Translate this page into:
Internalization of macromolecules into filarial parasites – Possible operation of host’s anti-fecundity immunity inside adult filarial nematodes
⁎Corresponding author. adityarmrc@gmail.com (Aditya K. Panda)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Objectives
Immune effector mechanisms operating against large extracellular systemic pathogens, such as adult stages of filarial nematodes, are not clearly understood. The only immune effect on the adult worms appears to be directed towards embryos in adult worms. The role of IL-4 or TLR-4 in immune responses that inhibit embryogenesis in adult filarial worms has been well-documented. This host’s immune responses that control embryogenesis (anti-fecundity immunity) are considered critical and were addressed in the current study. The current investigation demonstrated the possible operation of anti-adult immunity that could be functional against large stage adults.
Methods
Adult filaria worms were pulsed with macromolecules such as Concanavalin A, Human immunoglobulins, and Rabbit immunoglobulins for 6 hrs, and entry of these molecules was demonstrated using flow cytometry. Filaria worms were also preincubated with Latrunculin A, an endocytic inhibitor, to explain if macromolecules enter by endocytosis.
Results
Using the flow cytometry approach, we could show the entry of immunoglobulins into the uterine cavity of female Setaria digitata, a cattle-dwelling filarial parasite. Immunoglobulins were observed to bind to the surface of intrauterine stages of female worms. We also demonstrated the presence of bovine immunoglobulins in different embryonic stages in situ.
Conclusions
Overall, experimental evidence demonstrates the existence of the host’s immune molecules inside large-stage adult parasites. However, future studies are directed to understand the functional aspect of the presence of these effector molecules inside the adults.
Keywords
Anti-fecundity immunity
Setaria digitata
Immunoglobulins
Concanavalin-A
- LPS
-
Lipopolysaccharide
- IU
-
Intrauterine
- L3
-
Third stage larvae
- TLR
-
Toll like receptor
- Con-A
-
Concanavalin A
- FITC
-
Flourescein Isothyocyanate
- IgG
-
Immunoglobulin G
Abbreviations
1 Introduction
Host immune responses can influence the development of filarial parasites and their fecundity in human or experimental hosts (Kwarteng and Ahuno, 2017) Immune effector mechanisms against these large nematode parasites can be against larvae (anti-larval immunity) or microfilariae (anti-microfilarial immunity). Anti-larval immunity is directed towards incoming L3 larvae and reduces the possibility that the larvae develop into adult worms. In human filariasis, anti-microfilarial immunity has been demonstrated to be mediated by antibodies to the sheath of microfilariae, and there is an inverse association between circulating microfilaria and antibodies to the sheath (Ravindran et al., 1990). Although the immune response against larvae and microfilariae of filarial parasites has been understood with reasonable clarity (Ritter and Hubner, 2022), no mechanistic explanation is currently available explaining the mode of action of protective immune responses against large adult-stage filarial parasites.
The immune mechanism against large-stage adult nematode parasites is often mediated by host immune cells such as basophils and eosinophils (Yasuda and Kuroda, 2019; Peng and Siracusa, 2021), which act upon these parasites on their surface. The second possible effector mechanism is anti-fecundity immunity, which may operate inside the uterine cavity of adult parasites. This assumption was supported by the earlier studies, which indicated that sub-cutaneous infection of third-stage larvae of Brugia pahangi in IL-4-/- mice revealed that, at later time points of infection, the IL-4 −/- mice contained a large number of microfilariae in the peritoneal cavity than the wild type BALB/c mice and the differences in microfilariae levels appear to relate to differences in worm fecundity in the two strains of mice (Devaney et al., 2002). In another study in LPS-non-responsive C3H/HeJ and LPS-responsive C3H/HeN mouse strains, it was observed that the fertility of adult worms from C3H/HeJ mice after adult worm implantation in the peritoneal cavity was higher than those of C3H/HeN mice as indicated by the presence of higher numbers of microfilariae at the site of infection in C3H/HeJ mice suggesting a possible role of TLR-4 signaling in immune response that inhibits worm embryogenesis (Pfarr et al., 2003). In these two reports, the exact mechanism for difference in adult worms' fecundity was unknown. We assume this is due to the operation of anti-fecundity immunity inside filarial parasites. Using a flow cytometry-based assay system, we demonstrated the entry of macromolecules like Concanavalin-A and antibodies (Immunoglobulins) into the uterine cavity of live worms. We further propose that the entry of immunoglobulins into the worms may be through the cuticle through the process of endocytosis.
