Translate this page into:
Investigation of bioactive constituents and evaluation of different in vitro antimicrobial, antioxidant, and cytotoxicity potentials of different Portulacaria afra extracts
⁎Corresponding authors at: Department of Botany, Bacha Khan University, Charsadda, Khyber Pakhtunkhwa, Pakistan. banzeer.abbasi@f.rwu.edu.pk (Banzeer Ahsan Abbasi), tmahmood@qau.edu.pk (Tariq Mahmood)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The use of medicinal plants exists before the existence of humans. Healing with herbal treatments is of utmost significance because medicinal plants contain bioactive compounds. The objective of the current investigation was to evaluate the biological potential of methanolic (PAM) and chloroform (PAC) extracts of Portulacaria afra root, leaf, seed and flower. FT-IR (Fourier Transform Infrared Spectroscopy) spectroscopy was completed to detect the different functional groups. Total antioxidant capacity (TAC), total reducing power (TRP) and 2, 2-diphenyl-1-picrylhydrazyl (DPPH) assays, were done for the determination of antioxidant potential of medicinal plant P. afra. Five different bacterial strains were used to evaluate the antibacterial potential using disc diffusion method. Cytotoxicity assay was used to test the cytotoxic potential against brine shrimps and shown the maximum cytotoxic potential with lowest IC50 value (41.41 ± 0.80 µg/mL) in PAM. Radish seed germination phytotoxicity assay was performed and revealed the highest phytotoxic potential in PAM extract. ANOVA was applied via statistics version-8.1, and experimentations were done in triplicates. Maximum DPPH scavenging potential was observed in methanol extract of P. afra (PAM). The PAM extract has the highest flavonoid concentration (30.70 mg/g). PAM demonstrated maximum total reducing power (73.46 gallic acid equivalents per gram (GAE/g). Antibacterial activity was performed against different bacterial strains and PAM shown the best antibacterial activity with largest zone of inhibition (17 mm) contrary to Pseudomonas aeruginosa. The bioactive compounds found in P. afra extracts have antibacterial, antioxidant, and cytotoxic properties, revealing their ability to induce cell death in cancerous cell lines, indicating potential anticancer agents and making them intriguing candidates for future research and the possible creation of new therapeutics. we advise the use of these extracts against biofilms as these extracts have shown significant antibacterial activity against gram negative bacteria“.
Keywords
P. afra
Phytochemical
Antioxidant
Antibacterial
Cytotoxic
Phytotoxic
1 Introduction
In order to cure a variety of disorders, 80 % of people throughout the globe still employ medicinal plants as traditional remedies. Plants have been used by humans as herbal medicines to treat various diseases and enhance health since the ancient age (Gul et al., 2022). According to literature survey, the “Holy Quran” mentions over 22 different therapeutic herbs and are recommended to cure numerous diseases (Petrovska, 2012). A vast number of people in Pakistan utilize plant-based medications for both minor and serious illnesses as Allah Almighty has provided the country with a wide variety of therapeutic plants. Out of the 6,000 plant species, 600 to 700 are employed for medicinal reasons. Plants are a component of a number of medical systems, including the Siddha, Ayurvedic, and Unnani system (Shinwari, 2010). Medicinal plants' importance stems from their active constituents, including phytochemicals, which are non-nutritive active chemicals produced through primary or secondary metabolism. These chemicals cure ailments and protect against competitors (Sahoo and Soren, 2012).
In general, the family Portulacaceae is a plant family that has 580 species in 16 to 21 genera. The single genus in the family Portulacaceae, Portulaca, has 115 species that are found all over the globe (Mottaghipisheh and Stuppner, 2021). Succulent, usually spiny, and woody family. The weedy trailing pot plant Portulaca oleracea, sometimes known as pig weed, resembles Portulacaria afra (Jacq) (Baran, 2012). Portulacaria afra is used alone or in various combinations with other particular plants to cure 11 different skin conditions, including acne, ringworm, burns, shingles, ulcers, rashes, incisions, boils, characterization, and warts (Asong et al., 2019). According to reports, the P. afra plant may treat gastrointestinal diseases such colic as well as stomachaches (Rahmatullah et al., 2013, Van Vuuren et al., 2022). Traditional treatments for inflammatory diseases and obesity also involve P. afra leaf extracts (Olaokun et al., 2017). The leaves of P. afra being used to treatment of sore throats, coughs, colds, skin lesions, rashes and diarrhea, they are also believed to have, antibacterial, and antioxidant properties. They help to accelerate wound healing (Raimondo et al., 2009, Nciki et al., 2016). Portulacaria afra is also eaten by pachyderms, which are big mammals with very thick skin. It has a diverse nutritional composition, making it suitable as nourishment for those who raise sheep, goats, ostriches, cattle, and even horses. It is known as the ecosystem engineer because of its enormous economic significance (Baran, 2012). Poaceae, often known as the grass family, is monophyletic and has 12,000 species divided into 771 grass genera and 12 subfamilies (Garcia Mozo, 2017). Pakistan has 492 species of Poaceae, classified into 158 genera, with medicinal and commercial value (Cope, 1982). Some grasses have medicinal properties that include diuretics, anti-inflammatory, hypertensive, anti-ulcer, antidepressant, antioxidant, and anti-diabetic effects (Moreira et al., 2010). Due to its novelty, high medicinal and ethnobotanical importance, P. afra was selected for the current investigation. Numerous studies have been conducted on this plant but no phytochemical and biological research has been made worldwide. The purpose of the current research was to explore the photochemical and biological potentials of the whole plant for their optimal practice.
