Iron nanoparticles mitigates cadmium toxicity in Triticum aestivum; Modulation of antioxidative defense system and physiochemical characteristics
⁎Corresponding authors. anisalibot@gmail.com (Anis Ali Shah), ans.786@yahoo.com (Adnan Noor Shah)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Present study is carried out to check the effect of iron oxide nanoparticles (IONPs) on mitigating the heavy metal (HM) stress in wheat.
Method
For this purpose, IONPs were used in an attempt to ameliorate cadmium stress in wheat plants. IONPs were synthesized by using the husk of Cocos nucifera. The synthesized nanoparticles were characterized by using SEM and particle size analyzer. Wheat seedlings were treated with 10–1000 mg/L IONPs. The selected concentrations of IONPs (100, 300, and 500 mg/L) were used for next experiment of pot with soil drench irrigation. Growth parameters (shoot length, root length, number of roots, biomass, spike length, weight, and number of seeds/plant), biochemical and stress parameters (chlorophyll, sugar, proline, protein, phenolics, MDA, H2O2 and antioxidant enzyme analysis, elemental analysis) were studied.
Results
Results showed that the application of IONPs increased shoot length, root length, number of seeds, fresh weight, dry weight, spike length, and number of seeds of wheat plants by 37.5, 68, 81.25, 50, 100, 33, and 14.28% respectively with IONPs treatment. Biochemical attributes were also improved by using IONPs. Stress indicators showed that MDA and H2O2 were reduced by IONPs (by 31.91 and 30.76% respectively) whereas phenolics, proline, and antioxidant enzyme activity was increased (by 25, 40, and 55% respectively). IONPs treated plants had lesser or no cadmium content with the higher iron content of leaves
Conclusion
It showed that IONPs have strongly positive effects on mitigating cadmium stress in wheat plants.
Keywords
Antioxidant enzymes
Coconut-husk
Growth
HM stress
Nanoparticles
Yield
1 Introduction
At present, a major issue of the world population is enhanced addition of pollutants to air, water, and soil resources including various metallic pollutants. Some of them are essential but are toxic in higher conc. and some of them are toxic even in low concentrations like chromium, cadmium, etc. (Awa and Hadibarata, 2020; Ozaki et al., 2019). Cadmium is a toxic HM, which once absorbed by the plants, is entered into the food chain and then can stay there longer due to its longer biological half-life (30Years). It is toxic to human beings at concentrations that are not even phytotoxic (Elazab et al., 2021; Ismael et al., 2019), therefore, being silently bio-accumulated. Phytotoxic concentration may vary for different plants but on minimal level, it ranges from 3 to 8 ppm (Sukarjo and Wahyu, 2019). Cadmium at phyto-toxic concentrations is known to affect the photosynthetic machinery of the plants causing leaf chlorosis and growth suppression. Other than this, cadmium induces oxidative stress in plants, intensity of which depends upon Cd concentration in soil, soil pH and type of plant. This oxidative stress produces reactive oxygen species (ROS) in plant cells which in turn causes protein damage, DNA impairment, and finally causing plant death (Rasafi et al., 2021; Benakova et al., 2017).
Wheat (Triticum aestivum) is an essential cereal that fills the caloric demand of 1/3rd of the world population. The demand for wheat will increase by 70% in the coming few years. There are two main reasons for this increasing demand for wheat i.e., increased population and their increased purchasing power resulting in increased household consumption (Vitale et al., 2020). Therefore, there is a dire need to increase food crop production. Alongside, contamination of soil and irrigating water with pollutants like HMs are reducing crop production worldwide. Furthermore, due to water scarcity in underdeveloped countries, some farmers use wastewater to irrigate crops such as wheat, which increases the absorption of cadmium in wheat grains. Cadmium is absorbed by roots, taken into shoots through the vascular bundle, and stored in grains. Wheat organic materials, nutritional, and grain output are all affected by Cd levels (Rahman et al., 2021).
To stop HM absorption in crops, reliable approaches are needed. Cadmium stress from plants can be minimized by treating soils with various minerals like iron, zinc, and selenium, with other additives like biochar (Elazab et al., 2021). Iron plays the main role in the structure and function of chlorophyll, cytochrome and ferredoxin etc. Plants require iron to maintain normal growth parameters as iron performs various functions, including photosynthesis and respiration (Hochmuth, 2011). The role of iron is already well known in reducing the toxicity of HM from plants.
As cadmium competes with iron for its absorption, therefore, absorption of iron should be more efficient (Kim and Guerinot, 2007). According to prior research, nanotechnology can enhance agricultural output without affecting the environment by applying effective nutrition in the form of herbicides, nanofertilizers, or insecticides. Under metal stress conditions, nanoparticles have a potential role in increasing the yield of cereal stuff particularly wheat (Rizwan et al., 2019; Saxena et al., 2018). IONPs are smaller than conventional iron oxide particles, allowing plants to absorb more iron, thus hindering cadmium absorption.
Within the present study, IONPs were synthesized from the extract of coconut-husk, and these nanoparticles were used for the growth of wheat under Cd stress. The present study is aimed to find the effect of iron nanoparticles in mitigating the cadmium stress from wheat plants as it is hypothesized that iron in form of nanoparticles can effectively hinder the absorbance of cadmium by wheat plants.
2 Materials and methods
2.1 Chemicals and materials
The coconut-husk was purchased from a local market of Lahore, Pakistan. The cadmium (Cd) contaminated soil was collected from the industrial area and was tested for cadmium (Table 1). High-quality wheat seeds were obtained from Punjab Seed Corporation, Pakistan. All chemicals used in the present study were of analytical grade.
| Sr # | Metal | Content (mg/kg) |
|---|---|---|
| 1 | Cadmium | 7.5 |
| 2 | Chromium | 56 |
| 3 | Copper | 50 |
| 4 | Iron | 4 |
2.2 Synthesis of IONPs
The dried coconut-husk was grounded into fine powder. The plant extract was obtained at 600 W for 10 min of microwave irradiation (Sineo, MDS-6G). For the formation of IONPs, 30 ml of coconut-husk extract was mixed dropwise in aqueous solution of ferric chloride (1 mM) at room temperature, a significant color change was observed that indicated the synthesis of IONPs. This mixture was then centrifuged for 15 min at 13000 rpm and pellets were stored at 4 °C.
2.3 Characterization of nanoparticles
The formation of IONPs was verified using a UV/visible spectroscopic process. The size and shape of nanoparticles were confirmed by the particle size analyzer (Betasizer-90) and scanning electron microscopy (JSM-6480) respectively.
2.4 Selection of IONPs
This part was carried out to screen the non-toxic concentration of IONPs. 10% sodium hypochlorite solution (for 10 min) was used to sterilize wheat seeds. Seeds were washed thoroughly with distilled water. Sterilized filter papers were used to line petri plates. 10 seeds were placed per petriplate and watered with 2.5 ml of respective treatment. Treatments included control treatment (distilled water), iron chloride salt treatment (1–1000 mg/L) and IONPs treatment (1–2000 mg/L) in three replicates and were placed in an incubator. IONPs treatment with maximum germination percentage and rate were selected for the next experiment.