2 Methodology
2.1 Collection of adult-stage filaria worms (Setaria digitata) from cattle
Male and female bovine adult filarial parasites (Setaria digitata) (henceforth S. digitata) were collected from the peritoneum of slaughtered cattle and added to sterile Hank’s balanced salt solution containing antibiotics by following the procedure as mentioned earlier by our group (Mohanty et al., 2000; Mohanty et al., 2001).
2.2 Preparation of Intra-uterine stages of adult filaria worms
Intrauterine stages (IU stages, embryonic stages) of filarial worms (S. digitata) were prepared as documented earlier (Sahu et al., 2008). Briefly, individual female filarial worms collected from the peritoneum of cattle were appropriately washed in a sterile medium and chopped into small pieces in media to release all intrauterine stages from the uterine cavity. Large pieces were removed, and IUS were harvested and washed by centrifugation. Finally, the cell suspension was acquired in a flow cytometer (BD FACS Calibur), and different IU stages were identified.
2.3 Preparation of immune sera against intrauterine stages
The animal experiment was permitted by the institutional animal ethics committee of Regional Medical Research Centre (Indian Council of Medical Research) Bhubaneswar, Odisha, India, and all experiments were performed adhering to rules and regulations set by the board, taking care of minimum sufferings of animals. One rabbit was immunized with three doses (15 days apart) of intrauterine embryogenic stages of Setaria digitata (50,000 cells per dose) in Freund’s Adjuvant. Blood for sera was collected between days 40–45 after 1st immunization. Sera were stored at −20C till further use.
Assay for demonstrating entry of Concanavalin-A into female adult Setaria digitata.
To show Concanavalin A (Con-A) entry, metabolically active female adult S. digitata were cultured for 1 h, 3 h or 6 h in sterile medium containing 10 μg/ml of biotinylated Con-A. After culture, the individual worm was washed extensively in PBS and was dissected to harvest intrauterine stages. Embryogenic stages were probed with 250-fold diluted Streptavidin-FITC in buffer and were incubated for 30 min at RT. The cells were washed, taken in sheath fluid, and acquired in a flow cytometer. The binding of Streptavidin-FITC to 3 distinct populations (R1 Microfilariae, R2 early developmental stages of eggs, and R3 late stages of eggs) was analyzed.
2.4 Assay for demonstrating entry of immunoglobulins into female adult Setaria digitata
The institutional ethical committee of the Regional Medical Research Centre (Indian Council of Medical Research), Bhubaneswar, Odisha, approved the study's use of human blood, and the study subjects gave informed consent for the collection of blood samples.
Female worms were cultured for 6 h in a sterile medium with 10 % human filarial serum to show the entry of human immunoglobulins into worms. Intrauterine stages were harvested from worms and were incubated with 250-fold diluted anti-human IgG FITC in the buffer for 45 min at room temperature. To show entry of rabbit immunoglobulins into the uterine cavity of worms, adult worms were cultured in a sterile medium containing 10 % normal or immune rabbit serum (containing antibodies to IU stages) for 6 h. Worms were pre-incubated in a sterile medium containing 20 μM latrunculin A for 2 h, followed by incubating the worms in the medium containing immune serum for 6 h. After culture, embryogenic stages were harvested and incubated for 45 min with 250-fold diluted anti-rabbit IgG-FITC conjugate in buffer.
2.5 Demonstration of bovine IgG on the surface of intrauterine stages
For demonstrating the presence of bovine IgG on the surface of embryogenic stages, 50-fold diluted normal and immune rabbit serum (containing anti-bovine IgG) in buffer were added in separate tubes and incubated for 45 min at RT. The suspension was washed thrice in PBS and incubated with 250-fold diluted anti-rabbit IgG-FITC in buffer for 30 min at RT. Finally, the intrauterine stages were acquired and analyzed using flow cytometry to demonstrate cattle IgG on the surface of the worms.
2.6 Statistical analysis
The student’s t-test was applied for all experimental data to determine the statistical significance of differences between the groups. All statistical tests were performed using the Prism software package (Graph Pad Prism 4.0).