2 Materials and methods
2.1 Plant collection and extracts preparation
The P. afra (Acc. No. 130837) was collected from Renala Khurd district Okara, Punjab, Pakistan. Herbarium of Quaid-e-Azam University, Islamabad, Pakistan, assigned accession numbers in the month of March 2019.Whole plant were picked, washed carefully, dried out in the sun and powdered into a fine dust. Methanol (polar) and chloroform (nonpolar) (30 g/300 mL each) were used for extraction process. The resulting mixture was put on incubator shaker for 10 min at 200 rpm, kept at room temperature for 7 days and filtered. The filtrates were concentrated using evaporator (R-200 Buchi, Switzerland) through evaporation and were further kept at 4 °C for future use. Entire procedure was repeated thrice to obtain 900 mL filtrate of each plant extract.
-
Methanol: (CH3OH) Source: 64,271 Dermastadt, Germany, manufacturer: DAEJUNG CHEMICALS AND MATALS CO., LTD.
-
Chloroform: (CHCl3) Source: 186 Seohaean-ro, Siheung-si, Gyeonggi-do, Korea, manufacturer: Merck chemicals. CAS NO. 67–66-3
2.2 Fourier Transform Infrared spectroscopy (FTIR)
Fourier Transform Infrared Spectroscopy was accomplished through the Maitera and Chukkol approach (Maitera and Chukkol, 2016). Plant samples (0.5 mg) were pressed onto the diamond piece of the spectrometer. The absorbance spectra were produced using wave numbers between 4000 and 400 cm1.
2.3 Analysis of antioxidant assays
2.3.1 DPPH radical scavenging assay
Each plant sample of 20 µL was combined with 180 µL of DPPH reagent. The reaction mixture was incubated at various concentrations and absorbance was noted through a microplate reader at a wavelength of 517 nm (Vishwakarma et al., 2014). The positive control was ascorbic acid, while the negative control was DMSO. The following formula was employed to determine free radical scavenging activity:
2.3.2 Determination of total antioxidant capacity
A 100 µL plant sample solution was mixed with a 900 µL reagent solution (0.6 M sulfuric acid, 4 mM ammonium molybdate, 28 mM sodium phosphate). The resulting mixture was incubated at 95° C for 90 min. The absorbance of the sample solution was recorded at 630 nm with a microplate reader. Calibration curves were created using various concentrations of ascorbic acid and 100 µL DMSO was used as a blank. As a result, TAC was clearly represented as an ascorbic acid equivalent mg/g plant sample. The results were stated as AAE by via ascorbic acid calibration curve (y = 0.0 001x + 0.3365) (Uchoaet al., 2015).
2.3.3 Total reducing power assay
The total reducing power assay was evaluated by dissolving 100 µL stock solution in 250 µL solution (1 % potassium ferricyanide solution mixing in 200 µL 0.2 M phosphate buffer) and incubating the mixture at 50° C for 20 min. In the next step, 200 µL trichloroacetic acid was added and then mixture was centrifuged at 3000 rpm for 10 min. The supernatant layer (150 μL) was removed and mixed with 50 μL of 0.1 μL sodium chloride solution to acidify the reaction mixture. Gallic acid was adopted as a positive control and the results were clearly shown as µg ascorbic acid equivalent mg / g plant extract (Ravisankar et al., 2014).
