Translate this page into:
Interaction of nitric oxide synthase and mitochondrial ATP-sensitive potassium channels in protective impacts of combination therapy with irisin-preconditioning and melatonin-postconditioning in myocardial ischemia/reperfusion in aging
⁎Corresponding authors at: Cardiovascular Department, Jinan Central Hospital, Cheeloo College of Medicine, Shandong University, Jiefang Road, Jinan, Shandong, China (G. Su); Cardiovascular Department, Central Hospital Affiliated to Shandong First Medical University, Jiefang Road, Jinan, Shandong, China (K. Hu). suguohai65@sina.com (Guohai Su), hukeqing508@sina.com (Keqing Hu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Aging negatively affects cardioprotection in patients with ischemia/reperfusion damage. Pre- and post-conditioning with irisin and melatonin induced potent cardioprotection in aged hearts. Irisin/melatonin has anti-inflammatory, anti-apoptotic, and mitochondrial boosting effects. Activation of mitoKATP channels is the main contributor to this conditioning effect. eNOS/NO signaling pathway is only partially involved in this protection.
Abstract
Objectives
The main challenge for cardioprotection in patients with cardiac ischemia–reperfusion (I/R) damage is the existence of negatively-acting multiple risk factors such as aging. This work aimed to evaluate the efficacy of combined therapy with irisin and melatonin on cardiac I/R injury in aged rats by focusing on the involvement of mitochondrial ATP-sensitive potassium (mitoKATP) channels, and the eNOS/NO pathway.
Methods
Male aged Wistar rats (78 rats, 400–450 g) were experienced LAD ligation for 30 min followed by 3 h or 2 weeks reperfusion. Irisin (0.5 mg/kg/day) was administered for one week before ischemic insult. Melatonin (20 mg/kg) was administered 10 min before the onset of reperfusion. The releases of CK and LDH, mitochondrial function, inflammatory and apoptosis responses, the expression of eNOS, the levels of NO, cardiac function, and infarct size (IS) were evaluated.
Results
Combined therapy with irisin and melatonin significantly improved heart function, reduced IS, and limited the releases of CK and LDH (P < 0.01 for all). This combined therapy improved mitochondrial function, decreased inflammatory cytokines and apoptosis response, and increased eNOS/NO activity (P < 0.05 for all). Monotherapies had no significant impacts. Inhibition of mitoKATP channels and eNOS enzyme by 5HD and L-NAME, respectively, reversed the cardioprotective effects of combination therapy (P < 0.05), however, the effect of 5HD was more potent as compared to L-NAME (P < 0.05).
Conclusions
It can be suggested that combined therapy with irisin and melatonin can be proposed as a potential candidate to mitigate the I/R severity of aged hearts via activation of mitoKATP channels partly through the eNOS/NO signaling pathway.
Keywords
Ischemia/reperfusion injury
Aging
Irisin
Melatonin
Mitochondrial ATP-sensitive potassium channel
1 Introduction
Ischemic heart diseases (IHD) accompany background comorbidities such as aging. Aging has potential effects on the myocardium, as well as the development of ischemia/reperfusion (I/R) damage, and interferes with responses to cardioprotective strategies (Davidson et al., 2019). Therapeutic strategies which have promising results in young animal models of myocardial I/R commonly fail to diminish infarct size and improve outcomes in aged patients with I/R damage (Liu et al., 2012; Tocchi et al., 2017; Davidson et al., 2019).
Mitochondria have a critical contribution in determining the severity of I/R injury in the heart. During reperfusion, mitochondrial permeability transition pore (mPTP) opening serves as a key mechanism of cell death via overproduction of reactive oxygen species (ROS) and releasing pro-apoptotic and pro-inflammatory mediators (Gok et al., 2006; Saini et al., 2005). It has also been revealed that the insult of I/R may be associated with mitochondrial adenosine triphosphate-sensitive potassium (mitoKATP) channel-dependent mechanism. The opening of mitoKATP channels considerably contributes to the acute and delayed cardioprotection against myocardial I/R injury (Oldenburg et al., 2002; Zhang et al., 2007). Nitric oxide (NO) which itself confers a beneficial effect against cardiac I/R damage, triggers mitoKATP channel opening. Hence, modulation of mitoKATP channels has become an area of growing clinical interest in cardioprotection.
The myocardium and skeletal muscle secrete a proliferator-activated receptor-gamma coactivator-1alpha-dependent myokine called irisin into circulation during exercise (Boström et al., 2012). It has been documented that irisin had cardioprotective and infarct size limiting effects against I/R damage through the reduction of apoptotic proteins and upregulation of mitochondrial ubiquitin ligase in the post-ischemic myocardium (Lu et al., 2020). Besides, irisin improved the resistance of cardiomyoblasts against hypoxia/reoxygenation through inhibiting mPTP opening and mitochondrial apoptosis (Wang et al., 2017). Another study showed that irisin protected the myocardium against I/R damage by preserving mitochondrial function through the reduction of apoptosis and mitochondrial oxidative stress (Wang et al., 2018). Taken together, irisin may have good therapeutic potential in infarcted hearts of young animals by facilitating myocardial repair and function.