2.5 Pot experiment
Seeds were sown in pots filled with 6.0 kg of cadmium-contaminated soil. Three pots (5seeds/pot) as three replicates were selected. Then, these pots were treated by soil drench of IONPs (500, 300, 100 mg /L), positive control, and negative control at an interval of 15 days starting from day 0. The total experiment duration was from 20th November 2021 to 30th March 2022.
2.6 Phenotypic and yield analysis
Plants were carefully taken treatment-wise and roots were made free of entangled soil. Shoot length (SL), root length (RL), shoot weight (SW), and root weight (RW), number of roots (RN), spikes per plant, seeds per spike, and their weights were also carefully measured and noted.
2.7 Biochemical analysis
After phenotypic analysis, plants were carefully separated into roots, stems, and leaves. Furthermore, plants were also biochemically analyzed for chlorophyll and protein content by following the methods described by Awan et al. (2021).
2.8 Stress analysis
Wheat plants grown on cadmium affected soils and treated with IONPs were subjected to stress analysis which consisted of following.
2.8.1 Phenolic analysis
Testing mixture contained plant extract in acetone (1.25 ml), 0.75 ml of FC (Folin-Ciocalteu) reagent, and 1.25 ml of 7% Na2CO3. The mixture was stirred properly and the final volume was set at 3 ml with double distilled water and placed in the dark for 90 min. Then the absorbance was checked at 760 nm on a spectrophotometer. All values were plotted against the standard curve of Gallic acid.
2.8.2 Proline analysis
Proline content of the leaves from each replicate was analyzed by standard methods.
2.8.3 MDA analysis
When a plant is in stressful conditions, malondialdehyde (MDA) is a main lipid peroxidation product. By determining its level in plant tissue, plant resistance can be measured. The MDA content was calculated by following the method of Heath and Packer, (1968).
2.8.4 H2O2 analysis
The plant tissue (0.5 g) was frozen in liquid nitrogen and powdered with a pestle and mortar. 0.15 g of activated charcoal and 5 ml of 5% Trichloroacetic acid (TCA) were added during this grinding. The mixture thus obtained was centrifuged at 12000 rpm for 20 min at 4 °C. The supernatant was tested for hydrogen peroxide presence by adding 1 ml of colorimetric reagent (5 mg of 4-amino antipyrine, 2.5 mg of peroxidase, 5 mg of phenol, 25 ml of 100 mM CH3OH buffer at 5.6 pH) by taking its absorption at 505 nm on UV–Visible spectrophotometer. Standard curve of known concentration of hydrogen peroxide was used to find the final conc. of H2O2 in leaves.
2.8.5 Antioxidant enzyme analysis
For extraction and analysis of antioxidant enzymes in IONPs treated wheat plants, slightly modified methods of Elavarthi and Martin (2010) were used.
2.9 Iron and cadmium analysis
Powdered plant material (0.5 g) was added with 6, 3 and 15 ml of HNO3, HCl and H2O2 respectively. It was digested by microwaves of power level (400 W) at temperatures (130, 150, 180, and 210 °C) for 10, 5, 5, and 30 min respectively. Finally, the digested sample was filtered and analyzed for iron and cadmium content using Atomic absorption spectrometry.
2.10 Statistical analysis
Means were compared using one way ANOVA and Duncan’s multiple range test as a post hoc test to check the significance of the comparison of means at 5% level of significance. There were three replica for each treatment.
3 Results
3.1 Nanoparticles synthesis and characterization
In the current study, nanoparticles were synthesized with the aqueous extract of coconut-husk. Color change, particle size analyzer, UV–visible spectroscopy, and SEM analysis confirmed the nanoparticles synthesis. The color was changed from dark brown to black (Fig. 1a). UV–vis analysis of diluted suspension of nanoparticles showed peaks at 200–400 nm (Fig. 1a) while particle size analysis showed an average size of 60–120 nm (Fig. 1b). Particle size analysis also showed that it was almost mono dispersed, showing one intense peak. Fig. 1c is showing the SEM analysis of synthesized IONPs which confirmed that IONPs were round in shape.
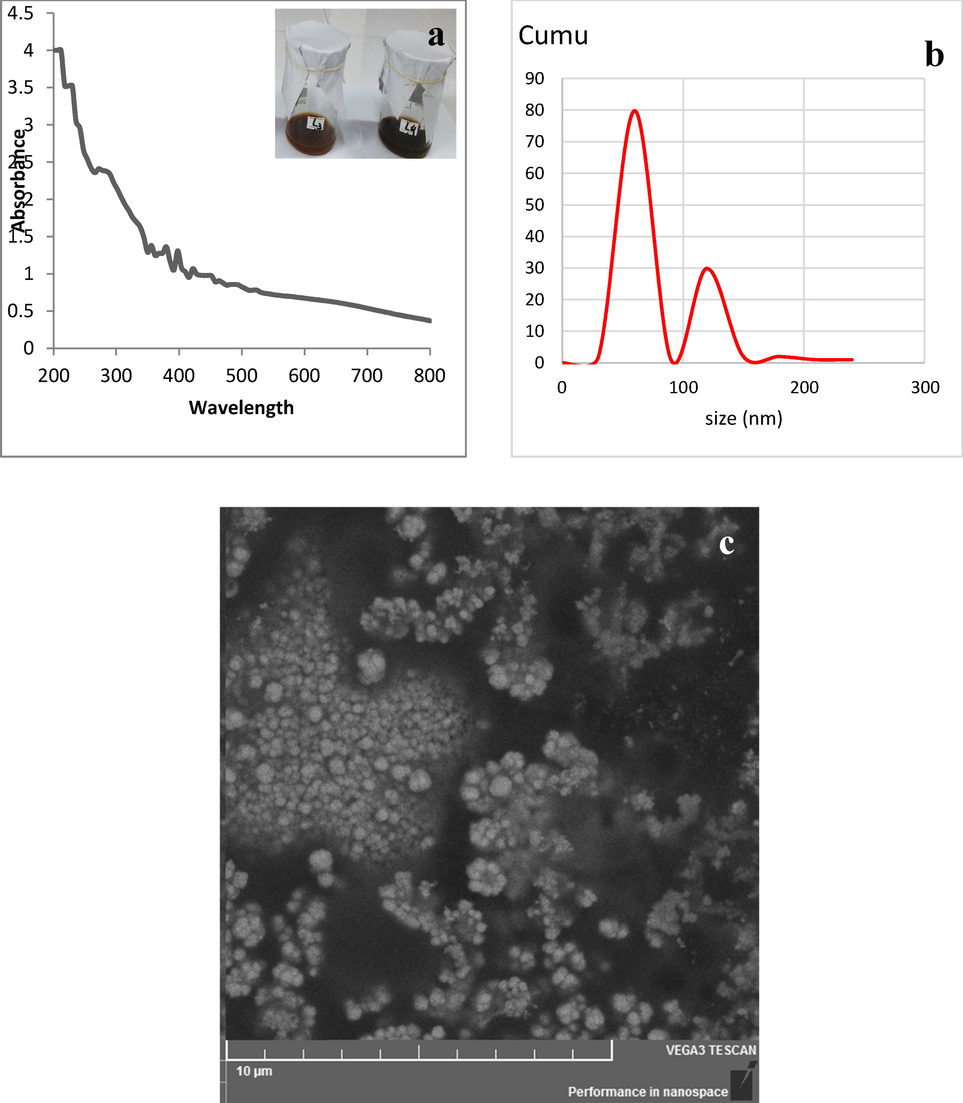
- (a) UV–Visible analysis, (b) Particle size analysis, and (c) SEM analysis of iron oxide nanoparticles.