3 Results
3.1 Concanavalin-A enters into the uterine cavity of adult s. Digitata and is bound to intrauterine stages
It is pertinent to note that, according to a report by Sahu et al., 2005, a flow cytometry-based technique was used to identify different embryonic stages of adult S. digitata. Although a flow cytometer is generally used to identify different cells (bacteria and eukaryotic cells), the author developed an assay by which different embryonic stages of adult S. digitata, such as microfilariae, early and late developmental stages, could be easily detected as three different clusters in the scatter plot. Using this novel method, we performed all experiments in the current research. The internalization of Con A into adult filarial worms was demonstrated by flow cytometry (Fig. 1). A significant proportion of microfilariae, early and late embryonic stages of adult worms was found to bind to Con A after incubating worms at different time points (1 h, 3hrs and 6hrs) (Fig. 1A) indicating that the molecule has entered into the uterine cavity of worms and then bound to IU stages. As shown in Fig. 1B, a gradual increase in the binding of embryonic stages to Con A was observed at different incubation time points, as noted from the mean intensity of fluorescence examined by flow cytometry.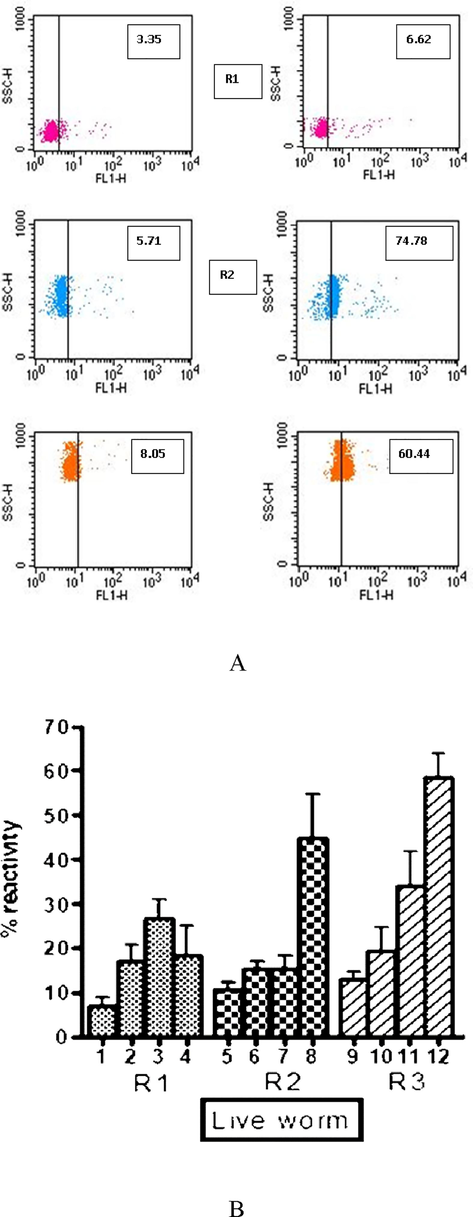
Entry of Con-A into uterine cavity of adult S. digitata. % Reactivity of Concanavalin A to different intrauterine stages of adult S. digitata. 1, 5 and 9 – Control unpulsed worm; 2, 6 and 10 – Binding of Con A to IU stages after 1 hr of incubating adult worms; 3, 7 and 11 − Binding of Con A to IU stages after 3 hrs of incubating adult worms; 4, 8 and 12 − Binding of Con A to IU stages after 6 hrs of incubating adult worms. Data of mean of experiments performed with five different adult worms are shown. Mean % reactivity ± SEM. % reactivity − % of intrauterine stages binding to ConA-FITC as examined by flow cytometry.
