution at different concentrations (0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 mg/ml) was mixed with 1 ml of 9 mmol salicylic acid in 70% ethanol, 1 ml of 9 mmol FeSO4 solution, and 1 ml of 9 mmol H2O2. The mixtures were then incubated at 37 °C for 30 min, after which absorbance was measured at 510 nm. Vitamin C (Vc) was used as a positive control and measurements were repeated three times for each group. The hydroxyl radical clearance rate (E) was calculated as follows: E = [Ab-(As-Ac) /Ac] × 100%, [where Ab: the absorbance of the mannan samples with distilled water; Ac: the absorbance of the mannan solution; and As: the absorbance after addition of the mannan solution sample].
2.6 Anti-proliferative activity of S. cervices mannan
The potential cytotoxicity of mannan was evaluated in vitro against two human carcinoma cell lines: liver (HepG2) and breast (MCF7) using the MTT assay. According to Mosmann (1983), Khan et al. (2016), and Basar et al. (2015), cells were washed with phosphate buffer saline and then harvested by trypsinization. The cells were then cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum. Cells were then cultured in 95% air and 5% CO2 at 37 °C. For the MTT assay, cells were seeded in 96-well plates with a volume of 1 ml/well and a density of 1.2 × 104 cells/well. The cells were allowed to grow for 24 hr before starting the experiments. The cells were then treated with different concentrations of the test samples (0.5, 1.0, 1.5, 2.0, 3.0 mg/ml) for 24 hr.
The stock solutions were diluted in a culture medium to obtain the final sample concentrations including the control (without test sample). Each sample was used to treat four wells with cells in each 96-well plate. After a treatment interval of 24 hr, the cytotoxicity of the samples on each carcinoma cell line was measured and analyzed. For this purpose, the medium in each well was replaced with MTT solution (500 μg/ml) and incubated for 2 hr. Toxicity was assessed by the ability of the cells to reduce the yellow dye MTT to a blue formazan product, as mentioned by Popescu et al. (2015). The MTT reagent was then removed and the formazan crystals produced by the viable cells were dissolved in 100 μl of dimethyl sulfoxide (DMSO). The absorbance (570 nm) was determined using the microplate reader (CLARIO Star Microplate reader, BMG Labtech, UK). The results were expressed as a percentage of MTT reduction as in equation Percentage MTT reduction = (absorbance of sample/absorbance of control) × 100.
3 Results
3.1 Isolation and identification of yeast Saccharomyces strain
Four yeast strains were isolated, purified, and further identified by performing various morphological and physiological tests that facilitated the identification of the yeast isolates. First, we isolated four Saccharomyces strains (RY1, RY2, RY3, RY4) and selected them based on specific colony characteristics for yeast, particularly Saccharomyces, such as fluffiness and smoothness and creamy to white color. The isolated colonies that grew on the surface of an agar plate showed smooth and regular edges and protruded above the surface of the agar. Further analysis of the isolated strains with the dye methylene blue showed that the yeast cells appeared blue. Additional microscopic examination of the isolated yeast cells revealed a spherical to cocci cell arrangement with an obvious nucleus. Buds were also present in most parts of the cells and mycelium was absent. Overall, this morphological analysis indicates that the isolates belong to the yeast Saccharomyces.
To confirm the genus of the isolated yeast, we also examined the physiological properties of the isolates using established biochemical assays. We demonstrated the ability of the yeast isolates to ferment carbohydrates such as sucrose, maltose, glucose, galactose, fructose, trehalose, and raffinose. The four strains showed their ability to produce acid and ester, ammonia production was not observed in all isolated strains. In addition, 18S rRNA gene sequencing was performed to confirm the identity of the yeast isolates. PCR amplification of genomic DNA was performed using universal fungal primer sets. Nucleotide sequence data obtained from DNA sequencing were submitted to GenBank (accession number LC479088.1). According to BLAST analysis, the RY1-specific amplicons showed 100% identity with S. cerevisiae (GenBank accession numbers CP046092.1, CP036478.1, and CP033481.1).
3.2 RY1 strain showed the highest yield of mannan
To select a yeast isolate to be considered for further analysis, the amount of mannan recovered from each isolate was quantified. As shown in Fig. 1, the mannan yield ranged from 2.0 − 4.0% of cell dry mass in the different isolates. RY1 exhibited the highest amount of mannan. On the other hand, the least amount of mannan was obtained from RY2, which showed the highest biomass production.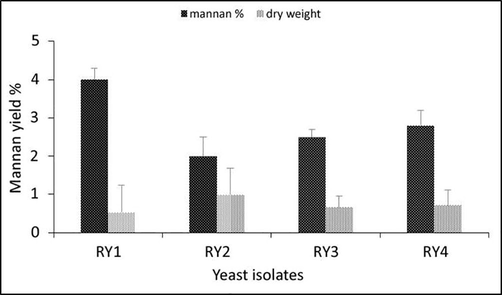
Yield of mannan extracted and mannan content in yeast isolates at the media of growth (YPD).