2.4 Antibacterial activity
Antimicrobial activity was done against five ATCC (American Type Culture Collection) bacterial strains S. aureus (ATCC 66332593), (P. aeruginosa (ATCC 27853), E. coli (ATCC 25922), L. monocytogenes (ATCC 13932) and S. enterica (ATCC 14028) and three MDR (multidrug resistant) bacterial strains (P. aeruginosa, Klebsiella pneumoniae and S. aureus). The bacterial strains were taken from Department of Microbiology, Quaid-e-Azam University, Islamabad. Nutrient agar media (28 g) was mixed in 1 L of distilled water, and the pH was set to 6.5. By using Whatman's filter paper # 1, spherical discs of approximately 6 mm size were made. The standard drug (2.5 mL) and sample solution (5 mL) were loaded onto a filter paper discs, dried, and placed on petri plates having bacterial inoculums and nutritional agar medium. All plates were incubated at 37 °C for 24 h. After the time limit was reached, the transparent zones of inhibition were accurately measured with a Vernier caliper, and the minimum MIC was calculated using broth microdilution method (Moonmun et al., 2017). Calculation of the Activity Index (AI) and Minimum Inhibitory Concentration (MIC). The MIC and AI for bacterial activity were computed. However, the following formula was employed to determine the activity index:
2.5 Cytotoxicity assay
Hatching of Artemia salina eggs were carried out in a bi-partitioned hatching tray having artificial sea water. 1 L of distilled water was mixed with 3.8 g of sea salt. Eggs were hatched after incubation period of 1–2 days. Total 10 nauplii transfer into each glass vials, in which evaporated plant material with 5 mL of sea water added. Dead nauplii were counted in all vials after incubation period of 24 h and percentage mortality rate was compared with positive control and LC50 values were noted after three repetitions of the experiment. Nauplii, sea water and vincristine sulphate in vials were taken as a positive control, whereas, as a negative control, methanol along with nauplii and sea water were used (Olowa and Nuñeza, 2013).
2.6 Phytotoxicity assay
In phytotoxicity assay, sterile filter paper (Whatman filter paper # 1) were placed in autoclaved Petri plates. After pouring the sample solution, it was permitted to vaporize then 5 mL of distilled water was poured. Radish seeds were sterilized in 1 % mercury chloride solution for 50–60 s, 15–20 seeds were placed then wrapped with parafilm. At 25 °C, petri plates were incubated. The % seed germination was calculated after 5 days, and the root length was carefully noted. Calculations were done after experiment was repeated three times (Ramalakshmi and Muthuchelian, 2013).
2.7 Statistics
The experiments were completed in triplicates and Microsoft excel version 2016 was used to estimate mean ± standard deviation. The results of the phytochemical analysis, antioxidant analysis, and biological assessment were analyzed using ANOVA in Statistical version 8.1, and the significance level was calculated using LSD. Probit analysis program devised by Finney, 1952 was used to evaluate the LC50 value while Graph prism version 5.01 was used to estimate IC50 value.
3 Results
The FTIR spectroscopy, antioxidant assays, DPPH radical scavenging assay, TAC, TRP, Antibacterial Activity, cytotoxicity and phytotoxicity assay of P. afra was evaluated at six different dilutions (1000 µg/mL to 31.25 µg/mL) for both methanol and chloroform extracts. All the results are expressed as mean ± SD (n = 3), with each letter (A-P, A-E, A-H, A-I, A-K, A-G, A-L, A-C, A-D) in the figures denoting the degree of significance at P < 0.05 (LSD).
3.1 Fourier Transform Infrared spectroscopy (FT-IR)
In PAM extract, various functional groups including N–H bond (1˚ amines), C-Cl stretch (alkyl halides), O–H stretch (alcohols, phenols), C–H stretch (alkanes), C–N stretch (aromatic amines), C–H rock (alkanes), C-N stretch (aliphatic amines) and C–Br stretch (alkyl halides) functional groups (Fig. 1A). PAC analysis showed the presence of O–H bond (alcohols, phenols), C–H rock (alkanes), C–H stretch (alkanes), C–H wag (CH2X) (alkyl halides), C = O stretch (α, β unsaturated esters), –C = C– stretch (alkenes), C–N stretch (aliphatic amines), C–H bend (alkanes) and C–Br stretch (alkyl halides) functional groups (Fig. 1B). All these functional groups showed the occurrence of particular class of complex molecules such as proteins, lipids and carbohydrates in the extracts.
FTIR analysis of: (a) methanol extract of P. afra; (PAM); (b) chloroform extract of P. afra (PAC).
3.2 Antioxidant assays
In order to scavenge the ROS, plant possess natural antioxidant potential to protect the cells otherwise ROS will destroy the cell functioning. Considering the above stated facts, the antioxidant analysis such as DPPH assay was done in order to discover the antioxidant potential of the selected plant.