3.2 Human immunoglobulins enter into the uterine cavity of adult s. Digitata and bound to intrauterine
Internalization of Con A, a macromolecule, prompted us to show if immunoglobulins enter adult worms. We demonstrated this phenomenon by pulsing the female adult worms with media containing human serum (10 %). Live worms were pulsed for 6 hrs in vitro with human sera containing filarial-specific antibodies. Non-filarial human serum was used as a control. As shown in Fig. 2 (A and B), significant binding of IgG to different embryonic stages of filarial works was noted. This indicates that human immunoglobulins have entered the worms' uterine cavity and are subsequently bound to IU stages.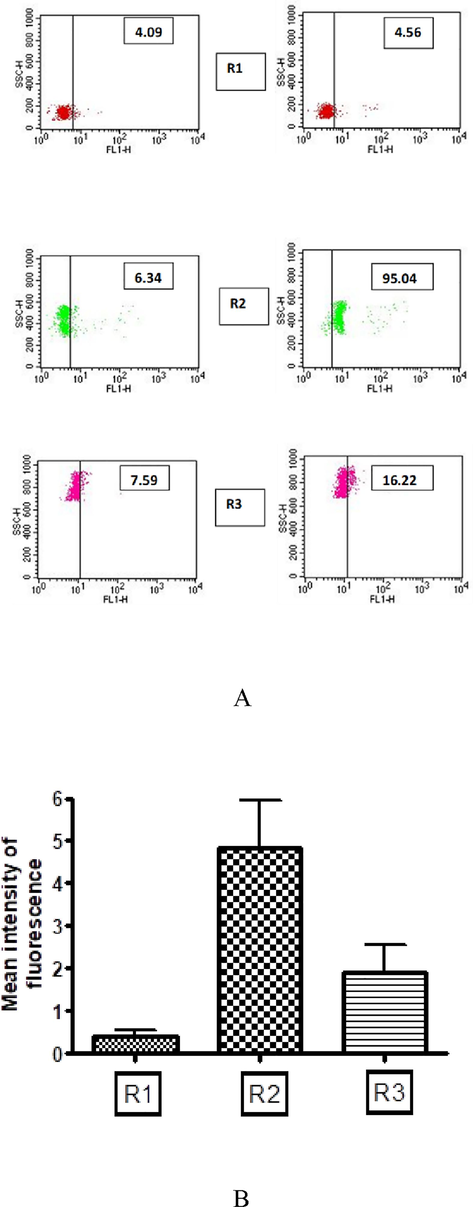
Immunoglobulins in human sera enter into live active worms. Binding of FITC labeled anti-human IgG to 3 distinct populations of IU stages was analyzed. (A) Data for experiment done with a single representative worm is shown. Scatter plot on left panels show background reactivity of anti-human-IgG – FITC conjugate to IU stages, Scatter plot on right panels show specific reactivity of antibodies in human filarial serum to R2 and R3 population of IU stages. Data of mean of experiments performed with five different adult worms are shown. Mean intensity of fluorescence ± SEM. (R1 Microfilariae, R2 early developmental stages of eggs and R3 late stages of eggs). Mean intensity of fluorescence – A value obtained in flow cytometer for overall fluorescence intensity of embryonic stages indicative of binding FITC conjugated antibody.
3.3 Rabbit immunoglobulins enter into the uterine cavity of adult s. Digitata and are bound to intrauterine stages
We also demonstrated the entry of rabbit immunoglobulins into the uterine cavity of filaria worms. Female adult worms were pulsed with 10 % rabbit serum containing antibodies to embryonic stages for 6 hrs. Embryonic stages were harvested, and the suspension was probed anti-rabbit IgG FITC to detect bound immunoglobulins on the surface of embryonic stages. Worms were also pulsed with normal rabbit serum (without containing antibodies to embryonic stages). Normal rabbit serum was used as this does not contain embryonic stage-specific antibodies. Therefore, the idea was that if immunoglobulins enter the uterine cavity of worms, embryonic stages specific immunoglobulins in immunized rabbit sera will bind significantly to embryonic stages. In contrast, immunoglobulins in normal rabbit serum (without specific antibodies) would either not bind or bind less. Fig. 3 showed a significant increase in the binding of IgG to IU stages when worms were pulsed with immune rabbit sera.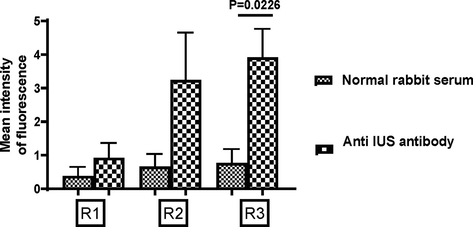
Immunoglobulins in rabbit sera enter into live active worms. Binding of FITC labeled anti-rabbit IgG to 3 distinct populations of IU stages were analyzed Data of mean of experiments done with four worms for normal rabbit sera and seven worms for reactivity with immune rabbit sera are shown. Mean intensity of fluorescence ± SEM; t-test. R1 Mf, R2 early developmental stages of eggs and R3 late stages of eggs).
