Investigations on cytokines and proteins in lactating cows with and without naturally occurring mastitis
⁎Corresponding author. sbilal@skuastkashmir.ac.in (Sheikh Bilal Ahmad),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Mastitis an inflammation of mammary gland in dairy herds is a key concern of economic losses worldwide caused by bacteria and its toxins. In this study, we investigated pro-inflammatory and anti-inflammatory cytokines (IL-2, IL-1β, IL-6, TGF-β, IL-10, TNF-α), lactoferrin and albumin, and milk composition in normal dairy cows and lactating cows with symptoms of sub-clinical and clinical mastitis. Lactating cows with clinical mastitis showed marked increase in IL-2, IL-6, IL-1β, TNF-α and a decrease in anti-inflammatory levels of TGF-β, IL-10, milk parameters fat, protein, SNF, lactose and increase in pH compared to normal lactating cows. Subclinical lactating cows showed significant alteration in TNF-α, IL-1β when compared to normal lactating cows. There was no significant difference between IL and 2 and IL-6 in normal and subclinical lactating cows. The subclinical cows also did not exhibit significant difference in TGF-β, albumin, milk fat, protein and pH when compared with normal lactating cows. Findings in the present study indicate that cytokines together with proteins lactoferrin and albumin can be considered as prospective markers in early detection of subclinical and clinical mastitis.
Keywords
Mastitis
Pro-inflammatory markers
Milk chemistry
Lactoferrin
Anti-inflammatory markers
Albumin
1 Introduction
Bovine mastitis is an inflammatory condition of mammary gland in lactating cows caused by bacteria and their toxins (Santos et al., 2003). It has huge economic implications in dairy industry (Dervishi et al., 2015). It leads to physical, chemical and pathological alterations in glandular tissue of udder and also compromises the quality of milk (Sharma, 2007). Bacterial toxins not only make milk unfit for human consumption but also increase the scope of zoonotic diseases like sore-throat, tuberculosis, Q-fever, brucellosis etc. The present treatment of bovine mastitis includes judicious or non-judicious use of antibiotics (Arshad, 1999; Yang et al., 2019; Kromker and Leimbach, 2017). While antibiotics can reduce mastitis, there has been steep rise in incidence of antibiotic resistant bacterial strains worldwide (Crisp et al., 2014). Further, mastitis enhances the risk of presence of antibiotic residues in milk because of high usage of antibiotics and their different withdrawal times. This seriously decreases milk quality due to an increase in somatic cell count (Yang et al., 2016), and enhances the enzymatic breakdown of milk protein and fat by proteolytic and lipolytic enzymes (Jaeger et al., 1994).
Based on severity, bovine mastitis has been categorized into two broad types viz. subclinical and clinical. Clinical mastitis is characterized by phenotypic changes in udder and milk, which are absent in subclinical mastitis (SCM) hence difficult to diagnose (Sharma and Sindhu, 2007). Subclinical form of the mastitis is more common than clinical mastitis, difficult to diagnose, persist longer in the herd, thus causing production losses (Sindhu et al., 2010; Ali et al., 2015). Subclinical mastitis also results in the alteration of physicochemical properties of milk (Singh et al., 2014). These changes in the milk influence the quality of by products produced from milk like cheese, yogurt and milk cream (Deeth, 2006).