3.2 Screening experiment/petriplate experiment
Fig. 2(a–c) is showing the screening experiment for the selection of the concentration of IONPs for pot experiment that is not toxic to plants. It is clear from the results that at lower concentrations of IONPs, the germination percentage was significantly lower. Whereas it increased with increasing doses of IONPs. IONPs at conc. range of 400–500 mg/L showed maximum germination percentage. Whereas a very higher conc. of IONPs like 1000–2000 mg/L did not germinate well or did not germinate at all. Shoot length (SL), root length (RL), and seedling weight data were also recorded (Not shown here), on basis of which IONPs conc. in the range of 100–500, mg/L were selected for pot experiment to treat cadmium stress in wheat.
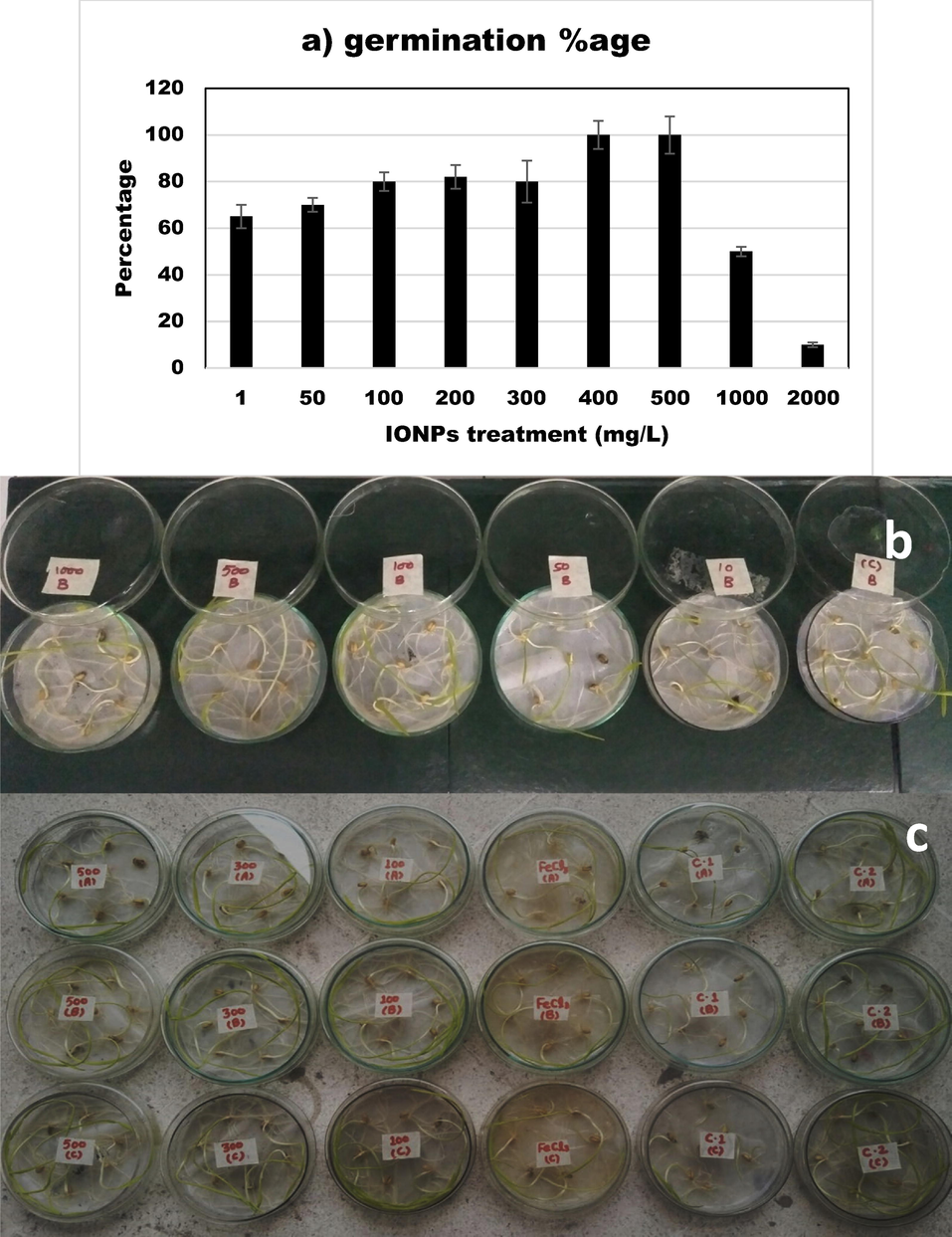
- (a–c) Effect of iron nanoparticles and iron salt on germination percentage of wheat seedlings.
3.3 Phenotypic and yield analysis
Results of growth or phenotypic analysis i.e., SL, RL, RN, FW, and DW showed that IONPs have a significantly positive effect on all these parameters under study. SL, RL, RN, FW, and DW in wheat plants increased by 37.5%, 68%, 81.25%, 50%, and 100% respectively on treatment with IONPs as compared to negative control or untreated plants (plants grown on cadmium stress but irrigated with water only). Whereas, when all above said growth parameters were compared with the positive control or with plants treated with iron salt solution (100 mg/L), it was found that SL, RL, RN, FW, and DW in wheat plants were 18.18, 15.8, 23.48, 57.89 and 25% higher in IONPs treated plants as compared to iron salt-treated plants (Figs. 3 and 4).