3.4 Entry of rabbit immunoglobulins into filarial worms is inhibited by Latrunculin A
Latrunculin A, an endocytic inhibitor, was used to examine if the entry of immunoglobulins into worms occurs by the process of endocytosis. As shown in Fig. 4A, significant binding of rabbit immunoglobulins IU stages (R1, R2, and R3 stages combined) was noticed (Red line). On the other hand, a decreased binding (green line) was observed in IU stages to antibodies in the presence of Latrunculin A, the endocytic inhibitor. Here, we must mention that, as it was optional to show how inhibition of rabbit immunoglobulins occurred for individual embryonic stages, we showed the results by combining all three developmental stages of female adult S. digitata.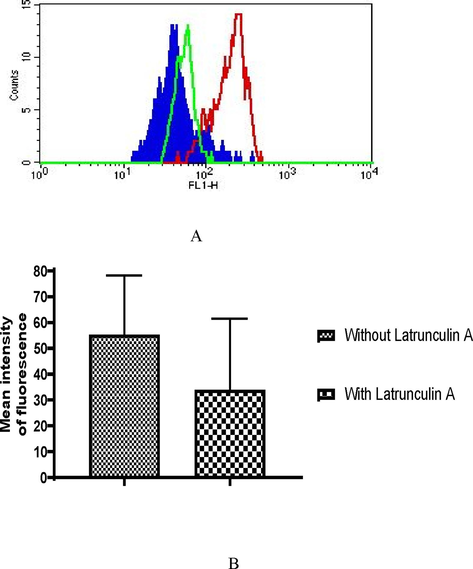
Inhibition of endocytosis of immunoglobulins in rabbit sera into uterine cavity of female adult S. digitata by Latrunculin A. Binding of FITC labeled anti-rabbit IgG to intra uterine egg population were analyzed. (I) Data for experiment done with a single representative worm is shown. Colour shaded area- background reactivity of anti rabbit IgG- FITC to eggs of an unpulsed worm. Red line- specific reactivity of filarial immune rabbit sera to embryogenic egg stages. Green line- specific reactivity of filarial immune rabbit sera to embryogenic egg stages in the presence of Latrunculin A. Numbers inside the histogram represent mean intensity of fluorescence. (II), Data of mean of experiments done with eight different worms for reactivity with immune rabbit sera and another eight worms for reactivity with filarial immune rabbit sera containing Latrunculin A are shown. Mean intensity of fluorescence ± SEM.
3.5 Bovine IgG is present on the surface of IU stages in adult filarial worms
The natural habitat of S. digitata used in our study is cattle. Our observation on the entry of human immunoglobulins and rabbit immunoglobulins into the uterine cavity of adult S. digitata predicted a possible operation and existence of the host’s anti-fecundity immunity inside filarial parasites. As S. digitata dwells inside cattle host, we were interested to examine how bovine immunity can operate inside the filarial parasite. To demonstrate that, IU stages were directly harvested from adult worms and were probed with anti-bovine IgG (a polyclonal antisera raised in rabbits). As shown in Fig. 5A (right panel), all three embryonic stages were found to bind to rabbit anti-bovine IgG. Fig. 5B represents the results of the mean of 8 different worms showing a significant binding (mean intensity of fluorescence), particularly to the R2 and R3 stages. The results indicate the presence of bovine IgG on the surface of IU stages, which might have occurred due to entry of IgG and subsequent binding to IU stages.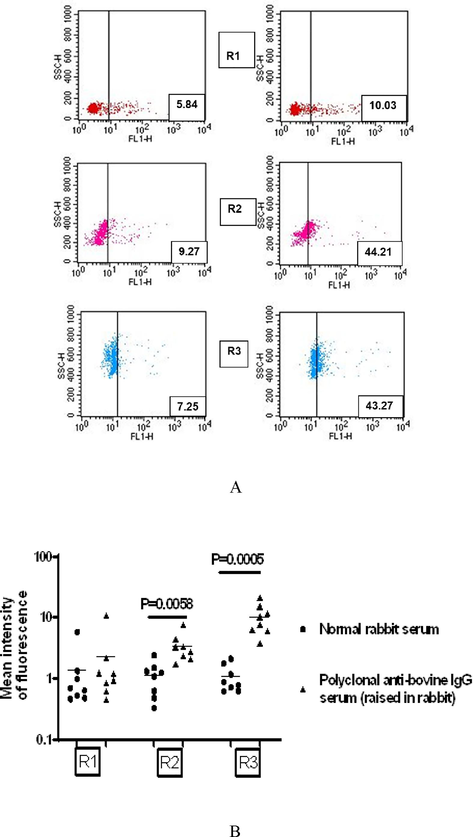
Bovine immunoglobulins are present in situ on the surface of intrauterine embryogenic stages of Setaria digitata. Binding of bovine IgG to 3 distinct populations of IU stages were analyzed. (A), Data for experiment done with a single representative worm is shown. Scatter plot on the left panels show background reactivity of normal rabbit serum; Scatter plot on the right panels show binding of a polyclonal anti bovine IgG serum (raised in rabbit) to three intrauterine populations. Numbers inside plots represent percentage reactivity. (B), Data of eight independent experiments performed with eight adult worms are shown. Each dot represents reactivity for a single worm. Mean intensity of fluorescence, t-test.