Melatonin or N-acetyl-5-methoxytryptamine is an endogenous hormone that has anti-inflammatory, anti-oxidative, and anti-apoptotic properties (Altun and Ugur-Altun, 2007). It has a beneficial effect in young animals against myocardial I/R damage-induced mitochondrial dysfunction, which appears to be due to the preservation of the integrity and content of cardiolipin molecules (Petrosillo et al., 2006). It has been revealed that melatonin has cardioprotective effects by inhibiting mPTP opening via inhibiting cardiolipin peroxidation (Petrosillo et al., 2009). Low melatonin level is associated with the increase in cardiovascular diseases in aging (Dominguez-Rodriguez et al., 2012). Therefore, it seems that melatonin may be a beneficial strategy for treating myocardial I/R damage or other cardiovascular diseases associated with mitochondrial oxidative injury in aging (Petrosillo et al., 2009).
Due to the poor prognosis of IHD in aged patients, using effective strategies to improve the outcomes of cardiac I/R damage is very important. Aging has interfering impacts on the individual cardioprotective modalities. Considering that irisin and melatonin have positive potentials in alleviating myocardial I/R damage in young animals, we supposed that combined therapy with irisin preconditioning and melatonin postconditioning may exert greater cardioprotection against I/R damage of aged myocardium and overcome the aging-induced loss of cardioprotection. The combination of melatonin and irisin would have more beneficial effects on the aging heart because they can activate distinct and parallel pathways regulating mitochondrial survival and biogenesis. So, the present work investigated the impact of melatonin postconditioning on cardioprotection in aged rats with I/R injury pretreated with irisin by focusing on the role of mitoKATP channels and eNOS/NO pathway in this context.
2 Materials and methods
2.1 Animal care
Experimental protocols and animal handling were done following approval of the local ethical committee (ethical code: 7020–21) in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (8th Edition, NRC 2011), according to the ARRIVE guidelines for animal studies (Kilkenny et al., 2010). Male Wistar rats (n = 78, 400–450 g) at 22–24 months of age were provided by the animal center, and maintained in standard cages under a cycle of 12 h lightness and 12 h darkness and a controlled room temperature and humidity and were fed with tap water and standard pellet chow. The procedures were performed according to the eighth edition of “Guide for the Care and Use of Laboratory Animals” (NRC, 2011), and validated by the local Ethics Board (Ethical code: 07020018).
2.2 Rat model of myocardial I/R
After being anesthetized using sodium-pentobarbital (50 mg/kg, intraperitoneally), the animals were ventilated with room air using a small animal ventilator (Cwe, Inc, Ardmore, PA, USA). After the left thoracotomy, the heart was exposed. To induce regional ischemia, the left anterior descending (LAD) coronary artery was occluded using a ligature for 30 min. ST-elevation on the electrocardiograms and pale discoloration of myocardial tissue in the ischemic areas were considered as successful induction of ischemia. Following 30 min of ischemia, the ischemic myocardium was reperfused by removing the ligature for 3 h or 2 weeks. Rats in the sham group experienced a similar surgical procedure, without LAD ligation.
2.3 Experimental protocols
Aged rats were randomly allocated into the five experimental groups in the first protocol, and eight experimental groups in the second protocol (n = 6 per group). In the first protocol, the reperfusion lasted for 2 weeks, and echocardiographical evaluation of cardiac function and infarct size (IS) were performed. Collecting blood samples for measuring lactate dehydrogenase (LDH) and creatine kinase (CK) serum levels were accomplished after 24 h of reperfusion. Echocardiographic changes require more time to occur, so reperfusion time was 2 weeks to evaluate this goal. In the second protocol, the reperfusion lasted for 3 h, and then the animals were sacrificed for biochemical and molecular assessments.
The groups in the first protocol are as follows (with 2 weeks reperfusion):
-
(1).
Sham: without experiencing I/R;
-
(2).
I/R: rats experienced 30 min LAD occlusion and 2 weeks reperfusion;
-
(3).
I/R + Iri: rats were pretreated with an intraperitoneal injection of recombinant irisin (Cloud Clone, Wuhan, China) at 0.5 mg/kg/day for one week, and at the time point of 30 min before I/R surgery (Lu et al., 2020), then the rats experienced 30 min LAD occlusion and 2 weeks reperfusion;
-
(4).
I/R + Mel: rats underwent 30 min LAD occlusion and 2 weeks reperfusion plus intraperitoneal injection of melatonin (Sigma-Aldrich, St Louis, USA) at 20 mg/kg, 10 min before the onset of reperfusion (Zhai et al., 2017); and
-
(5).
I/R + Iri + Mel: the I/R-treated rats received both irisin and melatonin at their corresponding time points.
The groups in the second protocol are as follows (with 3 h reperfusion):
Groups 1–5 are similar to the first protocol, except that the rats in groups 2–5 experienced 30 min LAD occlusion and 3 h reperfusion.
-
(6).
I/R + Iri + Mel + 5HD: the I/R-treated rats received both irisin and melatonin at their corresponding time points, and 5HD (Sigma-Aldrich, St Louis, USA), as a mitoKATP channel blocker, was administrated at 10 mg/kg, five minutes before ischemia;
-
(7).
I/R + Iri + Mel + L-NAME: the I/R-treated rats received both irisin and melatonin at their corresponding time points, and L-NAME (Sigma-Aldrich, St Louis, USA), as an eNOS enzyme inhibitor, was administrated at 16 mg/kg, five minutes before the reperfusion; and
-
(8).