3.3 DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging assay
DPPH potential of P. afra was analyzed at six different dilutions (1000 µg/mL to 31.25 µg/mL). Portulacaria afra exhibited IC50 value of 30.23 ± 0.49 µg/mL with DPPH scavenging activity ranged from 46.40 ± 0.54 µg/mL to 94.39 ± 0.31 µg/mL. PAC showed the IC50 value of 34.42 ± 0.61 µg/mL with minimum DPPH scavenging activity ranged from 45.46 ± 0.24 µg/mL to 90.60 ± 0.33 µg/mL µg/mL (Fig. 2) (Table 1). Ascorbic acid was taken as standard which depicted significant IC50 value (24.78 ± 1.13 µg/mL). Key:PAM: P. afra methanol extract; PAC: P. afra chloroform extract.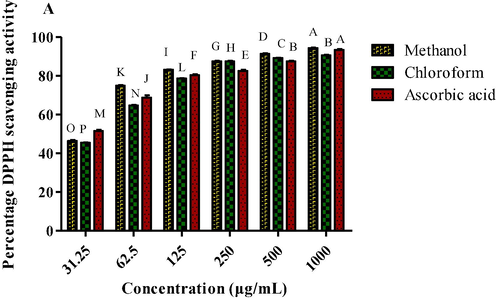
DPPH radical scavenging activity of methanol and chloroform extracts of P. afra at various dilutions.
Sr. No.
Plant Samples
IC50 (µg/mL)
1
PAM
30.23 ± 0.49C
2
PAC
34.42 ± 0.61A
5
Ascorbic acid
24.78 ± 1.13E
3.4 Total antioxidant capacity
The total antioxidant capacity of P. afra was evaluated at six different dilutions (1000 µg/mL to 31.25 µg/mL) for both methanol and chloroform extracts. PAM also showed antioxidant capacity (16.45 ± 1.25 AAE/g to 72.61 ± 2.02 AAE/g) and PAC revealed minimum TAC value (22.78 ± 1.25 AAE/g to 32.86 ± 0.62 AAE/g) as compared to other extracts (Fig. 3). TAC was evaluated employing phosphomolybdate assay and demonstrated as ascorbic acid equivalent mg/g of sample.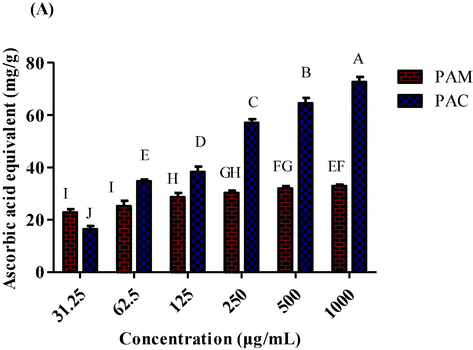
TAC of methanol and chloroform extracts of P. afra at various dilutions.
3.5 Total reducing power (TRP) assay
The total reducing power of P. afra was evaluated at six different dilutions (1000 µg/mL to 31.25 µg/mL) for both methanol and chloroform extracts. A higher ferric reducing capacity was indicated by higher absorbance of the extracts. Maximum reducing potential was revealed by PAM (73.46 ± 1.71 GAE/g to 48.95 ± 0.21 GAE/g) while, PAC showed the minimum TRP values (48.93 ± 0.965 – 26.71 ± 0.82 GAE/g) (Fig. 4).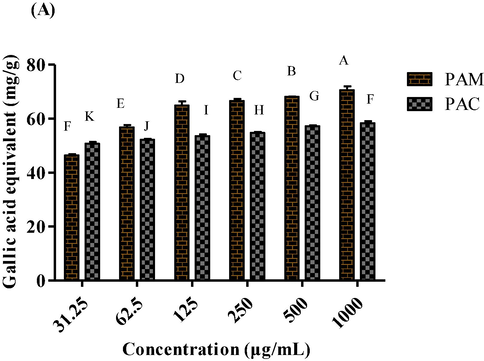
TRP of methanol and chloroform extracts of P. afra at various dilutions.
3.6 Antibacterial activity
The antibacterial activity of P. afra was assessed at five different dilutions (1000 µg/mL to 62.5 µg/mL) of methanol and chloroform extract using the disc diffusion method. Four ATCC strains (L. monocytogenes, P. aeruginosa, E. coli and S. aureus) and one MDR strain (K. pneumoniae) were used to investigate the potential of plant extracts. Maximum zone of inhibition was observed (17 ± 1.0 mm mean value) against P. aeruginosa (ATCC). Methanol extract of P. afra showed zone of inhibition of 12.33 ± 0.57 mm (mean value) and 12 ± 0.5 mm (mean value) against S. aureus (ATCC) and K. pneumoniae (MDR) (Fig. 5). Similarly, PAM also showed significant results against all bacterial strains as it showed inhibition zone of 10.33 ± 1.15 mm (mean value) against L. monocytogenes (ATCC) and minimum ZOI (7.66 ± 1.15 mean value) against E. coli (ATCC). Oxytetracycline which was used as a positive control showed the maximum zone of inhibition of 36 mm (mean value) and 26 mm (mean value) against S. aureus (ATCC) and P. aeruginosa (ATCC).PAC showed ZOI of 9.66 ± 1.15 mm (mean value) against P. aeruginosa (ATCC). DMSO was utilized as negative control which did not exhibited any zone of inhibition and for positive oxytetracycline standard drug was taken as positive control. Minimum inhibitory concentration (MIC) of all plant extracts were observed in contrast to five different bacterial strains (Table 2).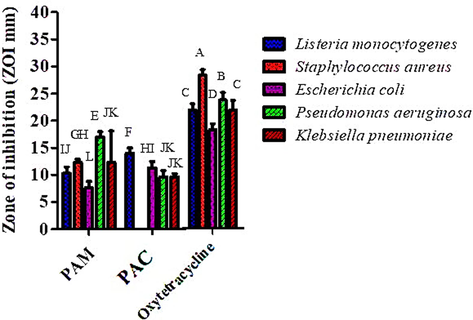
Antibacterial activity of methanol and chloroform extract of P. afra at various dilutions along with standard (oxytetracycline).