4 Discussion
We conducted the current study to demonstrate that anti-fecundity immunity exists and operates inside the uterine cavity of adult nematodes and proposed that it could be one of the host immune effector mechanisms against large adult parasites. Generally, during infection of hosts (human or experimental animals) with filarial parasites, the host’s immune effector mechanism to combat these parasites is poorly defined. As these parasites are multicellular in nature, it is believed that the host’s anti-filarial immunity against different developmental stages of parasites like L3 larvae, Microfilariae, and adult stage parasites could be operational from ‘outside’ of parasites, unlike unicellular pathogens where the host’s immunity act ‘inside’ of the parasites. However, the question arises if there is any mechanism in hosts that may block the production of embryos (microfilariae and eggs) in female adult filarial parasites, and parasites ultimately can not propagate inside hosts. One such possible mechanism is if the host’s molecules of effector immunity (chemokines, cytokines, interferons, and antibodies) can get access into the uterine cavity of female adult worms and act in embryonic stages. In other words, the anti-adult immunity may be influenced by the host’s anti-fecundity immunity during filariasis. However, there is no demonstrable evidence before examining if anti-fecundity immunity exists inside hosts during filariasis. In the current investigation, we examined whether host antibodies (immunoglobulins) can access adult worms. The use of flow cytometry to study the binding of antibodies to different embryonic stages of female adults becomes a tool for us to understand the entry of these molecules into the uterine cavity; however, internalization of immunoglobulins into adult worms was a surprising phenomenon for us as the mechanism of such entry into uterine cavity is not currently known. Previously, the uptake of several biomolecules into worms through cuticles has been well described. Initially, the cuticular surface was believed to be impermeable to high molecular weight polar solutes (Rogers et al., 1974; Pappas, 1975). However, the hypodermal membrane of adult worms of Brugia pahangi has several infoldings that increase the absorptive area of the worm (Vincent et al., 1975). There is adequate evidence to believe that radioactively labeled D-glucose, L-leucine, adenosine, and nucleic acid precursors are taken up in physiologically significant amounts by a transcuticular route of adult Brugia pahangi (Chen and Howells, 1981; Howells and Chen, 1981). According to study by Sasisekhar et al., (2005), in Setaria digitata, a hypodermally located protein named as SXP/RAL2 was identified which was of host origin.
To begin with, we wanted to examine if any large molecule such as Con-A, with a molecular weight of 108 kDa, can enter the uterine cavity of worms. Con-A, a lectin molecule, has a binding affinity for mannose residues, which are ubiquitously present in various components of filarial nematodes (van Die and Cummings, 2017) . Due to its binding specificity to embryonic stages of female adult worms, we selected this molecule to use in our experiment for pulsing female worms at different time points. The idea was that if Con-A entered the uterine cavity of female worms, it would bind the embryonic stages. We showed the binding of ConA to different IU stages of S. digitata, indicating that the molecule has entered the uterine cavity and then bound to embryonic stages. This observation revealed that not only small molecules could access nematode worms, but big molecules could also access worms.
We used filarial-specific antibodies to demonstrate the entry of these immunoglobulins into the uterine cavity. The assumption is that if immunoglobulins can enter the uterine cavity of worms, they should bind to epitopes present in the IU stages after their internalization. We pulsed female S. digitata in media containing filarial-specific antibodies for 6 hrs. This incubation time was selected based on data of ConA internalization, where we found a time-dependent entry of the molecule into worms with the highest amount of binding to embryonic stages at 6 hrs of incubation. We showed a significant binding of human filarial antibodies as well as rabbit filarial-specific antibodies to IU stages of S. digitata, demonstrating the phenomenon of operation of anti-fecundity immunity inside adult-stage filarial parasites. Our results were supported by previous findings that intravascular Schistosomes take up host immunoglobulins as part of enteric digestion and by surface Fc-receptor mediated mechanisms involving transport and processing within organelles as ‘elongate bodies.’(Thors et al., 2006).