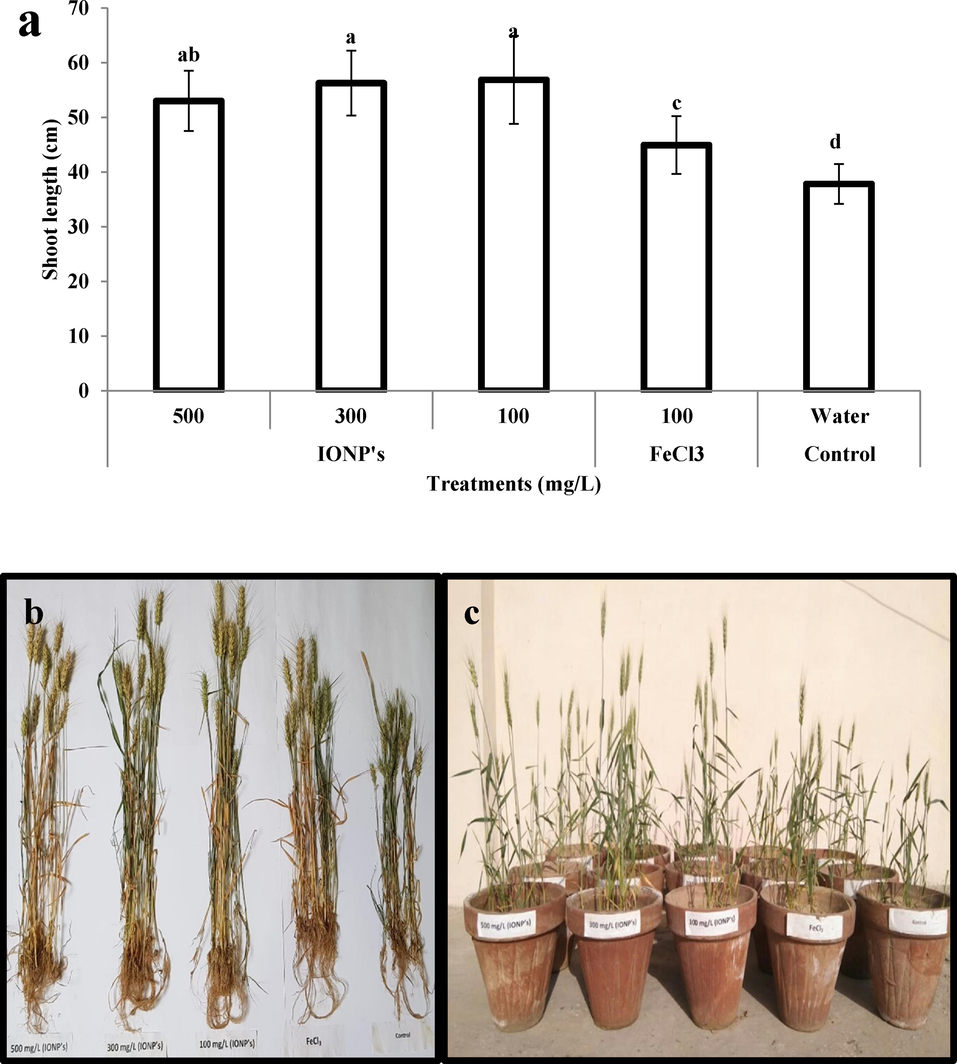
- (a–c) Effect of iron nanoparticles and iron salt on shoot length of wheat plants grown in cadmium stress.
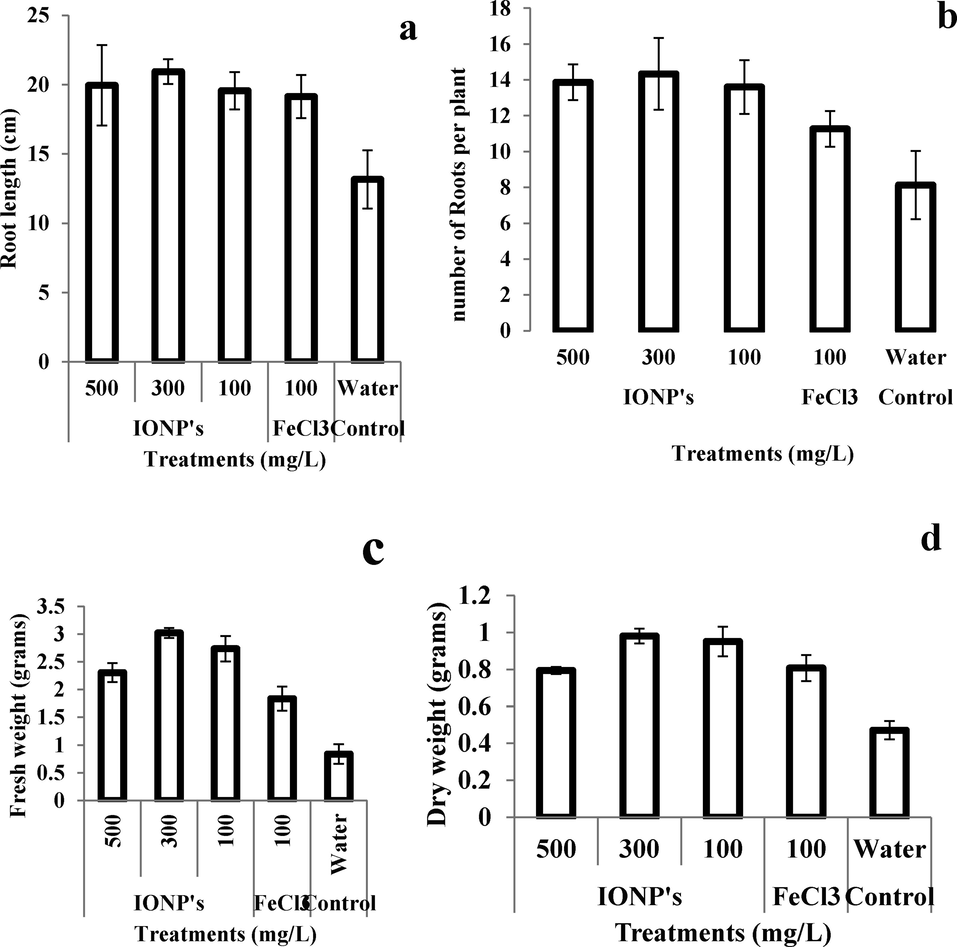
- Effect of iron nanoparticles and iron salt on (a) root length, (b) number of roots, (c) Fresh weight, and (d) dry weight per plant of wheat grown in cadmium stress.
Fig. 5(a–c) is showing the effects of IONPs on yield parameters of wheat plants grown under cadmium stress. The spike length was increased by 33% and 14.28% as compared to plants irrigated with water (negative control) and iron salt (positive control) Fig. 5a. Similarly, number and weight of seeds were also significantly increased after IONPs treatment (Fig. 5b). The number of seeds per spike was 300% higher as compared to negative control. Whereas the number of seeds per spike was 66.66% higher from negative control. Furthermore, seed weight per spike was also significantly improved (250 and 75%) as compared to negative and positive control respectively.
