Translate this page into:
Intragastric administration of dahuang zhechong pill modulates TGF-β1/smad signaling pathway in murine model of experimental silicosis
⁎Corresponding authors. Wangfei896@163.com (Fei Wang), chendayi0523@sina.com (Da-Yi Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Since DHZCP had significant inhibitory effects on liver and kidney fibrosis, we hypothesize that DHZCP could also inhibit pulmonary fibrosis. Therefore, the aim of this study is to examine the effect of DHZCP on silicosis, a type of pulmonary fibrosis caused by silica dust, and its underlying mechanism.
Methods
Pulmonary fibrosis was induced by inhalation of silica (SiO2) dust in mice which were then randomly divided into 5 groups: pulmonary fibrosis model group, high-dose DHZCP group, medium-dose DHZCP group, low-dose DHZCP group and Tetrandrine group. The normal control group mice were not exposed to SiO2 dust. After 28 days of continuous intragastric administration of DHZCP, the mice were sacrificed. The histopathology of lungs, the levels of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β) and hydroxyproline (HYP) in serum. Besides, the expression of transforming growth factor‑β1 (TGF-β1), α-smooth muscle actin (α-SMA), Smad2, Smad3 and Smad7 in lung tissue were examined.
Results
The mice with silicosis was generated with an observed inflamed lung tissues and elevated inflammatory cytokines. DHZCP significantly reduced serum levels of TNF-α, IL-6, IL-1β and HYP in mice with lung fibrosis. DHZCP treatment remarkably downregulated mRNA and protein levels of TGF-β1, α-SMA, Smad2 and Smad3 in lung tissue, while increased the protein level of Smad7.
Conclusions
These results demonstrated that DHZCP could alleviate pulmonary fibrosis induced by SiO2. The anti-fibrotic effects of DHZCP are conferred by decreasing inflammation and pulmonary fibrosis, which may be related to the TGF-β1/Smad pathway.
Keywords
Silica
Dahuangzhechong pill
Inflammation
Pulmonary fibrosis
TGF-β1/Smad pathway
1 Introduction
Silicosis is one of the most common occupational respiratory diseases due to long-term inhalation of crystalline silica (Chen et al., 2018; Li et al., 2019). Once silicosis occurs, it can eventually form a systemic disease mainly with diffuse fibrosis. The disease can still develop progressively after the patients were removed from the exposure factors (Barber et al., 2019) and implicated economic and health burden globally (Chen et al., 2018). There is no effective treatment for silicosis so far. Therefore, it is important to explore effective treatment to reduce silicosis fibrosis.
Silicosis is a process that forms fibrosis from chronic inflammation attributed by TGF-β1/Smad signal transduction. The inhaled silica particles are partially engulfed by alveolar macrophages (AMs) (Li et al., 2019; Rimala et al., 2005). The activated AMs release pro-inflammatory cytokines, such as IL-1β, IL-6, and TNF-α (Guo et al., 2013; Hornung et al., 2008; Leung et al., 2012), causing a persistent inflammatory response in the lung tissues (Davis et al., 1996; Guo et al., 2013; Karampitsakos et al., 2017; Li and Kan, 2017). In addition, some non-degradable SiO2 continues to stimulate the formation of chronic inflammation, which activates downstream signaling pathways such as TGF-β. TGF-β regulates a wide range of biological activities such as cell growth, apoptosis, differentiation and immunosuppression (Yan et al., 2018). Contributed to pulmonary fibrosis (Meng et al., 2016), TGF-β chemoattracts inflammatory cells and fibroblasts through different signal pathways, induces the transformation of fibroblasts to myofibroblasts and ultimately formation of extracellular matrix (ECM) over-deposition and tissue remodeling (Huaux, 2007; Piek et al., 1999). TGF-β1 is widely recognized as the initiator of pulmonary fibrosis formation and development (Fernandez and Eickelberg, 2012; Xaubet et al., 2003). This profibrotic effect is mostly thought to be mediated through the Smad signaling pathway. TGF-β sends signals through transmembrane receptors, which triggers the phosphorylation of Smad2/3 protein, regulates the transcription of important target genes including type I and III procollagen peptides (Allison, 2014; Yin et al., 2018), promotes the synthesis of TNF-α by cells, and up-regulates the expression of α-SMA in lung tissues. Smad2 and Smad3 are two major downstream regulators of TGF-β1-mediated tissue fibrosis, and Smad7 acts as a negative regulator of the TGF-β1/Smad pathway. Feedback regulators prevent Smad3 phosphorylation, thereby inhibiting the TGF-β signaling pathway and slowing the progression of pulmonary fibrosis (Hu et al., 2018). Imbalance of TGF-β1/Smads pathway is an important pathogenic mechanism of tissue fibrosis.