To understand the mechanism of internalization of immunoglobulins, we used an endocytic inhibitor, Latrunculin A (Hessien et al., 2023). Pre-incubation of adult filaria worms with Latrunculin A followed by pulsing with rabbit serum containing filarial-specific antibodies suggested a decreased binding of antibodies to IU stages as compared to worms without incubation with the endocytic inhibitor. This experiment clearly showed that the immunoglobulins have entered into the uterine cavity by endocytosis, and the entry is inhibited by Latrunculin A. The most significant illustration of anti-fecundity immunity operating inside the uterine cavity of adult filarial worms was the presence of bound bovine immunoglobulins in situ on the surface of embryogenic stages of S. digitata. This was demonstrated by harvesting intrauterine stages of worms and then probing with polyclonal anti-bovine IgG serum (raised in rabbit) or normal rabbit serum (as negative control). It is reasonable to assume that the presence of bovine Immunoglobulin in situ on the surface of microfilariae (R1) and eggs (R2 and R3) is due to the entry of antibodies through the cuticle and subsequent binding to IU stages. Although microfilariae in human blood circulation are found to have bound host IgG on sheath (Shenoy et al., 1996), no report has been made in the past on the presence of immunoglobulins in the developing stages in utero.
Instead of using adult filarial worms of humans, we used S. digitata in our investigation. As mentioned earlier, S. digitata is a parasitic nematode found in the peritoneal cavity of cattle. We used this parasite in our study due to three important reasons. First, the adult worms of human filarial parasites, such as Wuchereria bancrofti and Brugia malayi, are difficult to access as they dwell in the lymphatics of humans. Second, worm structure has a morphological similarity between S. digitata and W. bancrofti. The third and most important reason is, the comparative genomic analysis among S. digitata, W. bancrofti and B. malayi genomes revealed similarity in several genome sequences (Senanayake et al., 2020).
It is important to note that the exact entry route of these macromolecules, such as ConA and immunoglobulins, into female adult S.digitata is still controversial. Although we argue here that the entry could be through the cuticular surface of the worms (Due to previous evidence of entry of small molecules like D-glucose, Fructose, etc..), we have no demonstrable evidence to examine this. There are oral and anal pores of adult worms, and these macromolecules could be entering into worms through these routes. We anticipate that, after entering through the oral cavity, these molecules could enter into the digestive tract of the worms and afterward could diffuse into the uterine cavity. Although we could sufficiently demonstrate the host’s anti-fecundity immunity inside adult parasites, the functional significance of entry of immunoglobulins into the uterine cavity of S. digitata is currently not known to us. To comprehend the functional aspect, we can align our findings with previous studies. According to Devaney and Gillan et al. (2002), wild-type Balb/C mice had significantly fewer microfilariae of B. pahangi in their peritoneal cavity compared to IL4 −/- animals after subcutaneous injection of third-stage larvae. This difference may be due to compromised viability of embryonic stages of B. pahangi caused by IL4, potentially occurring after IL4 entered the uterine cavity of adult B. pahangi. Another possible functional significance may be that host effector immunity inside filarial parasites is a part of host-parasite interaction (Jo, 2019). There are prior instances of the formation of extracellular vesicles from different helminth parasites, and these vesicles can access the host cells for communication purposes and may be helpful for establishing infection inside the host. Parasitic trematodes, like Echinostoma Caproni and Fasciola hepatica, produce exosome-like vesicles that contain several important parasite-specific proteins, and these vesicles are found to be internalized by rat intestinal epithelial cells, most probably by the process of endocytosis (Siles-Lucas et al., 2017; Marcilla et al., 2012). We, therefore, assume that, as a part of host-parasite interaction, the host immunoglobulins could be internalized into the uterine cavity and bound to Intra-uterine stages.
5 Conclusions
The movement of host macromolecules and immune molecules into nematode parasites has not been reported in the literature so far. Unlike another immune effector mechanism against larvae and microfilariae of filarial parasites, which normally act ‘outside’ these parasites, we, for the first time, demonstrate the existence and operation of the host’s anti-fecundity immunity that works ‘inside’ the adult stage filarial parasites and this type of immunity could be one effector mechanism against large stage adult nematode parasites. However, it is crucial to investigate further the actual biological consequence of the operation of anti-fecundity inside nematode parasites.
Funding
None.