I/R + Iri + Mel + 5HD + L-NAME: the I/R-treated rats received both irisin and melatonin as well as both inhibitors, 5HD and L-NAME, at their corresponding time points as above.
It should be added that monotherapies had no significant effects on cardioprotection, so, 5HD and/or L-NAME were administered only in rats receiving combined therapy. In addition, according to pilot studies and previous reports (Lu et al., 2020; Zhai et al., 2017), the best cardioprotective effects of irisin and melatonin are achieved when they are administered pre-conditionally and post-conditionally, respectively. Therefore, in this study, these time points were considered for their injection.
2.4 Determination of heart function
Two weeks after reperfusion, echocardiography was carried out by a cardiologist who was blinded to the study design. The animals were reanesthetized, and assessment of the contractile function of the hearts was performed by Vevo 2100 system (VisualSonics, Toronto, ON, Canada). The measurement of ejection fraction (EF), left ventricular end-diastole inner diameter (LVIDd), left ventricular end-systole inner diameter (LVIDs), and fractional shortening (FS) was performed from three consecutive cardiac cycles.
2.5 Measurement of myocardial infarct size
Following completion of the cardiac function assessment, the Evans blue and triphenyl tetrazolium chloride (TTC) double-staining method was applied for the determination of the areas at risk (AAR) and IS. The ligature around the LAD was re-ligated, and the injection of 2 ml Evans blue (2%) was performed through the right jugular vein. The hearts were removed, frozen, sectioned, and stained with 1% TTC. Consequently, transverse sections of the left ventricles (1 mm thickness) were photographed. The AAR and IS were calculated by ImageJ software (Ver. 7.0, CA, USA). A percentage of the left ventricle was reported as an AAR, and a percentage of AAR was reported as IS.
2.6 Measurement of LDH and CK
After 24 h reperfusion, the blood samples were obtained from the rat's tail vein under anesthesia with sodium pentobarbital (40 mg/kg, intraperitoneally). After the samples being centrifuged and the serum is collected, a colorimetric method was applied to determine LDH and CK serum levels, with commercial assay kits (Nanjingjiancheng Bioengineering, China), in accordance with the protocol provided by the manufacturer. The recorded values were reported in IU/l.
2.7 Detection of cardiac mitochondrial function
Cardiac mitochondria from the AAR of LV were isolated after 3 h of reperfusion. Following homogenization of fresh tissue in buffer (pH 7.4) containing 70 mmol/L sucrose, 210 mmol/L mannitol, and 1 mmol/L ethylene-diamine-tetra-acetic acid (EDTA) in 50 mmol/L Tris/HCl, the samples were centrifuged at 1300g for 3 min. To obtain isolated mitochondria, the collected supernatants were then centrifuged at 10000g for 10 min. The pellets were collected and resuspended in a storage buffer (100 µL, pH 7.4) containing 70 mmol/L sucrose, and 210 mmol/L mannitol in 50 mmol/L Tris-HCl. To determine mitochondrial protein concentration, the Bicinchoninic Acid (BCA) assay was performed. Using a ROS assay kit (Beyotime, Shanghai, China), cardiac mitochondrial ROS production was measured by di-chlorohydro-fluorescein-di-acetate (DCFDA) dye (Yarana et al., 2012). By a mitochondrial membrane potential assay kit (Sigma, Germany) with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine-iodide (JC-1), the changes in mitochondrial membrane potential were determined according to the manufacturer’s protocol. Decreased red/green fluorescence intensity ratio was reported as depolarization of mitochondrial membrane (Yarana et al., 2012).
2.8 Determination of pro-inflammatory cytokines
Rat-specific enzyme-linked immunosorbent assay (ELISA) kits (MyBioSource Inc., USA) were applied to measure the levels of cytokines IL-6, IL-1β, and TNF-α in tissue homogenates, based on the manufacturer’s method. The concentrations of these cytokines were quantified by reference to standard curves. The relative absorbance of cytokines was read at 450 nm by a spectrophotometer, and the results were normalized and reported as pg/mg of protein in each sample.
2.9 Western blotting
Western blotting was conducted according to previous work (Palee et al., 2020). The concentrations of proteins from AAR samples were determined by applying an assay kit (Bio-Rad Laboratories, USA). Proteins (50 μg) from AAR samples were loaded for electrophoresis. Thereafter, transferring of proteins to nitrocellulose membranes was performed. The membrane was incubated for an hour at 23 °C and blocked with 5% skimmed milk, followed by overnight exposure to primary antibodies as follows: anti B cell lymphoma-2 (Bcl-2), anti-Bcl-2-associated × protein (Bax), anti cleaved caspase 3, anti endothelial nitric oxide synthase (eNOS), and anti-GAPDH (Cell Signaling Technology, USA). Then the membrane was exposed to a secondary antibody (Cell Signaling Technology, USA) for an hour at room temperature. Enhanced chemiluminescence substrate was applied to visualize bands. Alpha Ease ® FC Imaging System was used for band density analysis per their optical densities. Finally, the intensity of bands was normalized with GAPDH.