There are a number of tests available for detection of subclinical mastitis. International dairy federation (IDF) in 1971 suggested a criterion based on somatic cell count (SCC) and microbiological testing of milk for detection of subclinical infection of bovine mammary glands. SCC is an extensively used indicator for milk quality and udder health but varies somewhat according to milking frequency, lactational stage, season, age, breed, level of milk production, and nutrition (Kelly et al., 2000). Bacteriological tests are costly and time consuming and are not feasible to be used as routine test under field conditions. Due to interference of physiological dynamics on SCC and high cost of milk culture for diagnosis of sub-clinical mastitis, new biomarkers are required for identification of infected quarters (Pyörälä et al., 2011). Inflammatory response plays an essential role in host tissue infection by pathogens. Neutrophils are the key players of natural defense system, and their mobilization to the location of infection decides the fate of the infection. The movement of neutrophils is activated by inflammatory mediators which are produced by tissue in response to bacterial toxins or metabolites (Zbinden et al., 2014). A battery of such inflammatory mediators includes complement fragments, vasoactive amines, arachidonic acid metabolites, and cytokines. Cytokines facilitate the alignment of leukocyte to inflammatory areas produced in response to bacterial infections (Huber et al., 1991). Several cytokines, including interleukin-1β (IL-1β), interlukin-2 (IL-2), interlukin-10 (IL-10), interlukin-6 (IL-6), tumor necrosis factor (TNF-α), transforming growth factor (TGF-β), lactoferrin, interferon gamma and granulocyte–macrophage colony-stimulating factor facilitates the buildup of leukocytes at inflammation site (Shuster et al., 1997; Springer 1994). Cytokines have role in both inflammation and leucocyte migration, therefore they can have application in the diagnosis of sub clinical mastitis (Rainard et al., 2008).
2 Materials and methods
2.1 Animal experiments and groupings
The present study was carried in cross breed of dairy Jersey cows with and without mastitis. During the course of study, 90 lactating animals of dairy cows for sampling were selected on the basis of physio-chemical and clinical examination and divided into three groups. Each group comprised of 30 animals. Group 1 comprised of animals free from mastitis which served as control. Group 2 comprised of animals with sub clinical mastitis, while group 3 comprised of animals with clinical mastitis.
2.2 Sample (collection & preparation)
Samples of milk (from all the quarters) were collected aseptically from all animals assigned in the study. California Mastitis Test (CMT) was carried out on all the milk samples, to group the animals as mastitis free animals and mastitis animals. Later on mastitis animals were again subdivided into Sub-clinical and Clinical Mastitis subgroups based on obvious clinical signs and diagnostic test. About 17 ml of milk was collected from each animal out of which 15 ml was used for diagnostic tests viz: Electric Conductivity (EC), pH, CMT and SCC and remaining 2 ml was collected in microfuge tube and stored at −80 °C for estimation of bio-chemical parameters of milk. Blood samples were collected from healthy lactating animals to obtain baseline values of cytokine levels and also from animals suffering from clinical and sub-clinical mastitis. Approximately 10 ml of blood was collected in vials (heparinized) under aseptic conditions from the jugular vein of the cows. Blood was instantly centrifuged at 3,000 rpm to separate the plasma. The separated plasma was used for cytokine and protein estimation.
2.3 Diagnosis of clinical mastitis
A comprehensive clinical examination of the diseased dairy cows was carried out, which included examination of the mammary glands and their secretion and also changes in physical characteristics of milk (e.g. watery, off color, bloody appearance and presence of flakes, pus and clots), SCC and CMT were recorded by the method of Schalm et al., 1971. The severity of the cases was assessed on the scale of CMT score and SCC of milk samples. Cases with CMT score of 2 or 3 with SCC more than 4 × 105 /ml of milk were considered positive for mastitis. Animals not suffering from clinical mastitis were screened for sub-clinical mastitis using following tests
2.4 Electrical conductivity (EC) Test:
The electric conductivity of milk was carried out by digital electric conductivity meter as described by the manufacturer (Eutech, Singapore). The change in the electrical conductivity of milk due to change in Na+ and K+ ions during mastitis was recorded with the help of a hand held digital electric conductivity meter. Electrical conductivity was determined by taking the milk in the cup of the mastitis detector up to the marked brim. After pressing the button on the detector, the reading appeared on the screen which was recorded.
2.5 Milk pH
pH was measured in all raw milk samples using digital electric pH meter.
2.6 Biochemical analysis
Milk chemistry: The chemical constituents of milk like lactose (%), fat (%), casein (%), SNF (%) were estimated from normal healthy control group as well as from clinical and sub-clinical cases of mastitis and compared with normal healthy control group. The parameters were estimated using milk analyzer (Speedy Lab, Model 4828, Astoritechnica, Italy).