Dahuang Zhechong Pills (DHZCP) is a classic ancient recipe of “Jin Gui Yao Lue”, which promotes blood circulation, moves blood stasis and nourishes blood. Previous animal experiments have demonstrated the anti-fibrotic effect of DHZCP on the liver (Cai et al., 2010; Xing et al., 2012; Zhenghua et al., 2018) and this anti-fibrotic effect may be related to TGF-β1, α-SMA, etc (Li et al., 2003). Moreover, DHZCP significantly inhibited the expression of TGF-β1 gene in activated rat hematopoietic stem cells in vitro (Cheng et al., 2010). Since the effect of DHZCP in silicosis remains unknown, the present study aimed to investigate the anti-fibrotic and anti-inflammatory effects of DHZCP as potential drug for preventing and ameliorating SiO2-induced silicosis.
2 Materials & Methods
2.1 Animals
36 male Kunming mice (15–20 g), aged 4 weeks, were obtained from the Chengdu Da Shuo Laboratory Animal Co., Ltd. All mice were maintained under standard housing conditions with 12/12 h day‑night cycle and a controlled ambient temperature (23 ± 2 °C) with 50–70% humidity. The animals were given free access to water and food. All experimental procedures were approved by the Experimental Animal Ethics Committee of Chengdu University of Traditional Chinese Medicine (Sichuan, China), and complied with the National Institute of Health Guide for the Care and Use of Laboratory Animals.
2.2 Preparation of drugs
Dahuangzhechong pill (DHZCP), consisting of Eupolyphaga, Rhei Radix Et Rhizoma, Glycyrrhizae Radix Et Rhizoma, Toxicodendri Resina, Scutellariae Radix, Tabanus, Paeoniae Radix Alba, Hirudo, Rehmanniae Radix, Holotrichia, Persicae Semen, and Armeniacae Semen Amarum, were purchased from Beijing Tong Ren Tang Co, Ltd. (lot number: Z11020002). Tetrandrine (TD) were purchased from Zhejiang Jinhua Kangenbei Biological Pharmaceutical Co., Ltd (lot number:H33022075). The suspension of DHZCP and TD were aliquoted and stored at-20 °C and shaken well before use.
2.3 Experimental design and procedures
The purity of the silica dust (<5 μm in size, RHAWN, Shanghai, China) was used in the present study was > 99%. In the first phase, all mice were randomly divided into 2 groups: silica group (n = 30) and normal control group (NC, n = 6). The 30 mice in silica group were placed in an airtight dust cabinet, and the SiO2 dust was blown in the dust cabinet by a blower. The concentration of SiO2 dust was maintained at 800 mg/m3 (Zhang et al., 2017) and the mice were exposed to SiO2 dust 6 h per day for 40 days. The NC group mice were not exposed to the dust.
After 40 days of exposure to SiO2, the 30 mice in silica group were randomly divided into five groups: silicosis model control group (MC), high-dose DHZCP group (H-DHZCP), medium-dose DHZCP group (M−DHZCP), low-dose DHZCP group (L-DHZCP) and Tetrandrine group (TD). Mice were administered intragastrically with 1.17 mg/kg (H-DHZCP), 0.585 mg/kg (M-DHZCP) or 0.390 mg/kg (L-DHZCP) of DHZCP and 0.039 mg/g of Tetrandrine for 28 days. Mice in MC group and NC group received an equivalent volume of saline. Tetrandrine, an effective drug for the treatment of silicosis, was used as positive control in this study.
Mice were weighed and euthanized at 28 days post-DHZCP administration. Blood was collected before the mice were euthanized. The lung tissues were dissected, weighed and recorded. The left lung tissues were immobilized in 4% paraformaldehyde solution and right lung tissues were preserved at − 80 °C for western blotting and quantitative real-time PCR (RT-PCR) analysis.
2.4 Determination of the pulmonary index
All mice were weighed and recorded. The lung tissues were carefully separated after dissection, rinse with 0.9% saline and remove excess liquid. The lung tissues were weighed, and the pulmonary index was calculated. The formula is as follows:
2.5 Lung histopathology
The left lung tissues were fixed in 4% paraformaldehyde solution for 48 h, embedded in paraffin and cut into 5 µm thick sections. The sections were then stained with hematoxylin and eosin (H&E) and Masson trichrome staining to evaluate the histopathological changes in the lungs under light microscope (Olympus D72, Japan).
2.6 Enzyme-linked immunosorbent assay (ELISA)
Blood was collected before the mice were euthanized. The blood was placed at room temperature for 10 min before centrifugation at 4000 rpm, 4 °C and 10 min. TNF-α, IL-6, IL-1β, and hydroxyproline (HYP) concentrations in serum were detected in duplicate using ELISA kits (Shanghai Enzyme-linked Biotechnology, China) following the manufacturers’ instructions.