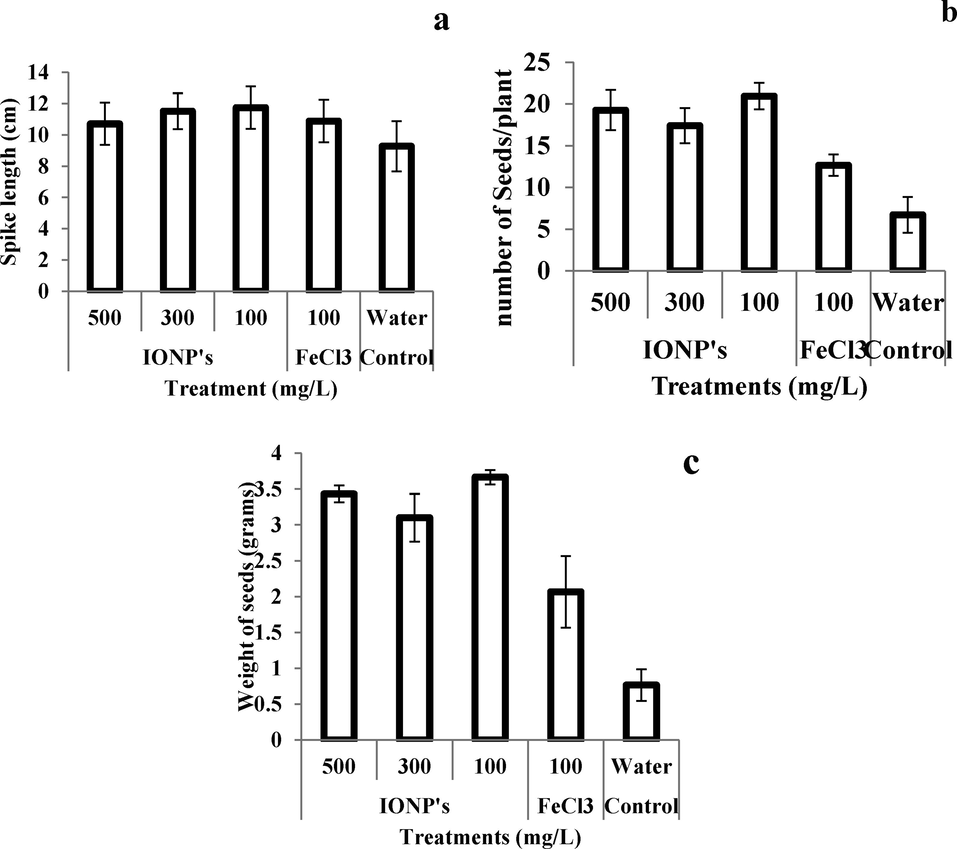
- Effect of iron nanoparticles and iron salt on (a) spike length, (b) number of seeds, and (c) weight of seeds per plant of wheat grown in cadmium stress.
3.4 Biochemical analysis
Chlorophyll content was almost 100% higher in wheat plants when irrigated with IONPs as compared to negative control (Fig. 6). The protein content of wheat leaves was 166% higher in IONPs treated plants in cadmium stress as compared to the negative control. Iron salt-treated plants had a higher content of chlorophyll and protein as compared to negative control plants but significantly lower than IONPs treated plants (21.1 and 60% respectively).
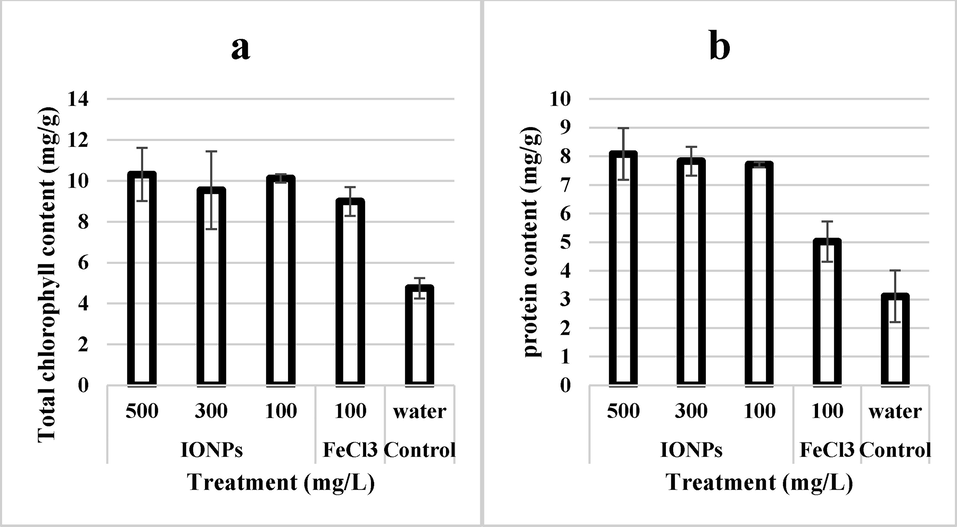
- Effect of iron nanoparticles and iron salt on (a) total chlorophyll content and (b) protein content of wheat grown in cadmium stress.
3.5 Stress analysis
Results of the present study showed that on treating the cadmium affected plants with IONPs, the stress indicators improved for wheat plants. Fig. 7a indicates that proline content is 25 and 5.2% higher in plants treated with IONPs as compared to negative and positive control treatments respectively. Similarly, total phenolics also enhanced on IONP’s treatment by 40 and 16.6% from negative and positive control respectively (Fig. 7b). It is clear from Fig. 7(c, d) that IONPs treatment of wheat plants significantly decreased the MDA and H2O2 content (31.91 and 30.76% respectively) as compared to the negative control. MDA and H2O2 contents were also significantly decreased (17.02 and 19.2%) in IONPs treated plants as compared to positive control as well.
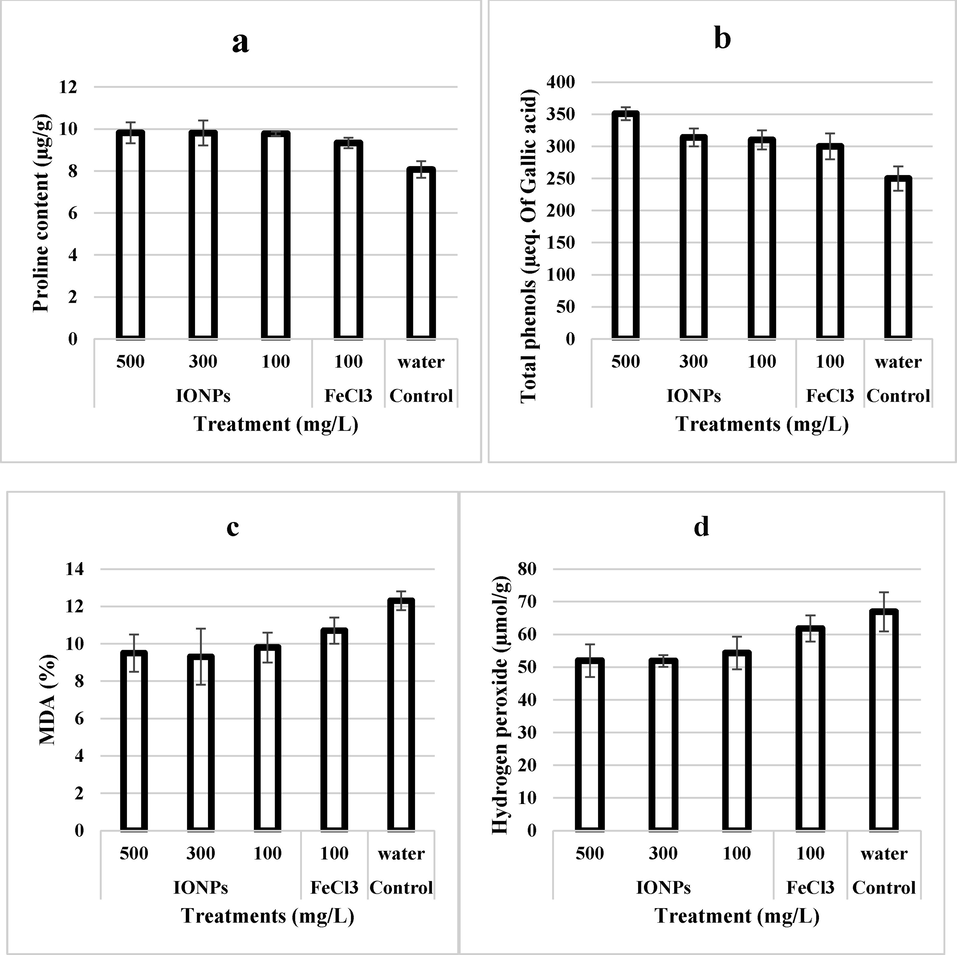
- Effect of iron nanoparticles and iron salt on (a) proline content, (b) Phenolics, (c) MDA, and (d) hydrogen peroxide content of leaves from wheat grown in cadmium stress.