2.10 Measurement of NO
Griess colorimetric method was applied for measuring NO levels following the NO assay kit protocol (Thermo-Fisher Scientific, Germany) (Castillo et al., 2000). Color development with Griess reagent (N-naphthyl ethylene diamine and sulfanilamide) in an acidic medium following nitrate conversion into nitrite by copperized cadmium granules is the principle of this method. The calibration of this assay was performed by standard solutions of sodium nitrite. Employing a 96-well microplate reader, the optical densities of NO metabolites were measured at 540 nm following 3 h incubation time, and the results were reported as µmol/mg protein.
2.11 Statistical analysis
The values were reported in means ± standard deviation (SD). Data analysis was performed with SPSS statistical software (Version 15.0, SPSS Inc., Chicago, IL). One-way analysis of variance (ANOVA) followed by Tukey post hoc test was employed to determine multiple comparisons between groups. P-value <0.05 was considered statistically different.
3 Results
3.1 Effect of combined conditioning on cardiac function of the aged rats
Our data revealed that induction of 30 min ischemia and 2 weeks reperfusion significantly decreased EF and FS in comparison with the Sham group (P < 0.01). Irisin preconditioning or melatonin postconditioning could not significantly increase EF and FS when compared with the I/R group. Nevertheless, combined therapy with irisin and melatonin significantly increased these parameters as compared to I/R (P < 0.01), I/R + Iri, and I/R + Mel (P < 0.05) groups (Fig. 1A and 1B).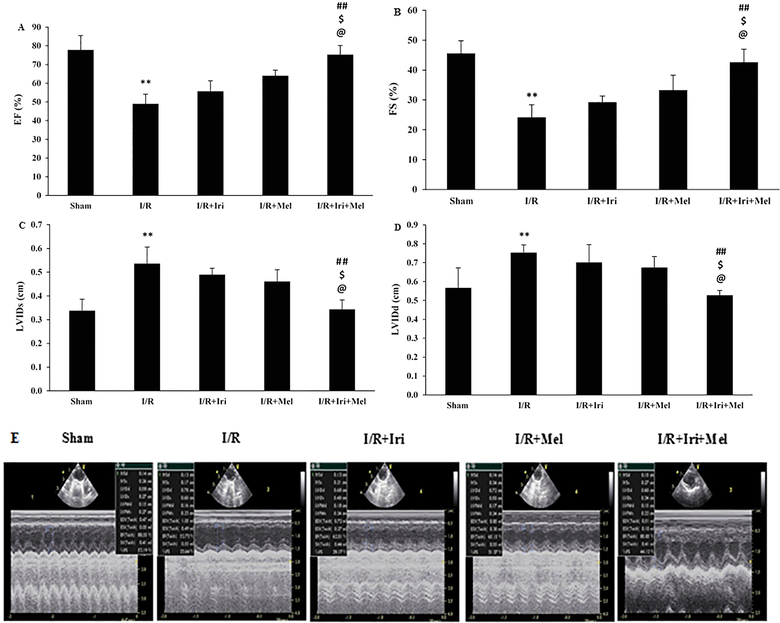
The changes of EF (A); FS (B); LVIDs (C); and LVIDd (D); and representative echocardiogram recordings (E) in experimental groups (n = 6 per group). The data were expressed as mean ± SEM. (** P < 0.01 vs. Sham group, ## P < 0.01 vs. I/R group, $ P < 0.05 vs. I/R + Iri group, @ P < 0.05 vs. I/R + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin; EF: Ejection fraction; FS: Fractional shortening; LVIDs: Left ventricular inner diameter in end systole; LVIDd: Left ventricular inner diameter in end diastole.
Fig. 1C and 1D show that LVIDs and LVIDd in the I/R group were significantly higher than the Sham group (P < 0.01 for both). In addition, individual treatments could not significantly decrease LVIDs and LVIDd in comparison with the I/R group. Combined therapy with irisin and melatonin caused a significant reduction in LVIDs and LVIDd when compared to I/R (P < 0.01 for both), I/R + Iri, and I/R + Mel (P < 0.05 for both) groups.