2.7 Western blotting
Lung tissues of mice were lysed 10 min with RIPA lysis buffer with 1% protease inhibitor PMSF. Following centrifugation at 4 °C and 12 000 rpm for 10 min, the supernatants were collected and used for western blot analysis. The protein concentration was determined by BCA protein assay kit (Beyotime Biotechnology). The proteins were transferred onto polyvinylidene fluoride (PVDF) membranes after separated by 10% SDS-PAGE. The membranes were blocked with 5% fat-free milk for 1 h at room temperature and incubated overnight at 4 °C with the diluted primary antibodies: rabbit anti-TGF-β1 (1:1000, Servicebio), mouse anti-α-SMA (1:1000, Servicebio), rabbit anti-Smad2 (1:1000, Servicebio), rabbit anti-Smad3 (1:1000, bioss), mouse anti-Smad7 (1:200, Santa Cruz, Inc.) and GAPDH (1:5000, Abcam). In the following days, the membranes were washed 3 times in Tris-Buffered Saline Tween-20 (TBST) and incubated with secondary antibodies (1:5000, Abcam) at room temperature for 2 h. The membranes reacted with enhanced chemiluminescence (ECL) substrate and the bands were visualized by Gene Gnome chemiluminescent Imaging System (Syngene, USA). The relative intensities of the bands were analyzed by using Image J software and normalized to β-actin.
2.8 Quantitative real-time PCR (RT-PCR)
Total RNA was extracted from lung tissues by using Direct-zol™ RNA MiniPrep Kit (ZYMO RESEACH, Germany). cDNA was synthesized by using 5X All-In-One RT MasterMix (ABM). The RT-PCR was performed using EvaGreen 2X qPCR Express MasterMix (ABM) with an ABI PRISM® 7900HT (Applied Biosystems, CA). The expressions of target genes were normalized to the reference gene GAPDH. The primers used in our experiment were shown in Table 1.
Gene
Forward primer 5′-3′
Reverse primer 5′-3′
Length (bp)
TGF-β1
CTCCCGTGGCTTCTAGTGC
GCCTTAGTTTGGACAGGATCTG
133
Smad2
ATGTCGTCCATCTTGCCATTC
AACCGTCCTGTTTTCTTTAGCTT
173
Smad3
CACGCAGAACGTGAACACC
GGCAGTAGATAACGTGAGGGA
101
Smad7
GGCCGGATCTCAGGCATTC
TTGGGTATCTGGAGTAAGGAGG
153
GAPDH
AGGTCGGTGTGAACGGATTTG
TGTAGACCATGTAGTTGAGGTCA
123
2.9 Statistical analysis
Statistical calculations were evaluated by using the SPSS 25.0 software. All data were presented as Means ± SD. The difference of means among groups was performed by one-way ANOVA analysis of variance followed by LSD or Tamhane’s test. A P value < 0.05 was considered as statistically significant.
3 Results
3.1 Effects of DHZCP on pulmonary index
Pulmonary index significantly increased in the SiO2-induced pulmonary fibrosis models (P < 0.01) as compared to control group (Fig. 1). After treatment with DHZCP, the pulmonary index decreased significantly (P < 0.01). Similarly, the pulmonary index also significantly reduced after treatment with tetrandrine (TD) (P < 0.01).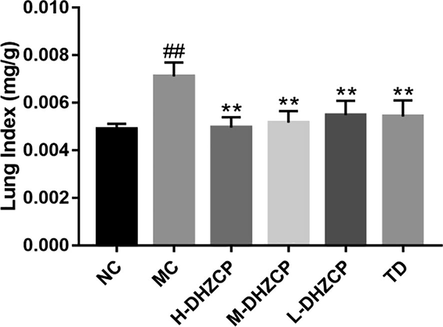
Effects of DHZCP on pulmonary index. NC: Normal control group; MC: silicosis model control group; H-DHZCP: High-dose DHZCP group (1.170 mg/g); M−DHZCP: Medium-dose DHZCP group (0.585 mg/g); L-DHZCP: Low-dose DHZCP group (0.390 mg/g); TD: Tetrandrine treated group (0.039 mg/g). Values are expressed as mean ± SD (n = 6). #P < 0.05 and ##P < 0.01 compared to NC group. *P < 0.05 and **P < 0.01 compared to MC group.