In the present study, enzymatic activity of SOD, CAT and POX was studied from wheat leaves grown in cadmium stress and treated with IONPs. Results clearly showed that IONPs treatments of wheat plants enhanced activities of SOD, CAT, and POX by 55, 55.55, and 62.5% as compared to control treatment respectively (Fig. 8).
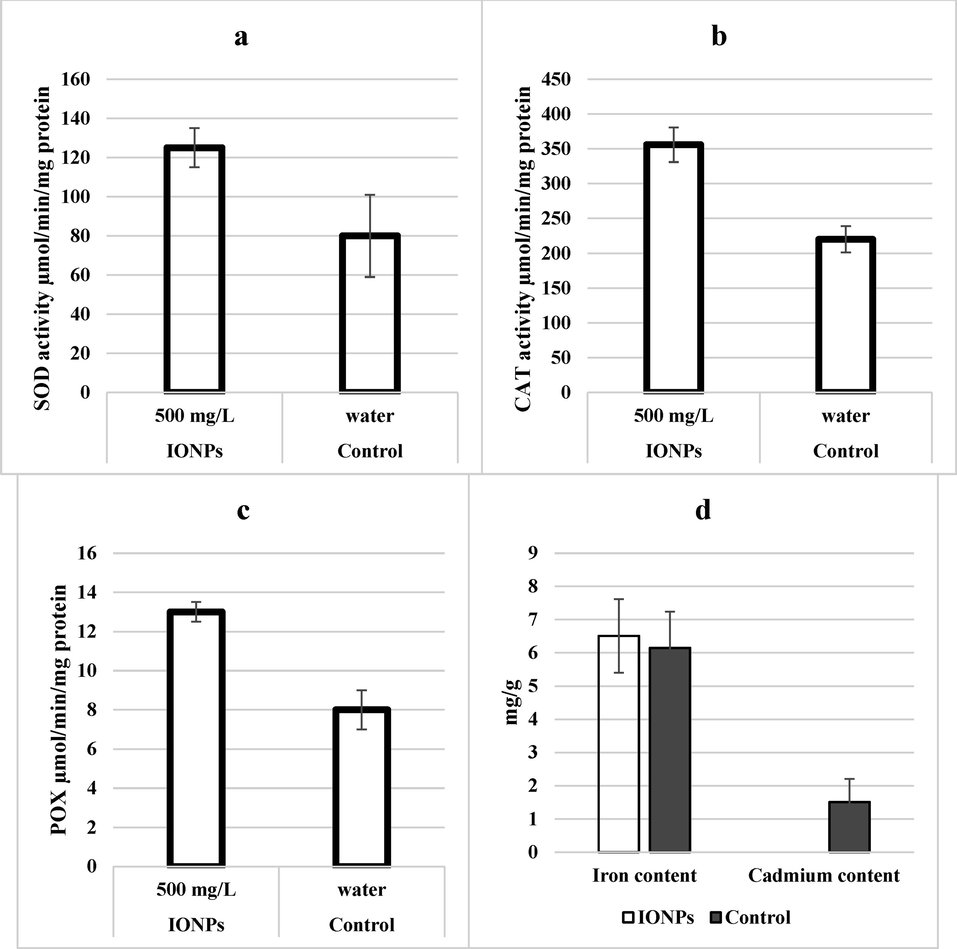
- Effect of iron nanoparticles and iron salt treatment on antioxidant enzymes (a) superoxide dismutase, (b) catalase, (c) peroxidase and (d) cadmium and iron content of leaves from wheat grown in cadmium stress.
3.6 Iron and cadmium analysis
Elemental analysis showed that iron content of IONPs treated wheat plants was 6.55% higher as compared to control whereas cadmium content was observed to be 0 mg/g of plant tissue in IONPs treated plants as compared to control treatment where it was 1.53 mg/g of plant material.
4 Discussion
Green synthesis refers to the synthesis of nanoparticles without using any hazardous chemicals and using a biological raw material like plants or microorganisms (Jadoun et al., 2021). In the present study, IONPs of 60–120 nm were formed by water extract of coconut-husk. Coconut-husk has been reported to have lignins, pectins, phenolics, and cellulose which can act as capping and reducing agents for the synthesis of nanoparticles. Anuar et al. (2020) and Sebastian et al. (2018) synthesized magnetite iron and silica nanoparticles respectively by using coconut-husk. Using coconut-husk as raw material makes this research very economical as globally coconuts are used in very large quantities and its husk is a by-product that is usually discarded. That’s why the present synthesis can be claimed green synthesis. Here we reported that the UV–visible spectrum of IONPs showed maximum absorption at 400 nm that is in accordance with the work reported by Sebastian et al. (2018).
Various HMs are part of a normal ecosystem, soil and irrigational water; and is silently being added to the food chain. They are also causing a heavy loss of food crops, thus creating the problem for food security worldwide (Rahman et al., 2021). One of the solution to this problem is nanotechnology. Objective of the present study was to check the effects of IONPs on ameliorating the cadmium stress from wheat plants. Results of growing wheat in cadmium affected soil showed that IONPs enhanced growth indicators of wheat.
As earlier discussed, iron is an essential microelement, which itself enhances the growth factors of plants (Hochmuth, 2011). The present study is showing the positive behavior of IONPs for coping with cadmium stress in wheat plants. These findings are indicating the significantly positive behavior of IONPs in ameliorating the stress of cadmium on wheat plants, even better than the iron salt itself.