3.3 Characterization of mannan using polymer analytical methods
Since strain RY1 had the highest yield of mannan, it was selected for further analysis. The mannan isolated from RY1 was characterized by polymer analytical methods, i.e. 13C and 1H NMR spectroscopy and IR spectroscopy. As shown in Fig. 2a, the IR spectrum of mannan showed the appearance of many bands found in similar glucans, the greatest intensity of O–H band at a lower frequency (3421 cm−1), the band at (2950 cm−1) associated with C–H stretching (alkane). The band associated with the carbonyl group at (1726 cm−1) and (1618 cm−1) for N–H stretching (amide) suggests that mannan oligosaccharides are closely associated with proteins. On the other hand, the band at (1410 cm−1) was assigned to C-O. Interestingly, the bands at (895 cm−1) and (808 cm−1), which were mainly used to assess purity, are characteristic of mannan.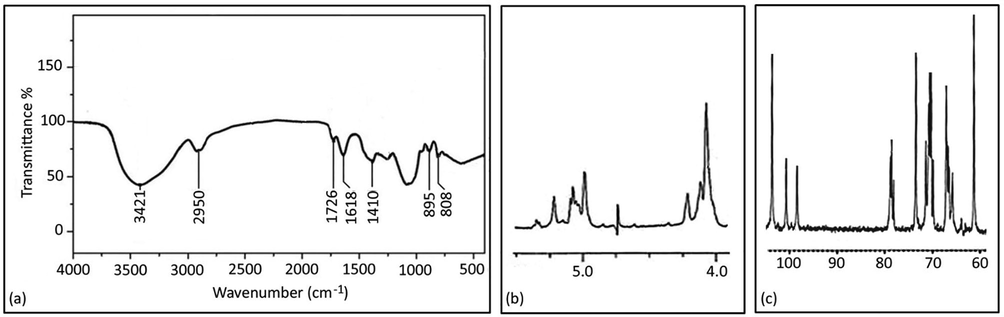
Infrared spectra of mannan (a), 1H NMR (b), and 13C NMR (c) spectra of mannan isolated from S. cerevisiae.
In this study, the stretching of the signals of the anomeric H atoms was analyzed as shown in Fig. 2b. A signal at 5.02 ppm was observed as a characteristic signal of α-1,2-linked mannose side chains of two or more subunits. We also identified a signal at 5.0–5.01 ppm characteristic of α-1,6-mannan in the base chain to which the mannose side groups are attached. For further identification of mannan, the 13C spectrum of mannan was analyzed here, as shown in Fig. 2c, six batches of signals were identified in the spectrum. The resonance signals between 98 and 103 ppm are assigned to the C-1 of the sugar ring. Interestingly, no resonance signals were detected at 82–97 ppm, indicating that the sugar residues are mannopyranose. Other signals detected at 68–78 ppm could be related to the substituted C-2, C-3, or C-4 atoms; and the signal detected at 61 ppm was assigned to the unsubstituted C-6 atom.
In this study, amino acid analysis was performed for the isolated mannoprotein, and compositional analysis showed that mannoprotein is a glycoprotein (Table 1). The results showed that mannoprotein contained 18 types of amino acids, with the percentage of serine, threonine, and aspartic acid being higher than other amino acids, 12.11, 19.89, and 13.59%, respectively.