Sr No.
Bacterial Strains
PAM
MIC
(µg/mL)
PAC
MIC
(µg/mL)
Oxytetracycline
MIC
(µg/mL)
1
L. monocytogenes (ATCC)
50
50
25
2
S. aureus (ATCC)
50
–
10
3
E. coli (ATCC)
–
50
25
4
P. aeruginosa (ATCC)
25
–
50
5
K. pneumoniae (MDR)
50
–
50
3.7 Cytotoxicity assay
In order to check the cytotoxic potential of plant extracts, brine shrimps lethality assay was carried out using six different dilutions (1000 µg/mL to 31.25 µg/mL) of P. afra (Fig. 6) and the results revealed the cytotoxic potential of plant extracts was directly associated to their dilutions. Further, LC50 values of tested samples were calculated (Table 3). Among PAM and PAC extracts, methanol extract revealed maximum cytotoxic potential (Fig. 6) with lowest IC50 value (41.41 ± 0.80 µg/mL). When compared to polar solvents, chloroform extracts have a higher cytotoxic potential. As a positive control, vincristine sulphate (0.64 ± 0.04 µg/mL) was used.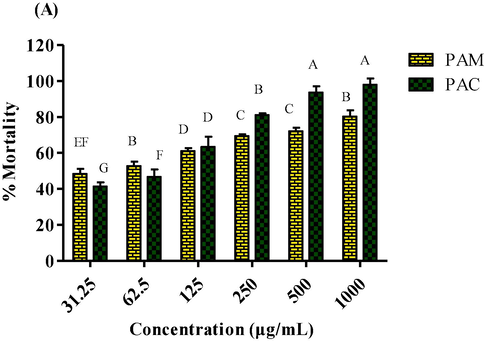
Cytotoxicity of: (a) methanol and chloroform extracts of P. afra at various dilutions.
Sr. No.
Plant Samples
LC50 (µg/mL)
1
PAM
41.41 ± 0.80C
2
PAC
58.18 ± 1.57A
5
Vincristine sulphate
0.64 ± 0.04E
3.8 Phytotoxicity assay
Repression of growth and development by wide variety of chemicals from the other plant is known as allelopathy or phytotoxicity. Phytotoxicity of P. afra was evaluated by means of radish seed germination assay at three different dilutions (1000 µg/mL, 100 µg/mL, and 10 µg/mL). In current study, minimum percentage seed germination and minimum root lengths were observed after incubation period of 5 days. It was observed that PAM revealed the best results by inhibiting the root lengths and also inhibiting the percentage seed germination more as compared to other extracts (Fig. 7A). It was observed that PAM showed the maximum percentage seed germination inhibition among extracts (Fig. 7B). Water was used as a control which showed maximum percentage seed germination and root lengths as well.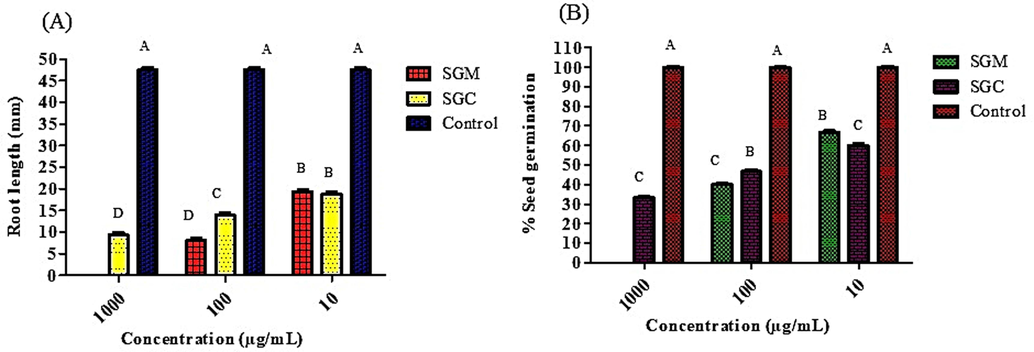
Phytotoxic effect and % germination of radish seeds of methanol and chloroform extracts of P. afra at various dilutions, (A) Phytotoxic effect, (B) percentage germination.