3.2 Effect of combined conditioning on AAR and IS in the aged heart
As shown in Fig. 2A, the AAR was similar among all groups. The expansion of IS to 53.67 ± 5.03% was detected in the I/R group as compared to the zero IS in the Sham group (P < 0.001). Alone treatments could not significantly reduce the IS in comparison to the I/R group. The IS was significantly reduced to 20.33 ± 2.50% in the group receiving combined therapy as compared to I/R, I/R + Iri, and I/R + Mel groups (P < 0.01 for all) (Fig. 2B).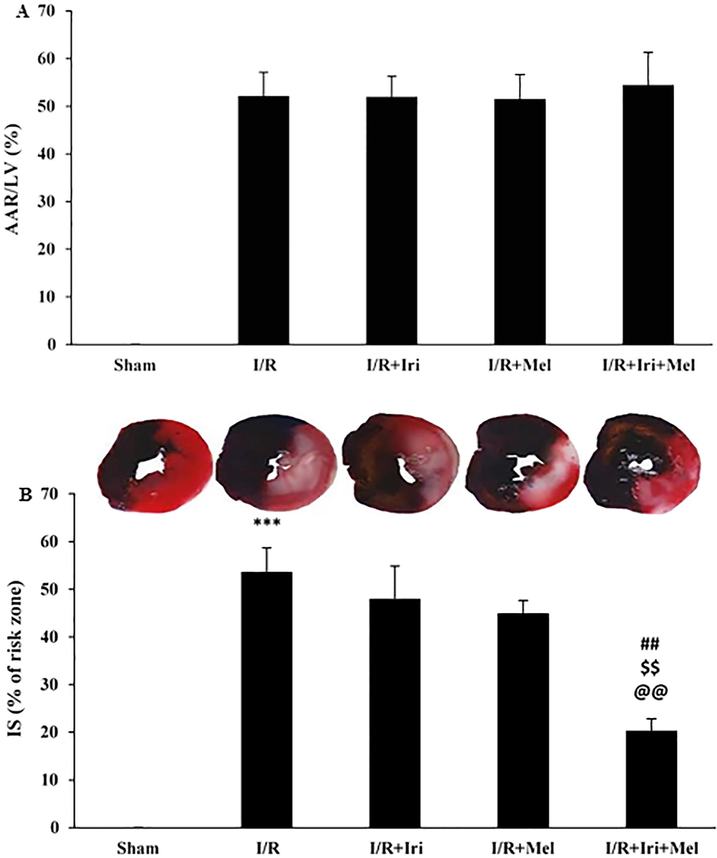
The percentages of area at risk (AAR) (A), and infarct size (IS) (B) in experimental groups (n = 6 per group). The data were expressed as mean ± SEM. (*** P < 0.001 vs. Sham group, ## P < 0.01 vs. I/R group, $$ P < 0.01 vs. I/R + Iri group, @@ P < 0.01 vs. I/R + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin.
3.3 Effect of combined conditioning on CK and LDH serum levels in the aged heart
The CK and LDH serum levels were increased after 24 h of reperfusion when compared to the Sham group (P < 0.001). Only combined therapy with irisin and melatonin caused a significant reduction in CK and LDH serum levels when compared to I/R, I/R + Iri, and I/R + Mel groups (P < 0.01 for both) (Fig. 3A and 3B).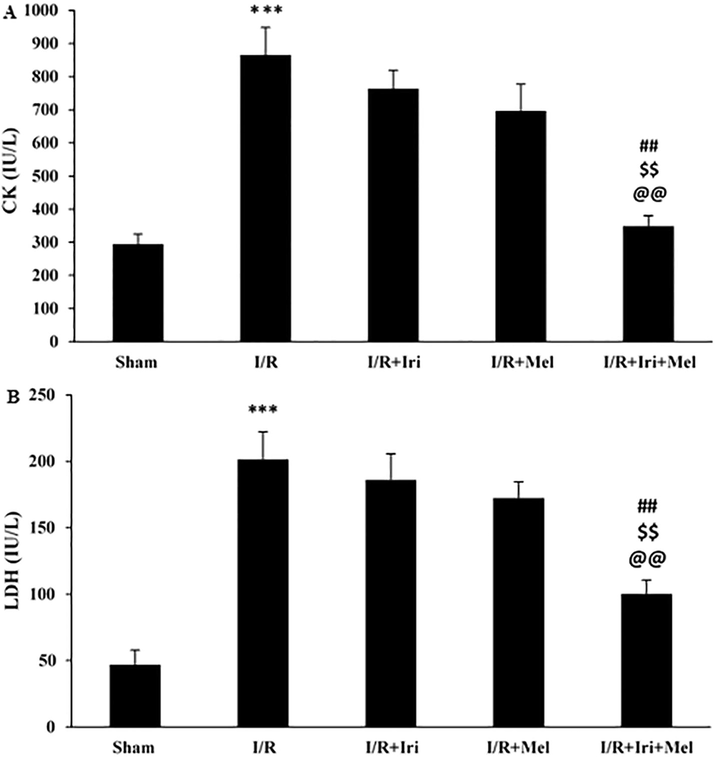
Serum levels of CK (A), and LDH (B) in experimental groups (n = 6 per group). The data were expressed as mean ± SEM. (*** P < 0.001 vs. Sham group, ## P < 0.01 vs. I/R group, $$ P < 0.01 vs. I/R + Iri group, @@ P < 0.01 vs. I/R + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin; CK: Creatine kinase; LDH: lactate dehydrogenase.