3.2 Effects of DHZCP on lung pathological changes
The alveolar septa in the lungs of mice exposed to SiO2 were significantly thickened and many inflammatory cells infiltrated into lung tissues (Fig. 2). In addition, collagen deposition accompanied by severe distorted lung structure with large fibrous area was observed in SiO2-induced silicotic mice (Fig. 2C and D). After treatment with DHZCP, infiltration of inflammatory cells and the deposition collagen in lung tissue decreased, and the lung tissue structures were less damaged as compared to MC group (Fig. 2E–L). These changes are more prominent in high dose DHZCP-treated and TD-treated mice (Fig. 2E and F and Fig. 2K and L).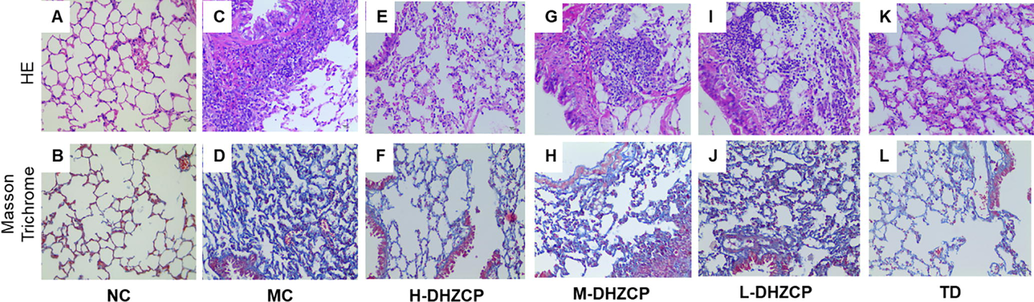
Effect of DHZCP on lung histopathology in SiO2-induced pulmonary fibrosis mice. Upper panel: H&E staining, lower panel: Masson Trichrome staining.
3.3 Effects of DHZCP on hydroxyproline (HYP) content
HYP is one of the markers of pulmonary fibrosis. MC group had higher HYP levels than that in NC group (P < 0.01) while high and medium doses of DHZCP treatment could reduce the elevated HYP levels in serum (P < 0.01) (Fig. 3). In addition, TD treatment also significantly decreased the level of HYP (P < 0.05), which was similar to that in the high dose group of DHZCP.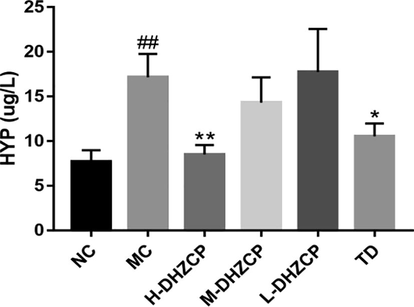
Effects of DHZCP on HYP content. Values are expressed as mean ± SD (n = 6). #P < 0.05 and ##P < 0.01 compared to NC group. *P < 0.05 and **P < 0.01 compared to MC group.
3.4 Effect of DHZCP on the levels of proinflammatory cytokines in serum
The levels of TNF-α, IL-6, and IL-1β in serum were detected by ELISA assay kits. We observed that these cytokines in serum were obviously increased after silica inhalation (Fig. 4A–C; P < 0.01). However, there was a significant reduction in the levels of these cytokines in DHZCP-treated animals compared with that in model groups (MC), especially in the high dose group of DHZCP (Fig. 4A–C; P < 0.01). Furthermore, the level of IL-1β in the medium dose group of DHZCP decreased significantly (Fig. 4C, P < 0.01). Besides, the levels of TNF-α (P < 0.05), IL-6 (P < 0.01) and IL-1β (P < 0.01) in mice serum decreased significantly after TD treatment. In short, these results showed that DHZCP abated the pulmonary fibrosis by its anti-inflammatory effects.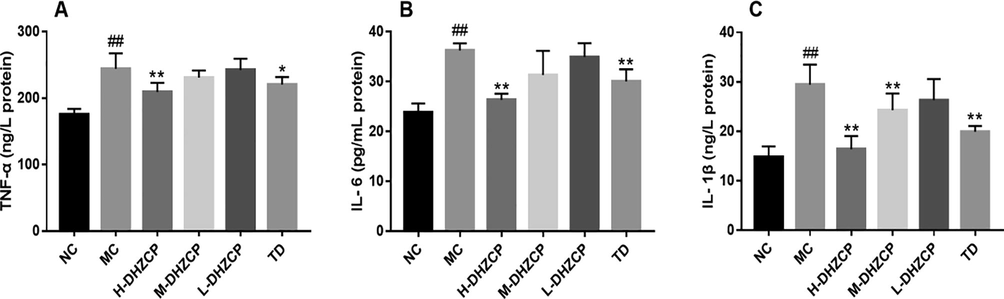
Effect of DHZCP on serum levels of inflammatory proteins. (A) Serum level of TNF-α. (B) Serum level of IL-6. (C) Serum level of IL-1β. Values are expressed as mean ± SD (n = 6). #P < 0.05 and ##P < 0.01 compared to NC group. *P < 0.05 and **P < 0.01 compared to MC group.