Estimation of cytokines (IL-2, TNF-α, IL-1β, IL-6, TGF- β and IL-10): TNF-α and TGF-β were estimated using an ELISA kit (Bioscience - USA), IL-1β was estimated using ELISA kit (Qayee-Bio -Korea), IL-2 was estimated by ELISA kit of Krishgen Biosystems (USA), IL-6 and IL-10 were assayed by the ELISA kit of Diaclone SAS (France) from plasma as per the instructions of the manufacturer.
2.7 Estimation of lactoferrin
Lactoferrin was estimated from plasma by using an ELISA kit from de Meditec Diagnostics (Germany) as per the instructions of manufacturer.
2.8 Estimation of albumin
Acute phase protein, albumin in plasma was determined by using commercial kit based on BCG (bromocresol green).
2.9 Statistical analysis
The data from individual groups is presented as the mean ± standard error of the mean (SEM). Differences between groups were analyzed using analysis of variance (ANOVA) followed by Student-Newman-Keuls Test and minimum criterion for difference was set at P < 0.05.
3 Results
3.1 Milk chemistry
There was a significant reduction in SNF and lactose in both subclinical and clinical animals when compared with normal cows. However fat, protein and pH did not showed any significant difference between subclinical and healthy lactating cows (Table 1).
| Component (%) | Normal | Subclinical | Clinical |
|---|---|---|---|
| Fat | 4.12 ± 0.08 | 3.73 ± 0.13 ns | 2.76 ± 0.19* |
| SNF | 8.78 ± 0.13 | 7.51 ± 0.11* | 5.47 ± 0.09** |
| Protein | 3.82 ± 0.12 | 3.53 ± 0.08 ns | 2.82 ± 0.10* |
| Lactose | 4.97 ± 0.07 | 4.32 ± 0.09* | 3.57 ± 0.12** |
| pH | 6.68 ± 0.04 | 6.92 ± 0.08 ns | 7.64 ± 0.13* |
Value are expressed as mean ± SE; n = 30 animals in each group.
**P < 0.01: Significance difference from control.
*P < 0.05: Significance difference from control.
Ns indicates non-significance from control.
3.2 Inflammatory cytokine level
The clinical mastitis resulted in marked increase (P < 0.001) in IL-2, IL-6, TNF-α, and IL-1β while as subclinical mastitis resulted in non-significant increase in IL-2 and IL-6 content when compared to samples of normal animals (Fig. 1).
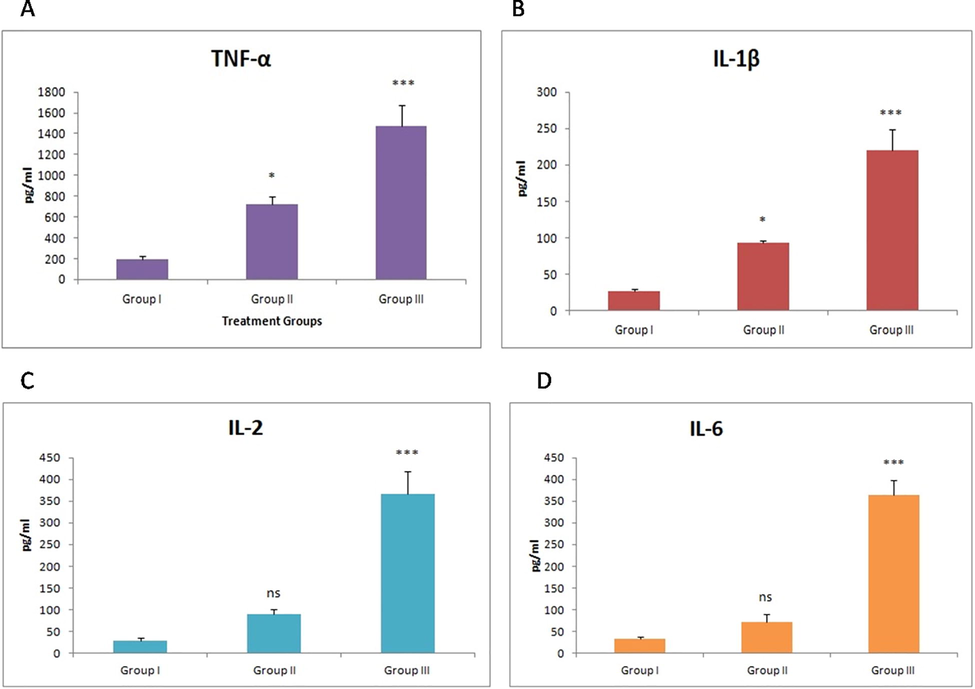
- Effect of mastitis on TNF-α, IL-1β, IL-2 and IL-6 levels. Values are expressed as mean ± SE; n = 30 animals in each group. *indicates significance from control group at P < 0.05, ***indicates significance at P < 0.001, ns indicates non-significance.