4 Discussion
The current study was directed in order to report the different therapeutic characters of P. afra. According to the best of our knowledge, antioxidant assays (DPPH, TAC and TRP), FTIR as well as biological activities such as antibacterial, cytotoxicity and phytotoxicity of the selected plant have been analyzed for the first time. The FTIR spectroscopy strengthens the perspective that plant have high proportion of bioactive compounds. Identified functional groups through FTIR showed that plant have various classes of phytochemicals. In the present study, P. afra extracts exhibited the presence of O–H, C-Br, C–H, C-N, C–H, N–H, C-Cl, -C = O, -C = C and C = O functional groups which are represented on graph as peaks. In biological system, phenolics and flavonoids have been reported to be associated with the antioxidant activities (Stroescu et al., 2013; Ashokkumar and Ramaswamy, 2014; Nagarajan and Kumar, 2017). For antioxidant evaluation tested plant samples were analyzed through DPPH free radical scavenging activity, TAC and TRP. In case of DPPH antioxidant activity methanol extracts showed significant potential. Methanol extract of P. afra revealed IC50 value of 30.23 ± 0.49 µg/mL and chloroform extract showed the minimum IC50 value of 34.42 ± 0.61 µg/mL. Our findings are comparable to Oliveira et al. (Oliveira et al., 2009) findings and Olaokun et al. (El-Shiekh et al., 2019) in which authors analyzed the DPPH radical scavenging activity of P. afra reporting IC50 value of 32.05 ± 3.89 µg/mL in acetone extract as well as higher percentage scavenging activity in leaves and stem of P. oleracea respectively. Previously Abas et al. (Abas et al., 2006), reported that P. oleracea had DPPH radical scavenging activity with IC50 = 0.89 ± 0.07 mg/mL and DPPH scavenging percentage inhibition of 94.8 ± 3.57 mg/mL which is comparative to our present findings of the plant belonging to same genus. Lim et al. (Lim et al., 2014) reported the P. grandiflora IC50 value ranged from 0.69 mg/mL to 2.14 mg/mL.
To determine the antioxidant capacity of all the selected plant extracts, the total antioxidant capacity (phosphomolybdenum assay) was done. Presently, it was found that P. afra methanol extract exhibited maximum TAC value of 72.61 ± 2.02 mg AAE/g and chloroform extract depicted TAC values of 32.86 ± 0.62 mg AAE/g. The TAC of a plant might be assigned to its TPC and TFC (Cai et al., 2004; Hendra et al., 2011). Cia et al. reported maximum TAC in P. oleracea (a member of same genus plant of P. afra) methanol extract which has been confirmed in the current research. Ferric reducing antioxidant power assay is one of the antioxidants determining assay. Reducing power of a plant is associated with its antioxidant capacity (Cai et al., 2004) and higher the reducing power the stronger the antioxidant activity (Guo et al., 2016). In P. afra, maximum TRP (70.54 ± 1.48 GAE mg/g) was observed in P. afra methanol extract. Hence, it can be suggested that in the food sector, selected plant extracts can be used as antioxidants as well as medicinal industries against various diseases. Present data also revealed that among both extracts, methanol extracts showed more significant antibacterial activity against five bacterial strains and P. afra showed maximum ZOI of 17 mm against P. aeruginosa. Chloroform extract of P. afra did not demonstrate any activity counter to S. aureus. Minimum ZOI was revealed by P. afra when tested against L. monocytogenes. Rahmatullah et al., (2013) and Lei et al., (2015) also noticed the antibacterial activity of P. grandiflora methanol extract and documented MIC values contrary to E. coli, S. aureus and also exhibited ZOI against different bacterial strains. Mekap et al. (2017) reported that the P. pilosa also exhibited ZOI against E. coli (14 mm), S. aureus (13 mm), P. aeruginosa (15 mm). Strong antibacterial potential of some species (i.e. P. grandiflora and P. pilosa) belonging to same genus have the similar results which have been evaluated in the present study.