3.5 Effect of DHZCP on the protein and mRNA expressions of TGF-β1, α-SMA, Smad2, Smad3 and Smad7
To understand the anti-fibrotic effects of DHZCP on silicosis mice, we examined the protein expressions of fibrosis-related proteins including TGF-β1, α-SMA, Smad2, Smad3 and Smad7 (Fig. 5). Inhalation of silica could increase the expression of TGF-β1 (Fig. 5A) and its downstream Smad2 (Fig. 5C) and Smad3 (Fig. 5D) in lung tissue, and a significant reduction in the level of Smad7 (Fig. 5E). While the proteins expression of TGF-β1 (Fig. 5A, PH-DHZCP < 0.05), Smad2 (Fig. 5C, PH-DHZCP < 0.01), Smad3 (Fig. 5D, PH-DHZCP < 0.01, PM-DHZCP < 0.01, PL-DHZCP < 0.01) and Smad7 (Fig. 5E; PH-DHZCP < 0.01, PM-DHZCP < 0.01, PL-DHZCP < 0.01) were significantly reversed by DHZCP intervention. It was also observed that level of α-SMA dramatically increased after inhalation of silica (Fig. 5C, P < 0.01) whereas treatment of high dose DHZCP led to a significant decrease of the levels of α-SMA (Fig. 5C, PH-DHZCP < 0.01). These results indicated that DHZCP could significantly reduce the expression levels of these proteins in a dose-dependent manner.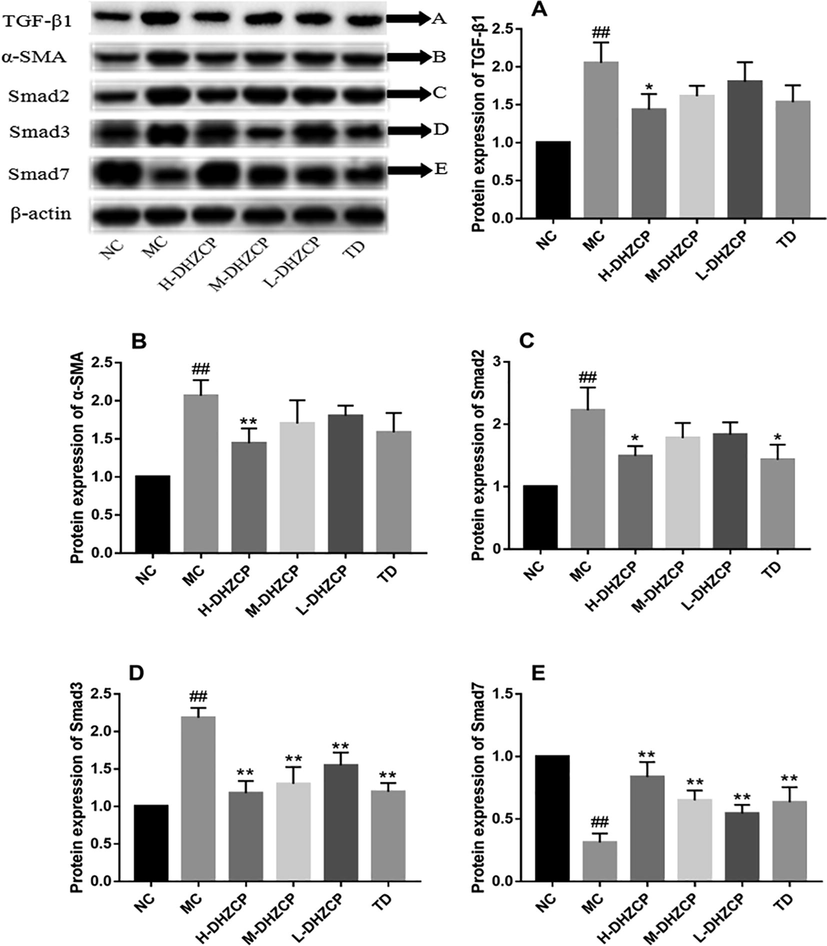
Effect of DHZCP on protein expressions of TGF-β1, α-SMA, Smad2, Smad3 and Smad7. (A) Western blot of TGF-β1, α-SMA, Smad2, Smad3 and Smad7 from lung lysates. Beta-actin serves as loading control. (B-F) Protein bands of (B) TGF-β1, (C) α-SMA, (D) Smad2, (E) Smad3 and (F) Smad7 were quantified by densitometry, expressed relative to beta-actin, and normalized to the NC group. Values are expressed as mean ± SD (n = 6). #P < 0.05 and ##P < 0.01 compared to NC group. *P < 0.05 and **P < 0.01 compared to MC group.