Earlier research has reported that iron can mitigate cadmium stress. It has been reported that iron minimized the absorption of cadmium by rice plants (Hussain et al., 2019). IONPs when mixed with biochar can further enhance crop growth and reduce the adverse effects of HM stress (Jia et al., 2020). The administration of IONPs to wheat seedlings in a hydroponic environment enhanced shoot and root lengths as compared to controls (Sebastian et al., 2018; Konate et al., 2017). Fe nutrition is known to decrease Cd in rice. Cd absorption and transport within plants are said to be reduced when Fe concentrations are higher (Bashir et al., 2018). When iron is supplied to cadmium-affected plants, their photosynthetic activity is recovered. Fe2+ competes with Cd2+ for absorption as both have same membrane binding site at cellular membranes. Therefore, an adequate supply of iron can reduce the absorption of cadmium by plants. It is, therefore, hypothesized that toxic effects of cadmium can be reduced by the speedy uptake of iron and iron in form of NPs is more surface-active, which in turn is more effective against Cd-stress (Solti et al., 2008).
IONPs release iron for the plants which itself is a micronutrient, having various constructive roles in plant metabolism from photosynthesis to respiration. Iron is used in chlorophyll synthesis, in the structure of electron carriers like cytochromes, ferredoxins, etc. (Hochmuth, 2011). Meanwhile, it has also been reported that iron as a mineral mitigates cadmium stress in plants because they compete for availability to plants. Cadmium is easily absorbed by plant roots in iron-deficient soils, which can be ameliorated by using iron fertilizers/nutrition (Elazab et al., 2021). Iron ions are not readily available to plants as they are converted into insoluble form when supplied in inorganic form. But IONPs offer a better form of iron fertilizer to increase germination etc.
Yield parameters are affected by Cd stress as reported in Mung bean (Aqeel et al., 2021), rice (Barman et al., 2020), and Brassica napus (Jhanji et al., 2012). In the present study, yield parameters were also affected in cadmium stress but when supplied with IONPs spike length per plant, the number of seeds per spike, and weight of seeds per spike significantly enhanced as compared to both positive and negative control. Vigorous growth of wheat plants under the effect of IONPs brings a number of physiological changes like increased metabolism, cell expansions, increased auxins, and enhanced biochemical activities which ultimately give rise to increased yield (Jalal et al., 2020).
Results showed that chlorophyll content, which was significantly lower in the negative control, was enhanced significantly by treating with IONPs. NPs at some specific concentration can positively enhance the rate of photosynthesis by increasing the activity of photosystems where size, and shape of NPs play a central role. Thus, it can be concluded that conc., size, and shape of nanoparticles are the central forces to determine their final role (Rastogi et al., 2019).
Cadmium when absorbed by the plants, destroys their photosynthetic machinery. This may be related to the negative effects of Cd on the activities of photosynthetic enzymes like ribulose bis-phosphate carboxylase, phosphoenolpyruvate carboxylase, and other carboxylic enzymes (Krantev et al., 2008). But in presence of IONPs cadmium absorption is reduced, which causes the photosynthetic machinery to work again.
Cadmium once accumulated in plants, causes damage to their growth, producing oxidative stress which in turn causes damage to cellular DNA and protein. Cadmium-induced stress in plants causes an increase in the MDA content and membrane permeability disturbing cell’s ionic and nutrient balance. Cadmium also causes a decrease in the chlorophyll concentration and results in leaf chlorosis, deactivation of carbohydrates and thus causing stunted growth. When cadmium is available in the soil, it also disturbs the water balance of plants. (Dad et al., 2021).
In the present study, protein content was quite lower in only water-treated plants, which clearly shows the protein damage due to cadmium stress. But on treating plants with IONPs protein content of wheat plants was significantly improved. A further look into the results shows that MDA and hydrogen peroxide content of water treated wheat plants was higher as compared to IONPs treated plants. It is already reported that cadmium stress in plants increases MDA and H2O2 content. These two compounds are ROS and clear indicators of oxidative stress (Davey et al., 2005). These stress indicators when produced, cause damage to cellar protein content. Therefore, here in the present study, higher content of MDA and H2O2 are higher in control treatment due to Cd stress; and they caused damage to plant proteins and ultimately decreased protein content. These results can be correlated to the work of Dad et al (2021) on the radish, showing the increase of MDA and cell membrane permeability under cadmium stress.
To face various internal and external stress issues, plants have various systems including the production of phenolics, proline, and antioxidant enzymes. Therefore, in the present study, these parameters were studied for wheat plants grown in cadmium stress and treated with IONPs. Increased phenolics under treatment of IONPs thus helped the plants to fight cadmium stress. Similar results were reported by Memari-Tabrizi et al. (2021) in summer savory by silicon nanoparticles, where the increased phenolics helped the plants to fight against cadmium stress. Farhat et al. (2021) also reported a correlation between enhanced phenolics and cadmium stress in wheat plants.
In the present study, the proline content of IONPs treated plants was higher as compared to both control treatments. Cadmium stress increased proline content in plants. Furthermore, earlier reports showed that higher proline content in bread wheat helped to mitigate cadmium stress (Farooq et al., 2020). Whereas it has also been reported that even the exogenous supply, or seed priming treatment with proline, enhances the activity of antioxidant enzymes, which indirectly helped the plant to mitigate stress (Khatun et al., 2020). Proline helps plant to fight stress by membrane stabilization, removing excess ROS, and creating osmotic adjustment.
Enhanced production of antioxidant enzymes helps plants to compete the HM stress induction. In this study, the activity of SOD, CAT, and POX was significantly higher from control treatment when treated with IONPs. It is also another indicator of the potential of IONPs to fight against stress and ROS species. SOD can convert more toxic oxygen to less toxic H2O2; CAT and POX can then neutralize it. In this way, oxidative damage to DNA and protein due to ROS can be reduced and plant growth can be restored (Konate et al., 2017). Earlier Rizwan et al. (2019) reported that IONPs reduced cadmium stress in wheat by enhancing antioxidant enzyme activity and decreasing MDA content. Furthermore, green synthesized nanoparticles are found more efficient in mitigating the stress in plants as compared to chemically-synthesized IONPs. Green synthesized nanoparticles have various capping agents at their surface that absorb cadmium on their surface in soil, thus hindering their absorption (Hussain et al., 2019). This situation resulted in HM immobilization in soil. Adsorption-dependent HM tolerance was also detected during the treatment of wheat and rice plants with magnetite nanoparticles (Konate et al., 2017). Due to their small size and larger surface area, IONPs are more reactive to interact with the higher number of cadmium ions (Manzoor et al., 2021).
5 Conclusion
Adverse effects of cadmium on the growth and yield of wheat plants can be well treated with IONPs. Cadmium stress was mitigated by IONPs treatment by increasing growth parameters and amending the stress indicators. IONPs are even more efficient than iron salt to mitigate cadmium stress in plants.
Author contributions
SM: Performed experiment. SJ: Designed experiment and supervised the research work. IN: Manuscript drafting and performing research work. SJ, AB, MB: Helped to explain stress physiology. AAS, AK, EN: Manuscript drafting. AAS, ANS, MN, WFAM: Review and Drafting.