3.3 Anti-inflammatory cytokine level
The clinical mastitis resulted in marked reduction (P < 0.001) in TGF-β and IL-10 levels when compared to normal lactating cows while subclinical lactating cows did not exhibit any significant variation in TGF-β levels but showed significant variation (P < 0.01) in IL-10 in comparison to normal lactating cows (Fig. 2).
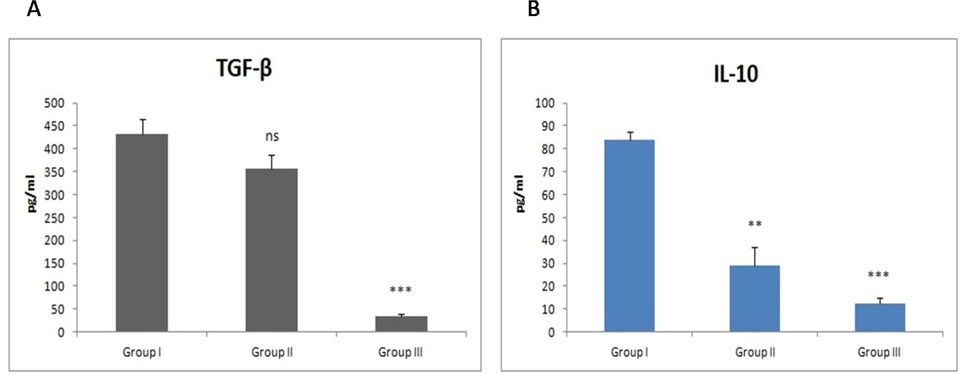
- Effect of mastitis on TGF-β and IL-10 levels. Values are expressed as mean ± SE; n = 30 animals in each group. ** indicates significance from control group at P < 0.01, ***indicates significance at P < 0.001, ns indicates non-significance.
3.4 Protein levels
Clinical mastitis resulted in significant (P < 0.01 and P < 0.05) increase in lactoferrin and protein levels against healthy lactating cows (Fig. 3). The lactating cows suffering from subclinical mastitis did not show any significant variation in lactoferrin and albumin levels.
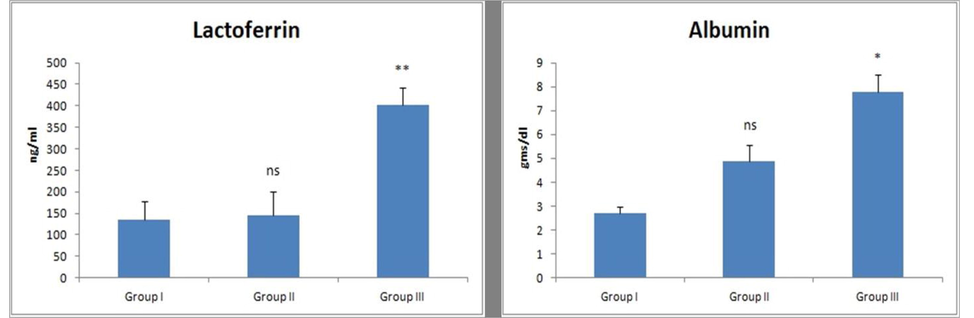
- Effect of mastitis on protein levels (lactoferrin and albumin). Values are expressed as mean ± SE; n = 30 animals in each group. * indicates significance from control group at P < 0.05, **indicates significance at P < 0.01, ns indicates non-significance.