Amino acids
Content %
Alanine
10.21
Arginine
0.98
Asparagine
1.10
Aspartic acid
13.59
Cysteine
0.06
Glutamic acid
11.42
Tyrosine
2.06
Glycine
3.33
Histidine
2.22
Isoleucine
4.72
Leucine
2.69
Lysine
2.96
Methionine
0.61
Phenylalanine
4.21
Proline
5.21
Serine
12.11
Threonine
19.89
Valine
5.40
3.4 The scavenging ability of mannan to superoxide anions and hydroxyl radicals
In this article, the ability of mannan isolated from the RY1 strain to scavenge hydroxyl radicals and superoxide anions was investigated. The results were expressed as the percentage of hydroxyl radical and superoxide anion scavenging activity presented antioxidant mechanism as shown in Fig. 3a and Fig. 3b, respectively. All mannan fractions isolated from RY1 showed hydroxyl-scavenging and superoxide anion ability in a dose-dependent manner, with the highest activity reported at 3.0 mg/ml.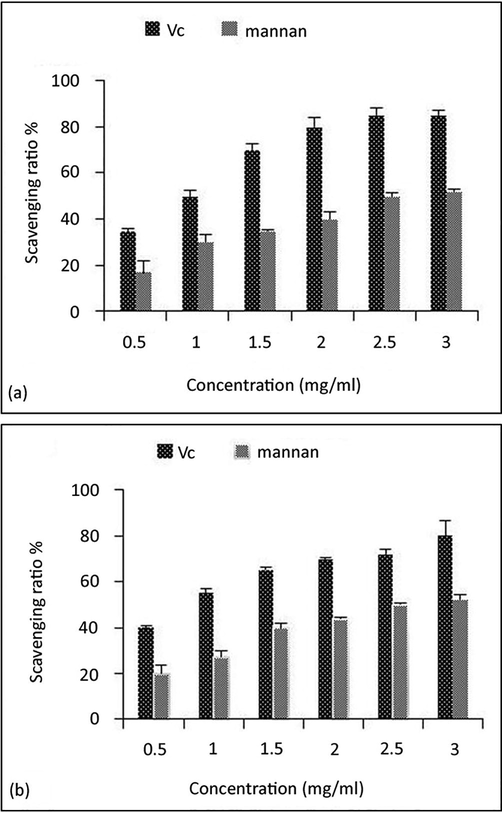
Scavenging ability of Vitamin C (Vc) and mannan to (a) hydroxyl radicals, (b) superoxide anion. Data are presented as means ± standard deviation.
3.5 The anti-proliferative capacity of mannan against human carcinoma cell lines
To further characterize mannan, the cytotoxicity of mannan against cancer cells was investigated. Mannan showed different levels of cytotoxicity against two human carcinoma cell lines, liver (HepG2) and breast (MCF7). Mannan showed concentration-dependent cytotoxicity against MCF7 and HepG2, with the maximum proliferation inhibitory effect of mannan observed at a concentration of 3 mg/ml (Fig. 4). Interestingly, mannan (3 mg/ml) induced severe morphological changes in both HepG2 and MCF7 cancer cells than the control ones as shown in Fig. 5.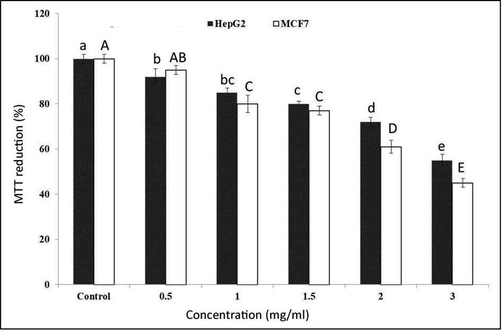
Anti-proliferative activity of mannan extracted from S. cerevisiae against HepG2 and MCF7 cell lines. Data are presented as means ± standard deviation. Different lower-case or upper case letters on bars indicate significant differences at the 0.05 probability level as revealed by the Duncan test.
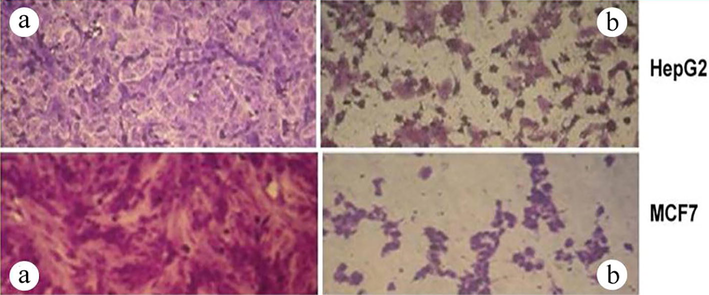
In vitro Anti-proliferative activity of mannan. Microscopic photographs of the tested cell lines: HepG2 and MCF7 were (a) control or (b) treated with mannan (3 mg/ml) for 24 hr using an inverted microscope at 40 × magnification.
4 Discussion
In this study, we isolated and identified the four yeast strains RY1, RY2, RY3, and RY4. The morphological analysis indicated that the isolates belong to the yeast Saccharomyces according to Walker and White (2005) and Abbott et al. (2015), physiologically the isolates showed their ability to produce acid and ester, and since Saccharomyces lacks urease enzyme (Navarathna et al., 2010), and all the strains studied were unable to utilize lactose. Therefore, the results of this biochemical characterization confirmed that the isolated strains belong to Saccharomyces. Thus, isolate RY1 was identified as S. cerevisiae based on morphological and molecular studies. Strain RY1 showed the highest yield of mannan. This could explain that the increase in biomass was at the expense of a decrease in the mannan component. The variability in mannan could be due to genetic differences between the tested isolates, which could influence their metabolic efficiency (Breunig et al., 2014).