3.4 Effect of combined conditioning on mitochondrial function in the aged heart and its reversal by 5HD and L-NAME
The production of mitochondrial ROS and the alterations of mitochondrial membrane potential were evaluated as indicators of mitochondrial function following 3 h reperfusion (Fig. 4A and 4B). Induction of I/R led to increased production of mitochondrial ROS, as well as the enhanced ratio of red to green fluorescent intensity when compared with those of the Sham rats (P < 0.01 for both). The levels of mitochondrial ROS and membrane potential did not differ between monotherapy groups and those of non-treated rats. However, these mitochondrial functional indices were significantly declined in rats receiving combined therapy in comparison to the I/R group (P < 0.05 for both). Since monotherapies had no significant effects on cardioprotection, 5HD and/or L-NAME were administered only in rats receiving combined therapy. The results demonstrated that the beneficial effects of combination therapy on mitochondrial ROS production and membrane potential were eliminated following 5HD administration (P < 0.05). Interestingly, administration of L-NAME was able to partially prevent the beneficial effects of combination therapy on mitochondrial indices (P < 0.05). The concomitant administration of these two inhibitors had a complete inhibitory effect on cardioprotection induced by the combined therapy (P < 0.001).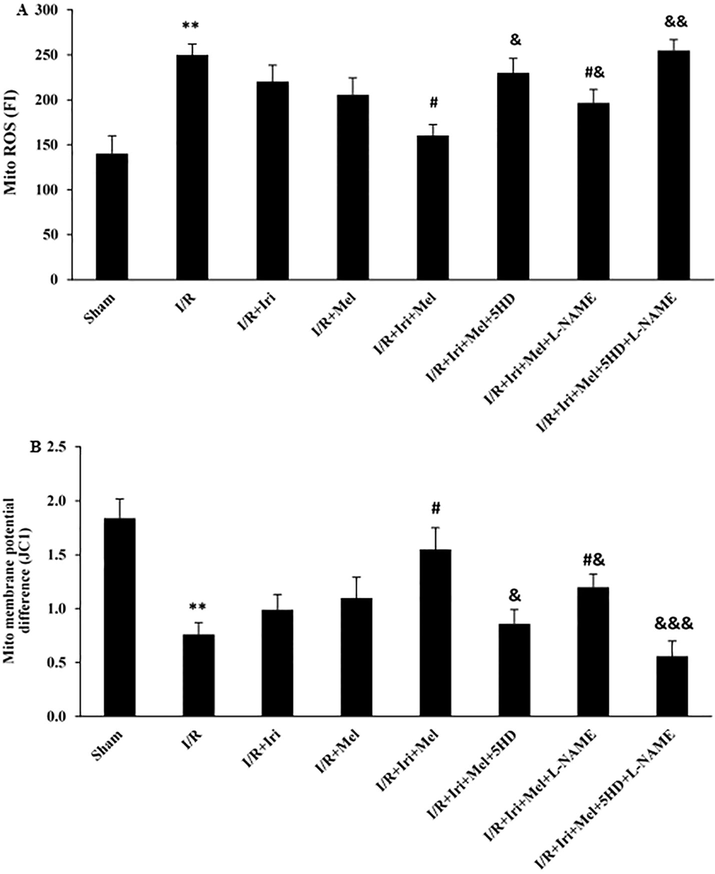
Mitochondrial ROS production in the heart assessed by DCFH-DA dye (A), and the alterations of mitochondrial membrane potential in the heart assessed by JC-1 dye (B) (n = 6 for each group). The data were expressed as mean ± SEM. (** P < 0.01 vs. Sham group, # P < 0.05 vs. I/R group, & P < 0.05, && P < 0.01, and &&& P < 0.001 vs. I/R + Iri + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin; 5HD: 5-hydroxydecanoate; L-NAME: NG-nitro-L-arginine methyl ester; ROS: Reactive oxygen species.
3.5 Effect of combined conditioning on inflammatory response in the aged heart and its reversal by 5HD and L-NAME
As shown in Fig. 5A-C, the myocardial levels of IL-1β, IL-6 and TNF-α did not differ between monotherapy and I/R groups. Nevertheless, the levels of these cytokines were significantly decreased in the group receiving combined therapy as compared to the I/R group (P < 0.05 for all). Co-administration of 5HD or L-NAME significantly attenuated the favorable effects of combination therapy on the levels of inflammatory cytokines (P < 0.05), however, the effect of 5HD in eliminating the inhibitory effects of combined therapy on these inflammatory cytokines was more potent as compared to L-NAME (P < 0.05). In addition, the administration of both 5HD and L-NAME at the same time completely neutralized the inhibitory effects of combined therapy on inflammatory cytokines (P < 0.001, and P < 0.01).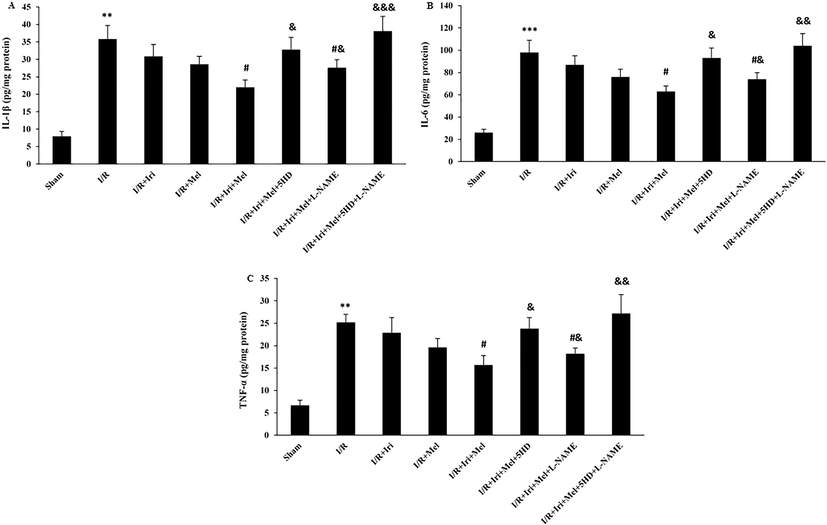
The levels of IL-1β (A), IL-6 (B), and TNF-α (C) in experimental groups (n = 6 per group). The data were expressed as mean ± SEM. (** P < 0.01 and *** P < 0.001 vs. Sham group, # P < 0.05 vs. I/R group, & P < 0.05, && P < 0.01, and &&& P < 0.001 vs. I/R + Iri + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin; 5HD: 5-hydroxydecanoate; L-NAME: NG-nitro-L-arginine methyl ester; IL: Interleukin; TNF-α: Tumor necrosis factor α.