Next, RT-PCR was used to detect the expression of TGF-β1/Smad pathway-related genes in lung tissue. SiO2-induced model group had a dramatic increase in the mRNA levels of TGF-β1 (Fig. 6A, P < 0.01), Smad2 (Fig. 6B, P < 0.01) and Smad3 (Fig. 6C, P < 0.01). Conversely, the level of Smad7 mRNA decreased significantly (Fig. 6D, P < 0.01). DHZCP treatment caused a significant decrease the mRNA levels in TGF-β1 (Fig. 6A, PH-DHZCP < 0.01, PM-DHZCP < 0.01, PL-DHZCP < 0.01), Smad2 (Fig. 6B; PH-DHZCP < 0.01, PM-DHZCP < 0.01, PL-DHZCP < 0.05) and Smad3 (Fig. 6C; PH-DHZCP < 0.01, PM-DHZCP < 0.01, PL-DHZCP < 0.01) while elevating mRNA level of Smad7 (Fig. 6D, PH-DHZCP < 0.01, PM-DHZCP < 0.01, PL-DHZCP < 0.01). DHZCP could significantly reduce the mRNA levels of these genes in a dose-dependent manner. Taken all together, the results showed that DHZCP could inhibit TGF-β1/Smad signaling pathway and fibrosis.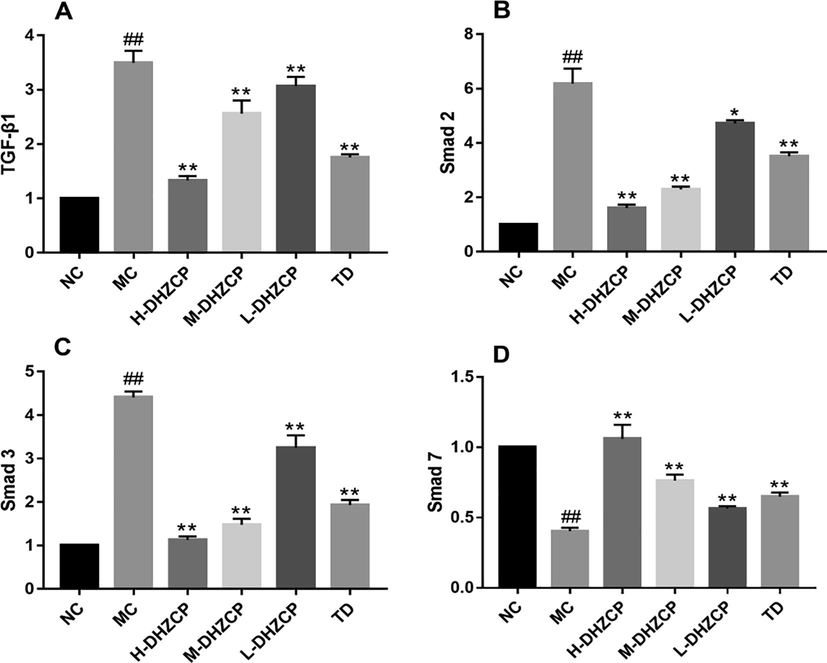
Effect of DHZCP on the expression of mRNA levels of (A) TGF-β1, (B) Smad2, (C) Smad3 and (D) Smad7. Values are expressed as mean ± SD (n = 6). #P < 0.05 and ##P < 0.01 compared to NC group. *P < 0.05 and **P < 0.01 compared to MC group.
4 Discussion
This study aims to investigate the therapeutic potential of DHZCP in ameliorating the silicosis and further exploring its molecular mechanisms involved.
Our study generated mice with silicosis via the inhalation of SiO2, and this was supported by an increased pulmonary index, inflammatory factor levels and TGF-β1/Smad-related protein expression levels as compared to mice without any SiO2 inhalation. As a good indicator of silicosis, an increase in the pulmonary index signified that the mice having silicosis had capillary congestion and cell swelling in the early stage. This was followed by elevated parenchymal weight of the lung due to the proliferation of collagen fibers in the middle and late stage of silicosis. Besides, the pathological examination of lung tissues from mice having silicosis illustrated that high abundance of inflammatory cells and collagen deposition. These characteristics were similar as discussed in the liver fibrosis, indicating the occurrence of lung fibrosis due to silicosis (Afdhal and Nunes, 2004). Moreover, HYP content, which is associated with the collagen fibers production and the formation of ECM, was increased in the silicotic mice (Sato et al., 2019). The intragastric administration of DHZCP could alleviate the silicosis in mice as evidenced in a decrease in pulmonary index, less inflamed lung tissues observation and a reduction in HYP content, thus indicating lesser progressive lung tissue damage and lesser collagen expression in response to silicosis.