Cytotoxicity is one of the in vitro biological screening tests used for analyzing extracts in drug discovery process. The brine shrimp lethality analysis was utilized for the confirmation of pesticidal compounds as well as antitumor compound (Bhosale et al., 2020). Methanol extract of chloroform extract of P. afra also revealed remarkable LC50 values showing good lethality potential indicating that the selected plant can be used for the formation of antitumor drugs. Rahmatullah et al., (2013) stated the cytotoxic potential of P. grandiflora methanol extract and P. oleracea ethanol extract revealed that Portulaca genus have significant cytotoxic potential. The brine shrimp lethality analysis is the authentic technique for the identification of bioactive compounds present in plant (Rahmatullah et al., 2013). Plant demonstrates allelopathic potential due to the presence of determined biochemicals called allelochemicals which influence the organism by different means such as survival, growth, germination and reproduction (Latif et al., 2017). To determine the phytotoxic potential of selected plant, radish seed assay was conducted. Results revealed that minimum root length and percentage seed inhibition was exhibited by methanol extracts followed by chloroform extracts. Toxicity level of medicinal plants increases by increasing their concentration (Gilani et al., 2010) correlates in present study, P. afra methanol extract showed minimum seed germination inhibition at 10 µg/mL and as concentration increases from 10 µg/mL to 1000 µg/mL the seed germination was completely inhibited due to toxicity level of plant. Previously, Shehata (Shehata, 2014) reported that seeds of P. oleracea has strong allelopathic potential confirmed in phytotoxic evaluation of selected plant. Plants which exhibit strong allelopathic potential might be used as biological control of weeds (Mahajan and Chauhan, 2013).
5 Conclusion
Research is being done on the phytochemicals that have been extracted from P. afra since it has been utilized as a source for drugs that are currently available on the market. It was determined that P. afra methanol extract has a significant potential to reduce oxidative stress based on the results of antioxidant assays. The FT-IR analysis found various classes of phytochemicals, which are in charge of P. afra biological and phytochemical activity. P. afra methanol extract has the highest level of phytotoxic potential. Further, antioxidant, antibacterial, cytotoxic and phytotoxic assays constitute scientific underpinnings and elaborated the various characteristics of medicinally characterized plant that plays a crucial part in defense mechanisms and can be effective against infections. Further research on the isolation and characterization of bioactive chemicals is still required in order to use them in the creation of drugs to treat wide a range of ailments.
Acknowledgments
This work was funded by Researchers Supporting Project number (RSPD2023R728), King Saud University, Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant and nitric oxide inhibition activities of selected Malay traditional vegetables. Food Chem.. 2006;95(4):566-573.
- [CrossRef] [Google Scholar]
- Phytochemical screening by FTIR spectroscopic analysis of leaf extracts of selected Indian medicinal plants. Int. J. Curr. Microbiol. App. Sci.. 2014;3(1):395-406.
- [Google Scholar]
- Medicinal plants used for skin-related diseases among the Batswanas in Ngaka Modiri Molema District Municipality, South Africa. S. Afr. J. Bot.. 2019;126:11-20.
- [Google Scholar]
- Portulacaria afra, the Elephant’s Food or Spekboom: a monograph which contains some of the areas of both knowledge and ignorance pertaining to this plant. Phoenix Bonsai. 2012;3(6):1-19.
- [CrossRef] [Google Scholar]
- Molecular docking studies, synthesis, toxicological evaluation using brine shrimp (Artemia salina L.) model and anti-inflammatory activity of some N-(substituted)-5-phenyl-1, 3, 4-thiadiazol-2-amine derivatives. Int. J. Sci. Res. Sci. Technol.. 2020;7(5):51-62.
- [Google Scholar]
- Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci.. 2004;74(17):2157-2184.
- [CrossRef] [Google Scholar]
- Nasir E., Ali S.I., eds. Flora of Pakistan. Vol 143. Karachi; 1982. p. :26-77.
- In-vitro screening of selected traditional medicinal plants for their anti-obesity and anti-oxidant activities. S. Afr. J. Bot.. 2019;123:43-50.
- [CrossRef] [Google Scholar]
- Poaceae pollen as the leading aeroallergen worldwide: a review. Allergy. 2017;72(12):1849-1858.
- [CrossRef] [Google Scholar]
- Phytotoxic studies of medicinal plant species of Pakistan. Pak. J. Bot.. 2010;42(2):987-996.
- [Google Scholar]
- Phytochemistry, biological activities and in silico molecular docking studies of Oxalis pes-caprae L. compounds against SARS-CoV-2. J. King Saud Univ.-Sci.. 2022;34(6):102136
- [CrossRef] [Google Scholar]
- Antioxidant efficacy of rosemary ethanol extract in palm oil during frying and accelerated storage. Ind. Crop. Prod.. 2016;2016(94):82-88.
- [CrossRef] [Google Scholar]
- Hendra, R., Ahmad, S., Oskoueian, E., Sukari, A., Shukor, M.Y., 2011. Antioxidant, anti-inflammatory and cytotoxicity of Phaleria macrocarpa (Boerl.) Scheff fruit. BMC Complement. Altern. Med. 11 ( 1 ), 110. http://www.biomedcentral.com/1472-6882/11/1-10.
- Allelopathy and the Role of Allelochemicals in Plant Defence. 2017;82(5):19-54.
- Separation and identification of four new compounds with antibacterial activity from Portulaca oleracea L. Molecules. 2015;20(9):16375-16387.