3.6 Effect of combined conditioning on apoptosis response in the aged heart and its reversal by 5HD and L-NAME
As shown in Fig. 6A-C, upregulation of Bax and cleaved caspase-3, and downregulation of Bcl-2 were observed following 30 min ischemia and 3 h reperfusion (P < 0.01 for all). Monotherapies had no significant influences. Simultaneous application of irisin preconditioning and melatonin postconditioning significantly suppressed Bax and cleaved caspase-3 expressions, and enhanced Bcl-2 expression, in comparison with the I/R group (P < 0.05 for all). The effect of combination therapy on the expressions of Bax, Bcl-2, and cleaved caspase-3 was reduced following 5HD or L-NAME administration (P < 0.05), however, L-NAME was not as potent as 5HD in inhibiting the combination effect on apoptosis response (P < 0.05). The concomitant administration of these two inhibitors had a complete inhibitory effect on the antiapoptotic response induced by the combined therapy (P < 0.01, and P < 0.001).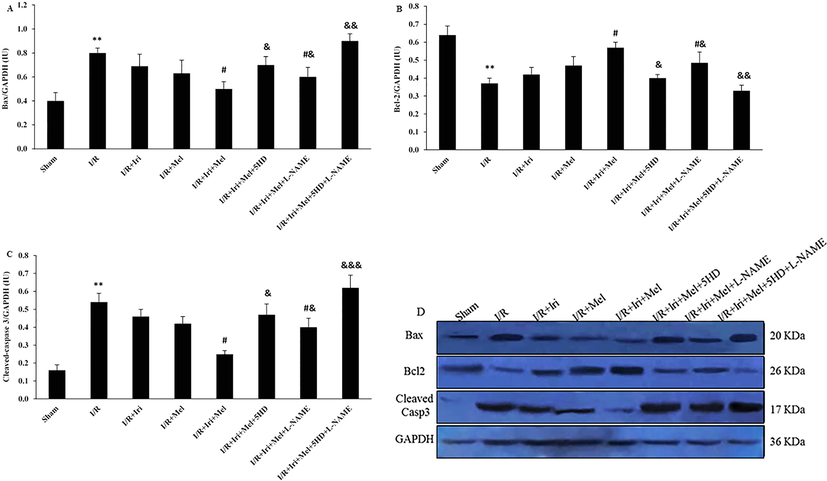
The expressions of Bax (A), Bcl-2 (B), and cleaved caspase 3 (C), and their representative bands correlated to the GAPDH band in experimental groups (n = 6 per group). The data were expressed as mean ± SEM. (** P < 0.01 vs. Sham group, # P < 0.05 vs. I/R group, & P < 0.05, && P < 0.01, and &&& P < 0.001 vs. I/R + Iri + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin; 5HD: 5-hydroxydecanoate; L-NAME: NG-nitro-L-arginine methyl ester; Bax: Bcl-2 associated × protein; Bcl-2: B-cell lymphoma 2.
3.7 Effect of combined conditioning on eNOS/NO pathway in the aged heart and its reversal by 5HD and L-NAME
In comparison to the Sham group, significant reductions in the expression of eNOS (P < 0.01) and the levels of NO (P < 0.05) were detected in the I/R group. Monotherapies had no significant effects on eNOS expression and NO levels in I/R rats. However, combined therapy with irisin and melatonin significantly increased eNOS expression and NO level when compared to the I/R group (P < 0.05 for both). These positive effects of combination therapy on eNOS expression and NO levels were eliminated following 5HD administration (P < 0.05 for both). Administration of L-NAME partially prevented the positive effects of combination therapy on eNOS expression and NO levels (P < 0.05). The concomitant administration of these two inhibitors had a complete inhibitory effect of combined therapy on these parameters (P < 0.01 for both) (Fig. 7A and B).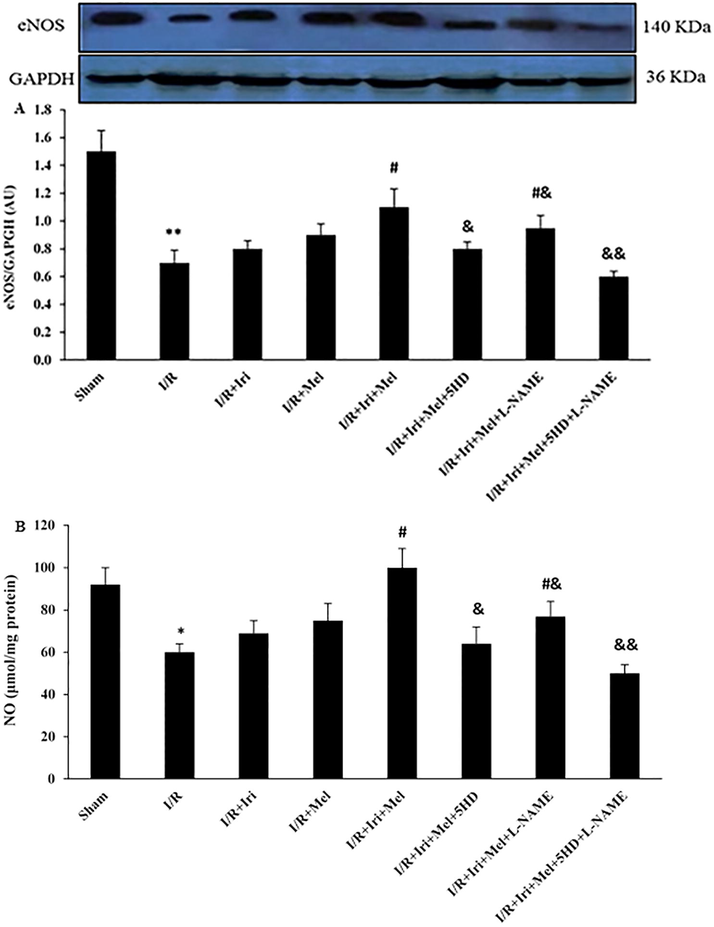
The expression of eNOS correlated to the GAPDH band (A), and the levels of NO (B) in experimental groups (n = 6 per group). The data were expressed as mean ± SEM. (* P < 0.05 and ** P < 0.01 vs. Sham group, # P < 0.05 vs. I/R group, & P < 0.05 and && P < 0.01 vs. I/R + Iri + Mel group). I/R: Ischemia/reperfusion; Iri: Irisin; Mel: Melatonin; 5HD: 5-hydroxydecanoate; L-NAME: NG-nitro-L-arginine methyl ester; eNOS: Endothelial nitric oxide synthase; NO: Nitric oxide.
4 Discussion
The present investigation revealed that the combination therapy with irisin preconditioning and melatonin postconditioning in aged rats with cardiac I/R injury decreased myocardial infarct size and CK and LDH releases, and improved myocardial performance following I/R. This combination therapy was found to improve mitochondrial function and suppress inflammatory and apoptosis responses by the opening of mitoKATP channels. The opening of these channels was partially (not completely) dependent on the increase in the activity of the eNOS/NO signaling.