In the early stages of inflammation, cells will release large amounts of cytokines such as IL, TNF and other inflammatory factors for the purposes of attracting other immune cells to prevent the invasion of pathogens besides preparing for the wound healing process (Efron and Moldawer, 2004). In addition, many fibrotic factors, including IL-1, IL-6, TNF-α, TGF-β1, and their signaling cascades play important roles in pulmonary fibrosis disease (Yang et al., 2013). The mice experiencing inhalation of SiO2 had high levels of TNF-α, IL-6 and IL-1β, indicating a highly inflamed lung tissue environment and this have progressed to silicosis. Nonetheless, DHZCP treatment reduced the level of these inflammatory cytokines, indicating that DCHZP has a potent anti-inflammatory effect.
In silicosis, the regulation of cytokine and their signaling process was well-documented (Zhai et al., 2004). As an indicator of silicosis, α-SMA is a marker of myofibroblasts which regulates myofibroblasts' contractile activity (Zheng et al., 2009). Its content can indirectly reflect the degree of myofibroblast proliferation, deposition of ECM and pulmonary fibrosis. Moreover, TGF-β1 is the most recognized regulatory factor in pulmonary fibrosis. It can induce the expression of protease inhibitors, eventually lead to abnormal accumulation of ECM and fiber formation. This consequences in the excessive accumulation of ECM in the interstitial and alveolar spaces (Rittié, 2015). Besides, Smads protein is a downstream signal-regulating protein which functions to transfer TGF-β signal from cell membrane to the nucleus and consequences in many biological effects. Smad2 and Smad3 belong to the receptor-regulated Smads, and its phosphorylation of Smad indicates an activation of Smad pathway (Aschner et al., 2014; Li, 2002; Li et al., 2015). Despite that, Smad7 is a negative regulator of TGF-β which blocks R-Smad phosphorylation, thereby blocking the TGF-β/Smad signaling pathway. In our study, the treatment either with DHZCP or tetrandrine had effectively reduced the expression of α-SMA, TGF-β1, Smad2 and Smad3 while elevated the expression of Smad7. This implied that both DHZCP and tetrandrine direct the TGF-β1/Smad pathway to alleviate the silicosis.
This is the first report on the study of DHZCP on silicosis. So far, DHZCP has been reported in clinical and laboratory studies of liver fibrosis, renal interstitial fibrosis, pulmonary interstitial fibrosis, myocardial fibrosis and pancreatic fibrosis. These experiments have shown that inhibition of TGF-β1 pathway is the major target of DHZCP, thereby reducing ECM deposition, potentiating anti-fibrosis effect and protecting the organ tissues from scarring (Xing et al., 2012). Of course, DHZCP exerts its potential via the regulation of different pathway apart from TGF-β1/Smad pathway. Previous experiments illustrated that DHZCP's inhibitory effect on liver fibrosis may be related to inhibition of p38 MAPK phosphorylation, PI3K/Akt pathway and balancing the MMP/TIMP1 (Gong et al., 2020; Cai et al., 2010; Pan et al., 2005). The inhibitory effect of DHZCP on fibrosis was also illustrated through the removal of oxygen free radicals (Xing et al., 2012). Therefore, these suggest the potential of DHZCP as a multiple signaling pathway targeting drug in alleviating fibrosis.
5 Conclusions
DHZCP can alleviate the process of pulmonary fibrosis induced by SiO2 in silicosis mice via the reduction in inflammatory effects, fibrotic effects, and inhibition of TGF-β/Smad signaling pathways.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Evaluation of liver fibrosis: a concise review. Am. J. Gastroenterol.. 2004;99(6):1160-1174.
- [Google Scholar]
- Regulation of fibrotic signalling by TGF-β receptor tyrosine phosphorylation. Nat. Rev. Nephrol.. 2014;10(484–484):1.
- [Google Scholar]
- Protein tyrosine phosphatase α mediates profibrotic signaling in lung Fibroblasts through TGF-β Responsiveness. Am. J. Clin. Pathol.. 2014;184:1489-1502.
- [Google Scholar]
- Epidemiology of silicosis: reports from the SWORD scheme in the UK from 1996 to 2017. Occup. Environ. Med.. 2019;76:17-21.
- [Google Scholar]
- Effects of dahuangzhechong pills on cytokines and mitogen activated protein kinase activation in rats with hepatic fibrosis. J. Ethnopharmacol.. 2010;132:157-164.
- [Google Scholar]
- Transplantation of adipose-derived mesenchymal stem cells attenuates pulmonary fibrosis of silicosis via anti-inflammatory and anti-apoptosis effects in rats. Stem Cell Res. Ther. 2018:9.
- [Google Scholar]
- Intervention of Dahuang Zhechong Pill on Early Hepatic Fibrosis via Paracrine Pathway. Lishizhen Med. Mat. Med. Res.. 2010;21:296-299.