CRediT authorship contribution statement
Bikash Ranjan Sahu: Writing – original draft, Software, Methodology, Formal analysis, Data curation, Conceptualization. Nityananda Mandal: Writing – review & editing, Methodology, Data curation, Conceptualization. Ahmad O. Babalghith: Visualization, Formal analysis, Conceptualization. Noha E. Abdel-razik: . Abdullah F. Aldairi: Writing – review & editing, Formal analysis, Data curation, Conceptualization. Farkad Bantun: Formal analysis, Conceptualization. Raju K. Mandal: Visualization, Formal analysis, Conceptualization. Shafiul Haque: Visualization, Software, Resources, Project administration, Conceptualization. Aditya K. Panda: .
Acknowledgements
The authors (SH and RKM) gratefully acknowledge Jazan University for providing the access of Saudi Digital Library for this study.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Brugia pahangi: uptake and incorporation of nuclei acid precursors by microfilariae and macrofilariae in vitro. Exp. Parasitol.. 1981;51(2):296-306.
- [Google Scholar]
- Interleukin-4 influences the production of microfilariae in a mouse model of Brugia infection. Parasite Immunol.. 2002;24(1):29-37.
- [Google Scholar]
- Mechanistic-Based Classification of Endocytosis-Related Inhibitors: Does It Aid in Assigning Drugs against SARS-CoV-2? Viruses. 2023;15(5)
- [Google Scholar]
- Brugia pahangi: feeding and nutrient uptake in vitro and in vivo. Exp. Parasitol.. 1981;51(1):42-58.
- [Google Scholar]
- Interplay between host and pathogen: immune defense and beyond. Exp. Mol. Med.. 2019;51(12):1-3.
- [Google Scholar]
- Immunity in filarial infections: lessons from animal models and human studies. Scand. J. Immunol.. 2017;85(4):251-257.
- [Google Scholar]
- Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS One. 2012;7(9):e45974.
- [Google Scholar]
- Setaria digitata infections in cattle: parasite load, microfilaraemia status and relationship to immune response. J. Helminthol.. 2000;74(4):343-347.
- [Google Scholar]
- Human bancroftian filariasis - a role for antibodies to parasite carbohydrates. Clin. Exp. Immunol.. 2001;124(1):54-61.
- [Google Scholar]
- Membrane transport in helminth parasites: a review. Exp. Parasitol.. 1975;37(3):469-530.
- [Google Scholar]
- Involvement of Toll-like receptor 4 in the embryogenesis of the rodent filaria Litomosoides sigmodontis. Med. Microbiol. Immunol.. 2003;192(1):53-56.
- [Google Scholar]
- Antibodies to microfilarial sheath in bancroftian filariasis–prevalence and characterization. Ann Trop. Med. Parasitol.. 1990;84(6):607-613.
- [Google Scholar]
- Editorial: Host immune response and protective immune responses during filarial infections. Front. Immunol.. 2022;13:1102121.
- [Google Scholar]
- Studies with Brugia pahangi. 5. Structure of the cuticle. J. Helminthol.. 1974;48(2):113-117.
- [Google Scholar]
- Protective immunity in human filariasis: a role for parasite-specific IgA responses. J Infect Dis. 2008;198(3):434-443.
- [Google Scholar]
- Setaria digitata: identification and characterization of a hypodermally expressed SXP/RAL2 protein. Exp. Parasitol.. 2005;111(2):121-125.
- [Google Scholar]
- The Genome of Setaria digitata: A Cattle Nematode Closely Related to Human Filarial Parasites. Genome Biol. Evol.. 2020;12(2):3971.
- [Google Scholar]
- Isolation and characterization of exosomes derived from fertile sheep hydatid cysts. Vet Parasitol. 2017;236:22-33.
- [Google Scholar]
- Immunoglobulin uptake and processing by Schistosoma mansoni. Parasite Immunol. 2006;28(9):421-428.
- [Google Scholar]
- The Mannose Receptor in Regulation of Helminth-Mediated Host Immunity. Front. Immunol.. 2017;8:1677.
- [Google Scholar]
- The ultrastructure of adult Brugia malayi (Brug, 1927) (Nematoda: Filarioidea) J Parasitol. 1975;61(3):499-512.
- [Google Scholar]
- Role of eosinophils in protective immunity against secondary nematode infections. Immunol. Med.. 2019;42(4):148-155.
- [Google Scholar]