In the present work, the mannan produced from RY1 was characterized using various analytical methods, such as the IR spectrum of mannan, which showed the appearance of many bands; this result is consistent with those reported by Kath and Kulicke (1999). Further identification of mannan was performed using high-field NMR spectroscopy to analyze the structure of the polysaccharides and to evaluate the ratio of the linked side chain. Following previous studies by Vinogradov et al. (1998), the composition of mannan was analyzed by 1H and 13C NMR. Kath and Kulicke (1999) showed that the resonances of the anomeric H atoms of mannan fall in the range of 4.9–5.5 ppm. The anomeric H atoms were determined following the published data for mannan, based on the results of periodic acid oxidation and Smith degradation (Liu et al., 2014). We also detected a signal at 5.02 ppm consistent with terminal α-1,2-linked mannose and for α-1,2-linked mannose with a mannose substitution in the third position consistent with the study of Kath and Kulicke (1999). Other signals were detected at 61, 68–78 ppm, which is consistent with the earlier results of Kath and Kulicke (1999).
The results showed that Manno protein contained 18 types of amino acids and the proportion of serine, threonine and aspartic acid was higher than other amino acids. This was consistent with the studies of Wang et al. (2005). The ability to scavenge hydroxyl radicals increased dramatically with the increase in mannan concentration. At a concentration of 3.0 mg/ml, the interception ratio was 50%, indicating that mannan has a significant ability to remove superoxide anions. Moreover, the higher concentration of mannan increased the ability to scavenge superoxide anions, which is in agreement with Galinari et al. (2018). Mannan showed a concentration-dependent antiproliferative capacity against MCF7 and HepG2. In addition, the cells exhibited irregular shapes, were aggregated, detached from the substrate, and floated in their culture medium. In addition, the cells exhibited normal apoptotic properties characterized by chromatin condensation near the nuclear envelope associated with cytoplasmic vacuolization.
Antioxidants are compounds that retarded the development of reactive oxygen species (ROS) that can oxidize other molecules. ROS can be formed in three phases: Initiation, propagation, and termination (Lugasi, 1997). Galinari et al. (2018) reported that mannan from various sources has antioxidant activity and can scavenge superoxide anions and hydroxyl radicals.
The proliferation inhibitory properties of polysaccharides from filamentous fungi and yeasts have also been described. For example, Gan et al. (2011) reported that treatment of a human gastric cancer cell line with polysaccharides from the filamentous fungus Pholiota dinghuensis resulted in 46.85% inhibition of cancer cells. Moreover, Yang et al. (2014) observed 92.38% and 98.79% inhibition of proliferation of HeLa and Hep-G2 cell lines, respectively, after treatment with 1.0 mg/ml polysaccharides extracted from the filamentous fungus Cordyceps militaris after 72 hr of incubation.
5 Conclusion
It could be concluded that S. cerevisiae mannan has a bio-therapeutic activity that can be used in the treatment of cancer. Moreover, ribosomal DNA nuclear gene 18S rRNA is considered a valuable genetic marker for the identification of the current isolate.
Acknowledgment
This study was supported by the Researchers Supporting Project number (RSP-2021/25), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Coevolution of yeast mannan digestion: Convergence of the civilized human diet, distal gut microbiome, and host immunity. Gut Microbes. 2015;6(5):334-339.
- [Google Scholar]
- Comparative cytotoxicity of Glycyrrhiza glabra roots from different geographical origins against immortal human keratinocyte (HaCaT), lung adenocarcinoma (A549) and liver carcinoma (HepG2) cells. Phytother. Res.. 2015;29(6):944-948.
- [Google Scholar]
- Yeast: An Experimental Organism for 21st Century Biology. Genetics. 2011;189(3):695-704.
- [Google Scholar]
- Isolation and phenotypic characterization of Lactobacillus species from various dairy products. Curr. Res. Bacteriol.. 2010;3(2):84-88.
- [Google Scholar]
- Antioxidant, antiproliferative, and immunostimulatory effects of cell wall α-d-mannan fractions from Kluyveromyces marxianus. Int. J. Biol. Macromol.. 2018;109:837-846.
- [Google Scholar]
- Production, preliminary characterization and antitumor activity in vitro of polysaccharides from the mycelium of Pholiota dinghuensis. Carbohydr. Polymers. 2011;84(3):997-1003.
- [Google Scholar]
- Extraction and deproteinization of mannan oligosaccharides. Zeitschrift für Naturforschung C. 2010;65(5–6):387-390.