4 Discussion
Mastitis is an inflammatory condition of the mammary gland which leads to massive economic losses in dairy sector (DeVliegher et al., 2012). Mastitis results when pathogenic microorganism penetrates through the mammary gland, mostly by disturbing the physical barriers such as the teat canal (Goldammer et al., 2004) once the barrier is disrupted it requires quick and competent defenses system to prevent the spread of pathogenic organisms and further injury to the tissue of mammary gland (Aitken et al., 2011). The response of the immune system to the attacking pathogen is a critical factor for establishing persistent and severe infection (Bannerman, 2009). Epithelial and endothelial cells play a crucial role as the first-line of resistance against infections, by activating inflammatory mediators including cytokines (Corl et al., 2008; Griesbeck-Zilch et al., 2008). In the mammary gland, the cytokines with epithelial cells identify the invading pathogens through Toll-like receptors (TLRs) (Rainard and Riollet, 2006; Griesbeck-Zilch et al., 2008; Goldammer et al., 2004). Activated TLRs promotes the expression of inflammatory cytokines and other mediators related to cell differentiation, immune response, and apoptosis (Yang et al., 2008; Cates et al., 2009).
At the beginning of infection innate immune response is dominated by up regulating the pro-inflammatory cytokines (Dego et al., 2002) that initiates the mobilization of neutrophils to the infection site in the udder (Heringstad et al., 2003, Sordillo, 2005). These pro-inflammatory cytokines have a crucial role in combating the primary infection (Bannerman et al., 2004; Shuster et al., 1993; Johnzon et al., 2018). An interesting finding in the present study was the increased levels of TNF-α, and IL-1β in subclinical mastitis suggesting their role in the early stage of the development of mastitis. In the current study, striking increase in the levels of the pro-inflammatory cytokines IL-2, IL-6, TNF-α, and IL-1β was observed in lactating cows suffering from clinical mastitis. Variations in the concentrations of these pro-inflammatory coincided with development of clinical signs of disease. In these cows’ transformation in the mammary gland tissue, increase in pH and significant decreases in fat, SNF, protein and lactose were observed as reported previously (Trigo et al., 2009). Our study demonstrated non-significant increase in IL-2 and IL-6 markers in cows suffering from subclinical mastitis which is in contradict to earlier study (Trigo et al., 2009). The non-significant alteration in TGF-β in cows with subclinical mastitis may be because of its important functioning in moderating the inflammatory response (Bannerman, 2009).
The lactoferrin an iron binding glycoprotein with bacteriostatic activity enhances mammary gland immunity and activates various molecules via several pathways (Kanyshkova et al., 2001). It exercises its bacteriostatic potential by challenging mastitis causing bacteria for available iron or through binding on the bacterial surface (Ward et al., 2002). Lactoferrin has been reported to inhibit secretion of IL-1 and TNF-α from monocytes in response to endotoxin, and also to prevent the mobilization and recruitment of neutrophils at the site of inflammation (Lonnerdal and Iyer, 1995). Lactoferrin increase corresponds to the severity of infection thereby has crucial function of combating the disease (Erdei et al., 1994). The elevated serum lactoferrin therefore, indicates that it is exerting an immune-modulating function by inhibiting the production of cytokines and subsequent inflammation. Albumin has been reported to be a suitable criterion for detection of tissue damage in mastitis. The significant elevation in albumin indicates severity of the infection and the amount of tissue damage in the lactating cows suffering from clinical mastitis (Shamay et al., 2005).