Acknowledgment
The authors would like to extend their appreciation to the Deanship of Scientific Research at King Khalid University for funding this project through the research group program under grant number (R.G.P. 2/143/43).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The physical and optical studies of crystalline silica derived from the green synthesis of coconut husk ash. Appl. Sci.. 2020;10(6):2128.
- [Google Scholar]
- Elucidating the distinct interactive impact of cadmium and nickel on growth, photosynthesis, metal-homeostasis, and yield responses of mung bean (Vigna radiata L.) varieties. Environ. Sci. Pollut. Res.. 2021;28:27376-27390.
- [Google Scholar]
- Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut.. 2020;231:47.
- [Google Scholar]
- A preliminary study of influence of zinc oxide nanoparticles on growth parameters of Brassica oleraceae var italic. J. Saudi Soc. Agric. Sci.. 2021;20:18-24.
- [Google Scholar]
- Genotypic variation among 20 rice cultivars/landraces in response to cadmium stress grown locally in West Bengal, India. Plant Physiol. Biochem.. 2020;148:193-206.
- [Google Scholar]
- Bashir, S., Zhu, J., Fu, Q., & Hu, H. (2018). Cadmium mobility, uptake and anti-oxidative response of water spinach (Ipomoea aquatic) under rice straw biochar, zeolite and rock phosphate as amendments. Chemosphere, 194, 579-587.
- Effects of Cd and Zn on physiological and anatomical properties of hydroponically grown Brassica napus plants. Environ. Sci. Pollut. Res.. 2017;24(25):20705-20716.
- [Google Scholar]
- Influence of Iron-Enriched biochar on Cd sorption, its ionic concentration and redox regulation of radish under cadmium toxicity. Agriculture. 2021;11:1.
- [CrossRef] [Google Scholar]
- High-throughput determination of malondialdehyde in plant tissues. Anal. Biochem.. 2005;347(2):201-207.
- [Google Scholar]
- Spectrophotometric assays for antioxidant enzymes in plants. In: Sunkar R.I., ed. Plant Stress Tolerance, Methods and Protocols. Springer Publishers; 2010. p. :273-280.
- [Google Scholar]
- Iron and zinc supplies mitigate cadmium toxicity in micropropagated banana (Musa spp.) Plant Cell Tissue Cult.. 2021;145:367-377.
- [Google Scholar]
- Moringa leaf extract and ascorbic acid evoke potentially beneficial antioxidants especially phenolics in wheat grown under cadmium stress. Pak. J. Bot.. 2021;53(6):2033-2040.
- [Google Scholar]
- Application of zinc and biochar help to mitigate cadmium stress in bread wheat raised from seeds with high intrinsic zinc. Chemosphere. 2020;260:127652
- [Google Scholar]
- Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys.. 1968;125(1):189-198.
- [Google Scholar]
- Iron (Fe) nutrion of plants. UF/IFAS Extension. University of Florida; 2011. p. :SL353.
- Responses of wheat (Triticum aestivum) plants grown in a Cd contaminated soil to the application of iron oxide nanoparticles. Ecotoxicol. Environ. Saf.. 2019;173:156-164.
- [Google Scholar]
- Cadmium in plants: uptake, toxicity and its interactions with selenium fertilizers. Metallomics. 2019;11:255.
- [Google Scholar]
- Green synthesis of nanoparticles using plant extracts: a review. Environ. Chem. Lett.. 2021;19:355-374.
- [Google Scholar]
- Yield and phenological indices of wheat as affected by exogenous fertilization of zinc and iron. Revista Brasileria de Ciencias Agrarias. 2020;15(1):e7730.
- [Google Scholar]
- Role of nitric oxide in cadmium-induced stress on growth, photosynthetic components and yield of Brassica napus L. J. Environ. Biol.. 2012;33:1027-1032.
- [Google Scholar]
- Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland. Sci. Total Environ.. 2020;740:139534
- [Google Scholar]
- Exogenous proline enhances antioxidant enzyme activities but does not mitigate growth inhibition by selenate stress in tobacco BY-2 cells. Biosci. Biotech. Bioch. 2020
- [CrossRef] [Google Scholar]
- Kim, S. A., & Guerinot, M. L. (2007). Mining iron: iron uptake and transport in plants. FEBS letters, 581(12), 2273-2280.
- Magnetic (Fe3O4) nanoparticles reduce heavy metals uptake and mitigate their toxicity in wheat seedling. Sustainability. 2017;9(5):790.
- [Google Scholar]
- Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol.. 2008;165:920-930.
- [Google Scholar]
- Iron oxide nanoparticles ameliorated the cadmium and salinity stresses in wheat plants, facilitating photosynthetic pigments and restricting cadmium uptake. Sci. Total Environ.. 2021;769:145221
- [Google Scholar]
- Foliar-applied silicon nanoparticles mitigate cadmium stress through physio-chemical changes to improve growth, antioxidant capacity, and essential oil profile of summer savory (Satureja hortensis L.) Plant Physiol. Biochem.. 2021;165:71-79.
- [Google Scholar]
- Immutable heavy metal pollution before and after change in industrial waste treatment procedure. Sci. Rep.. 2019;9:4499.
- [Google Scholar]
- Alleviatory effects of silicon on the morphology, physiology and antioxidative mechanisms of wheat (Triticum aestivum L.) Sci. Rep.. 2021;11:1958.
- [Google Scholar]
- Cadmium stress in plants: A critical review of the effects, mechanisms, and tolerance strategies. Crit. Rev. Environ. Sci. Technol. 2021:1-52.
- [CrossRef] [Google Scholar]
- Phytotoxic effect of silver nanoparticles in Triticum aestivum: Improper regulation of photosystem I activity as the reason for oxidative damage in the chloroplast. Photosynthetica. 2019;57:209-216.
- [Google Scholar]
- Zinc and iron oxide nanoparticles improved the plant growth and reduced the oxidative stress and cadmium concentration in wheat. Chemosphere. 2019;214:269-277.
- [Google Scholar]
- Applications of nanotechnology in agriculture. J. Nanosci. Nanoeng. Appl.. 2018;8:20-27.
- [Google Scholar]
- A green synthetic route to phenolics fabricated magnetite nanoparticles from coconut husk extract: Implications to treat metal contaminated water and heavy metal stress in Oryza sativa L. J. Clean. Prod.. 2018;174:355-366.
- [Google Scholar]
- Impact of iron supply on the kinetics of recovery of photosynthesis in Cd stressed poplar (Populus glauca) Ann. Bot.. 2008;102:771-782.
- [Google Scholar]
- The critical limit of cadmium in three types of soil texture with shallot as an indicator plant. In: International Conference on Biology and Applied Science Proceedings. 2019.
- [CrossRef] [Google Scholar]
- Economics of wheat breeding strategies: Focusing on Oklahoma hard red winter wheat. Agronomy. 2020;10(2):238.
- [Google Scholar]