- [Google Scholar]
- Polymer analytical characterization of glucan and mannan from yeast Saccharomyces cerevisiae. Die Angewandte Makromolekulare Chemie. 1999;268(1):69-80.
- [Google Scholar]
- Efficacy and safety of the probiotic Saccharomyces boulardiifor the prevention and therapy of gastrointestinal disorders. Ther. Adv. Gastroente. 2011;5(2):111-125.
- [Google Scholar]
- Cytotoxicity of the roots of Trillium govanianum against breast (MCF7), liver (HepG2), lung (A549) and urinary bladder (EJ138) carcinoma cells. Phytother. Res.. 2016;30(10):1716-1720.
- [Google Scholar]
- Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev.. 2002;26(3):239-256.
- [Google Scholar]
- A Study of Saccharomyces cerevisiae cell wall glucans. J Institute Brewing. 2009;115(2):151-158.
- [Google Scholar]
- Purification of soluble β-Glucan with immune-enhancing activity from the cell wall of yeast. Biosci. Biotechnol. Biochem.. 2001;65(4):837-841.
- [Google Scholar]
- Structural characterization and antineoplastic activity of Saccharomyces cerevisiae mannoprotein. Int. J. Food Prop.. 2014;18(2):359-371.
- [Google Scholar]
- The derivatization and antioxidant activities of yeast mannan. Int. J. Biol. Macromol.. 2018;107:755-761.
- [Google Scholar]
- Lugasi, A., 1997. Natural antioxidants chemistry, health effects, and applications. Edited by F. Shahidi. VIII and 432 pages, numerous figures and tables. AOCS Press, Champaign, Illinois, 1997. Food/Nahrung 41(5), 321–321.
- Immune response of broiler chicks fed yeast derived mannan oligosaccharides and humate against Newcastle disease. World Appl Sci J. 2012;18(6):779-785.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65(1–2):55-63.
- [Google Scholar]
- Structure of the N-linked oligosaccharides that show the complete loss of α-1,6-polymannose outer chain from och1, och1 mnn1, and och1 mnn1 alg3 mutants of Saccharomyces cerevisiae. J. Biol. Chem.. 1994;268:26338-26345.
- [Google Scholar]
- Evolutionary aspects of urea utilization by fungi. FEMS Yeast Res.. 2010;10(2):209-213.
- [Google Scholar]
- Structure of the O-polysaccharide from the lipopolysaccharide of Providencia alcalifaciens O28. Carbohydr. Res.. 2011;346(16):2638-2641.
- [Google Scholar]
- On the photocatalytic reduction of MTT tetrazolium salt on the surface of TiO2 nanoparticles: Formazan production kinetics and mechanism. J. Colloid Inter. Sci.. 2015;457:108-120.
- [Google Scholar]
- Saccharomyces boulardii stimulates intestinal immunoglobulin A immune response to Clostridium difficile toxin A in mice. Infect. Immun.. 2001;69(4):2762-2765.
- [Google Scholar]
- Determination of yeasts antimicrobial activity in milk and meat products. Adv. J. Food Sci. Technol.. 2011;3:442-445.
- [Google Scholar]
- Yeast-Derived -Glucan augments the therapeutic efficacy mediated by anti-vascular endothelial growth factor monoclonal antibody in human carcinoma xenograft models. Clin. Cancer Res.. 2008;14(4):1239-1247.
- [Google Scholar]
- Molecular Cloning: A Laboratory Manual. CSHL Press; 2001.
- Yeast Mnn9 is both a priming glycosyltransferase and an allosteric activator of mannan biosynthesis. Open Biol.. 2013;3(9):130022
- [Google Scholar]
- Structural analysis of the intact polysaccharide mannan from Saccharomyces cerevisiae yeast using 1H and 13C NMR spectroscopy at 750 MHz. Carbohydr. Res.. 1998;307:177-183.
- [Google Scholar]
- Separation, purification and structural analysis of β-glucan from oat. Food Sci. 2005;26:90-93.
- [Google Scholar]
- Structure and antioxidant activity of polysaccharide POJ-U1a extracted by ultrasound from Ophiopogon japonicus. Fitoterapia. 2012;83(8):1576-1584.
- [Google Scholar]
- Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gefland D.H., Sninsky J.J., White T.J., eds. PCR Protocols. A Guide To Methods And Applications. San Diego, California: Academic Press; 1990. p. :315-322.
- [Google Scholar]
- Optimization of fermentation process of Cordyceps militaris and antitumor activities of polysaccharides in vitro. J. Food Drug Anal.. 2014;22(4):468-476.
- [Google Scholar]