- [CrossRef] [Google Scholar]
- Antioxidant activity and total phenolic content of different varieties of Portulaca grandiflora. Int. J. Phytopharm.. 2014;4(1):01-05.
- [Google Scholar]
- The role of cultivars in managing weeds in dry-seeded rice production systems. Crop. Prot.. 2013;49:52-57.
- [CrossRef] [Google Scholar]
- Phytochemical and Fourier Transform Infrared Spectroscopy analysis of Faidherbia Albida (Del) as a preservative agent. World J. Res. Rev.. 2016;3(3):25-29.
- [Google Scholar]
- Phytochemical and pharmacological profile of Portulaca pilosa Linn.: a review. J. Environ. Life Sci.. 2017;2(2):46-51.
- [Google Scholar]
- Quantitative phytochemical estimation and evaluation of antioxidant and antibacterial activity of methanol and ethanol extracts of heliconiarostrata. Indian J. Pharm. Sci.. 2017;79(1):79-90.
- [Google Scholar]
- Chemical composition and cardiovascular effects induced by the essential oil of Cymbopogon citratus DC. Stapf, Poaceae, in rats. Rev. Bras.. 2010;20(6):904-909.
- [CrossRef] [Google Scholar]
- A comprehensive review on chemotaxonomic and phytochemical aspects of homoisoflavonoids, as rare flavonoid derivatives. Int. J. Mol. Sci.. 2021;22(5):2735.
- [Google Scholar]
- Fourier transform infrared spectroscopy analysis of garlic (Allium) Int. J. Zool. Stud.. 2017;2(6):11-14.
- [Google Scholar]
- Plants used to treat skin diseases in northern Maputaland, South Africa: antimicrobial activity and in vitro permeability studies. Pharm. Biol.. 2016;54(11):2420-2436.
- [CrossRef] [Google Scholar]
- Phytochemical screening, antioxidant, anti-inflammatory and glucose utilization activities of three South African plants used traditionally to treat diseases. Biol. Med.. 2017;9(412):2-20.
- [CrossRef] [Google Scholar]
- Phytochemical characterization and radical scavenging activity of Portulaca oleraceae L. leaves and stems. Microchem. J.. 2009;92(2):129-134.
- [CrossRef] [Google Scholar]
- Brine shrimp lethality assay of the ethanolic extracts of three selected species of medicinal plants from Iligan City, Philippines. Mortality. 2013;1(T2):T3.
- [Google Scholar]
- Historical review of medicinal plants’ usage. Pharmacogn. Rev.. 2012;6(11):1.
- [CrossRef] [Google Scholar]
- A comparative analysis of medicinal plants used to treat gastrointestinal disorders in two sub-districts of Greater Khulna Division, Bangladesh. Adv. Nat. Appl. Sci.. 2013;4(1):22-29.
- [Google Scholar]
- Red list of South African plants 2009. South African National Biodiversity Institute; 2009.
- Studies on cytotoxic, phytotoxic and volatile profile of the bark extract of the medicinal plant, Mallotustetracoccus (Roxb.) Kurz. Afr. J. Biotechnol.. 2013;12(43):6176-6184.
- [CrossRef] [Google Scholar]
- Antioxidant activites and phytochemical analysis of methanol extract of leaves of Hypericum hookerianum. Int. J. Pharm. Pharm. Sci.. 2014;6(4):456-460.
- [Google Scholar]
- Phytochemicals and gut microbial populations in non-ruminants. In: Dietary Phytochemicals and Microbes. Dordrecht: Springer; 2012. p. :371-389.
- [Google Scholar]
- Allelopathic potential of Portulaca oleracea L. seed extracts on germination and seedling growth of Cichoriumendivia L., Lactua sativa L., Echinochloa crus-galli L., and Brassica tournefortiiGouan. J. Exp. Biol. Agric. Sci.. 2014;2(4):388-396.
- [Google Scholar]
- Medicinal plants research in Pakistan. J. Med. Plants Res.. 2010;4(3):161-176.
- [CrossRef] [Google Scholar]
- Optimization of fatty acids extraction from Portulaca oleracea seed using response surface methodology. Ind. Crop. Prod.. 2013;43:405-411.
- [CrossRef] [Google Scholar]
- Antioxidant activity and phytochemical profile of Spondias tuberosa Arruda leaves extracts. Am. J. Plant Sci.. 2015;6(19):3038.
- [CrossRef] [Google Scholar]
- Traditionally used polyherbals in a southern African therapeutic context. J. Ethnopharmacol.. 2022;288:114977
- [Google Scholar]
- Comparative study of qualitative phytochemical screening and antioxidant activity of Mentha arvensis, Elettariacardamomum and Allium porrum. Indo. Am. J. Phram.. 2014;4:2538-2556.
- [Google Scholar]