In conclusion this study provides an insight in changes in cytokines induced in subclinical and clinical mastitis in cross breed of dairy cows. Our data demonstrates significantly increased levels of plasma TNF-α, and IL-1β in subclinical mastitis, whereas IL-2, IL-6, TNF-α, and IL-1β were significantly raised in clinical mastitis. The increased levels of lactoferrin and albumin were also observed in cows with mastitis. We suggest that the role of TNF-α, and IL-1β detection in cows with subclinical mastitis should be studied in large population for their possible role as biomarkers of udder infection and mastitis.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University, Riyadh, Saudi Arabia for funding this work through Research Group Number (RG-1441-396).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Immunopathology of mastitis: insights into disease recognition and resolution. J. Mammary Gland. Biol. Neoplasia. 2011;16:291-304.
- [Google Scholar]
- Prevalence and antibiogram of bacterial pathogens from subclinical mastitis in buffaloes. Buffalo Bull.. 2015;34:41-44.
- [Google Scholar]
- Arshad, G.M., 1999. A population based active disease surveillance and drug trails of mastitis in cattle and buffaloes of District Sargodha. M. Sc. Thesis. Department: Vet. Clinical Medicine and Surgery, University Agricultural, Faisalabad, Pakistan.
- Pathogen-dependent induction of cytokines and other soluble inflammatory mediators during intra-mammary infection of dairy cows. J. Anim. Sci.. 2009;87:10-12.
- [Google Scholar]
- Innate immune response to intramammary infection with SerratiamarcescensandStreptococcusuberis. Veterinary Research 2004. 35(6): 681-700. Veterinary Research. 2004;35(6):681-700.
- [Google Scholar]
- Functional characterization of bovine TIRAP and MyD88 in mediating bacterial lipopolysaccharide - induced endothelial NF- kappa B activation and apoptosis. Comp. Immunol. Microbiol. Infect.. 2009;32:477-490.
- [Google Scholar]
- Platelet activating factor production and proinflammatory gene expression in endotoxin-challenged bovine mammary endothelial cells. J. Dairy Sci. 2008:3067-3078.
- [Google Scholar]
- Crisp, K.T., Atalla, H., Miglior, F., Mallard, B.A., 2014. Bovine mastitis: frontiers in Immunogenetics 5: 493.
- Factors involved in the early pathogenesis of bovine Staphylococcus aureus mastitis with emphasis on bacterial adhesion and invasion. Vet. Quart.. 2002;24:181e98.
- [Google Scholar]
- Innate immunity and carbohydrate metabolism alterations precede occurrence of subclinical mastitis in transition dairy cows. J. Anim. Sci. Technol.. 2015;57:46.
- [Google Scholar]
- Invited review: mastitis in dairy heifers: nature of the disease, potential impact, prevention and control. J. Dairy Sci.. 2012;95:1025-1040.
- [Google Scholar]
- Lactoferrin binds to porinsOmpF and OmpC in Escherichia coli. Infect. Immun.. 1994;62:1236-1240.
- [Google Scholar]
- Mastitis increases mammary mRNA abundance of -defensin 5, Toll-like-receptor 2 (TLR2), and TLR4 but Not TLR9 in cattle. Clin. Diagn. Lab. Immunol. 2004:174-185.
- [Google Scholar]
- Griesbeck-Zilch, Meyer, H.H., Kuhn, C.H., Schwerin, M., Wellnitz, O., 2008. Staphylococcus aureus and Escherichia coli cause deviating expression profiles of cytokines and lactoferrin messenger ribonucleic acid in mammary epithelial cells. J. Sci. 91, 2215–2224
- Selection responses for clinical mastitis and protein yield in two Norwegian dairy cattle selection experiments. J. Dairy Sci.. 2003;86:2990-2999.
- [Google Scholar]
- Regulation of trans endothelial neutrophil migration by endogenous interleukin-8. Science. 1991;254:99-102.
- [Google Scholar]
- The effect of lipopolysaccharide-induced experimental bovine mastitis on clinical parameters, inflammatory markers, and the metabolome: a kinetic approach. Front. Immunol.. 2018;9:1487.