- [Google Scholar]
- Cytokines and wound healing: the role of cytokine and anticytokine therapy in the repair response. J. Burn Care Rehabil.. 2004;25(2):149-160.
- [Google Scholar]
- The impact of TGF-beta on lung fibrosis: from targeting to biomarkers. Proc. Am. Thorac. Soc.. 2012;9:111-116.
- [Google Scholar]
- DahuangZhechong pill attenuates CCI4-induced rat liver fibrosis via the PI3K-Akt signaling pathway. J. Cell. Biochem.. 2020;121(2):1431-1440.
- [Google Scholar]
- Neutralization of interleukin-1 beta attenuates silica-induced lung inflammation and fibrosis in C57BL/6 mice. Arch. Toxicol.. 2013;87:1963-1973.
- [Google Scholar]
- Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol.. 2008;9:847-856.
- [Google Scholar]
- New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact.. 2018;292:76-83.
- [Google Scholar]
- New developments in the understanding of immunologyin silicosis. Curr. Opin. Allergy Clin. Immunol.. 2007;7:168-173.
- [Google Scholar]
- Toll-like receptors in the pathogenesis of pulmonary fibrosis. Eur. J. Pharmacol.. 2017;808:35-43.
- [Google Scholar]
- Smad7 inhibits fibrotic effect of TGF- on renal tubular epithelial cells by blocking smad2 activation. J. Am. Soc. Nephrol.. 2002;13:1464-1472.
- [Google Scholar]
- Metformin attenuates gefitinib-induced exacerbation of pulmonary fibrosis by inhibition of TGF-β signaling pathway. Oncotarget. 2015;6:43605-43619.
- [Google Scholar]
- Effect of dahuang zhechong pill on transforming growth factor-beta 1 in hepatic stellate cells in rats. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:763-766.
- [Google Scholar]
- Traditional chinese medicine for pulmonary fibrosis therapy: progress and future prospects. J. Ethnopharmacol.. 2017;198:45-63.
- [Google Scholar]
- Genetic loss of Gas6/Mer pathway attenuates silica-induced lung inflammation and fibrosis in mice. Toxicol. Lett.. 2019;313:178-187.
- [Google Scholar]
- Effects of dahuangzhechong pill on expression and activity of matrix metalloproteinase in rat hepatic stellate cells. Chin. J. Intergr. Trad. West Med.. 2005;25:1100-1103.
- [Google Scholar]
- Specificity, diversity, and regulation in TGF-beta superfamily signaling. FASEB. 1999;13:2105-2124.
- [Google Scholar]
- Basic pathogenetic mechanisms in silicosis current. Curr. Opin. Pulm Med.. 2005;11:169-173.
- [Google Scholar]
- Another dimension to the importance of the extracellular matrix in fibrosis. J. Cell Commun. Signal.. 2015;9:99-100.
- [Google Scholar]
- Generation of bioactive prolyl-hydroxyproline (Pro-Hyp) by oral administration of collagen hydrolysate and degradation of endogenous collagen. Int. J. Food Sci. Technol.. 2019;54(6):1976-1980.
- [Google Scholar]
- Transforming growth factor-beta1 gene polymorphisms are associated with disease progression in idiopathic pulmonary fibrosis. Am. J. Respir Crit. Care Med.. 2003;168:431-435.
- [Google Scholar]
- Evaluation of the liver protection and toxicity of Da-Huang-Zhe-Chong pill in rats. Pharm. Biol.. 2012;50:344-350.
- [Google Scholar]
- Bone morphogenetic protein-7 inhibits silica-induced pulmonary fibrosis in rats. Toxicol. Lett.. 2013;220:103-108.
- [Google Scholar]
- Aloperine protects mice against bleomycin-induced pulmonary fibrosis by attenuating fibroblast proliferation and differentiation. Sci. Rep.. 2018;8:6265.
- [Google Scholar]
- Differences in cellular and inflammatory cytokine profiles in the bronchoalveolar lavage fluid in bagassosis and silicosis. Am. J. Ind. Med.. 2004;46(4):338-344.
- [Google Scholar]
- The effects of silica dust on the expression of NF-κB p65 mRNA and TGF-β1 mRNA in mice. Toxicology. 2017;31:94-97.
- [Google Scholar]
- Carbon monoxide modulates alpha-smooth muscle actin and small proline rich-1a expression in fibrosis. Am. J. Respir. Cell Mol. Biol.. 2009;41:85-92.
- [Google Scholar]
- Effect of dahuang zhechong pills on long non-coding RNA growth arrest specific 5 in rat models of hepatic fibrosis. J. Trad. Chin. Med.. 2018;38:190-196.
- [Google Scholar]