- [CrossRef] [Google Scholar]
- Correlation between bovine milksomatic cell count and polymorphonuclear leukocyte level for samples of bulk milk and milk from individual cows. Journal of Dairy Science. 2000;83:300-304.
- [Google Scholar]
- Mastitis treatment-reduction in Antibiotic usage in dairy cows. Reprod. Domest. Anim.. 2017;3:21-29.
- [Google Scholar]
- Lactoferrin: molecular structure and biological function. Annu. Rev. Nutr.. 1995;15:93-110.
- [Google Scholar]
- Pyörälä, S., Hovinen, M., Simojoki, H., Fitzpatrick, J., Eckersall, P,D., Orro, T., 2011. Acute phase proteins in milk in naturally acquired bovine mastitis caused by different pathogens. Vet. Res. 168, 535.
- Mol. Immunol.. 2008;45:4020-4027.
- Effect to somatic cell count on proteolysis and lipolysis in pasteurized fluid milk during shelf-life storage. J. Dairy Sci.. 2003;86:2491-2503.
- [Google Scholar]
- Bovine Mastitis. Edn. Philadelphia, USA: Lea and Febiger; 1971.
- Expression of albumin in non-hepatic tissues and its synthesis by the bovine mammary gland. J. Dairy Sci.. 2005;88:569-576.
- [Google Scholar]
- Alternative approach to control intramammary infection in dairy cows- A review. Asian Journal of Animal and Veterinary Advances. 2007;2(2):50-62.
- [Google Scholar]
- Occurrence of clinical and subclinical mastitis in buffaloes in state of Haryana (India) Ital. J. Anim. Sci.. 2007;6:965-967.
- [Google Scholar]
- Complement fragment C5a and inflammatory cytokines in neutrophil recruitment during intramammary infection with Escherichia coli. Infect. Immunol. J.. 1997;65:3286-3292.
- [Google Scholar]
- Cytokine production during endotoxin-induced mastitis in lactating dairy cows. Am. J. Vet. Res. 1993;L 54:80-85.
- [Google Scholar]
- Coagulase gene based molecular detection of Staphylococcus aureus directly from mastitic milk samples of Murrah buffalo. Buffalo Bull.. 2010;29:52-59.
- [Google Scholar]
- Economic losses due to important diseases of bovines in central India. Vet. World. 2014;7:579-585.
- [Google Scholar]
- Factors affecting mammary gland immunity and mastitis susceptibility. Livestock Prod. Sci.. 2005;98:89-99.
- [Google Scholar]
- Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301-314.
- [Google Scholar]
- Trigo, G., MárciaDinis, M., Angela, F.A., Andrade, E., Costa, R., Paula, F.P., Tavares, D., 2009. Leukocyte populations and cytokine expression in the mammary gland in a mouse model of Streptococcus agalactiae mastitis. J. Med. Microbiol. 58, 951–958.
- Exogenous melatonin reduces somatic cell count of milk in Holstein cows. Sci. Rep.. 2016;7:43280.
- [Google Scholar]
- Bovine TLR2 and TLR4 properly transduce signals from Staphylococcus aureusandE. coli, but S. aureusfails to both activate NF-kappa B in mammary epithelial cells and to quickly induce TNFalpha and interleukin-8 (CXCL8) expression in the udder. Mol. Immunol. 2008:1385-1397.
- [Google Scholar]
- Yang, W.T., Ke, C.Y., Wu, W.T., Lee, R.P., Tseng, Y.H., 2019. Effective treatment of bovine mastitis with intramammary infusion of Angelica dahurica and Rheum officinale extracts. Evd. Based Compl. Alter Med., Article ID 7242705, 8 p.
- The inflammatory response of primary bovine mammary epithelial cells to Staphylococcus aureus Strains is linked to the bacterial phenotype. PLoS ONE. 2014;9:e87374.
- [Google Scholar]