It has been shown that aging can modify or abolish the infarct size-limiting effects of strategies in experimental studies, and probably cause adverse outcomes in clinical trials. Since aging defects the cardioprotective mechanisms, so the translation of infarct size-limiting effects of strategies in experimental studies into patients with cardiac I/R insults is challenging (Petrosillo et al., 2005). Therefore, it is important to design appropriate animal models of myocardial I/R with comorbidities and considering the combination therapy to mimic clinical settings (Ferdinandy et al., 2007). In the present study, combination therapy with irisin preconditioning and melatonin postconditioning was administered to increase cardioprotection in the myocardial I/R model in aged rats. The cardioprotective effects of both irisin and melatonin against I/R insults have been shown in previous preclinical studies (Wang et al., 2018; Lu et al., 2020; Sahna et al., 2005; Lee et al., 2002; Zhai et al., 2017). Evaluation of endpoints in individual treatments revealed that pretreatment with irisin or postconditioning with melatonin in aged rats tended to decrease infarct size, CK, and LDH, and improve myocardial performance, but these effects were not statistically significant. However, combination therapy had more potent cardioprotective effects and could increase the resistance of aged myocardium against I/R damage, which was proved by a significant reduction of myocardial infarct size, the releases of CK and LDH, and improvement of myocardial function. So, it seems that the effectiveness of individual treatments was diminished under aging conditions. These findings are in line with the previous works demonstrating that aging diminishes the cardioprotective effects of agents which are protective in young rats (Liu et al., 2012; Schulman et al., 2001; Liu et al., 2006).
Damaged mitochondria lead to the excessive production of ROS and releasing pro-death factors such as inflammatory and apoptotic mediators, and subsequently cardiomyocyte death (Ferdinandy et al., 2007; Kuznetsov et al., 2019). Our data showed that combined therapy with irisin and melatonin in aged hearts significantly improved mitochondrial dysfunction (as evidenced by reduced mitochondrial ROS generation and increased its membrane potential), decreased inflammatory cytokines (as evidenced by enhanced levels of three main proinflammatory cytokines), and attenuated apoptosis response (as evidenced by Bcl-2 upregulation and Bax and cleaved caspase-3 downregulation). Irisin preconditioning or melatonin postconditioning did not significantly influence these parameters. Pretreatment with irisin possibly normalizes some negative alterations associated with aging in cardiomyocytes such as mitochondrial dysfunction, metabolic disturbances, cellular oxidative status (Wang et al., 2018; Lu et al., 2020), and hence this can raise the potency of postconditioning with melatonin in improving mitochondrial function and suppressing inflammatory/apoptosis responses in aged I/R rats. Effective co-activation of multiple mechanisms related to cardioprotection may occur following combined conditioning. The combination of melatonin and irisin may activate distinct and parallel survival pathways resulting in improvement of mitochondrial homeostasis and correction of reperfusion-induced pathophysiologies.
To investigate the mechanisms of greater protection by combined therapy with irisin and melatonin in aged rats, their combined effects were evaluated in the presence of 5HD as a mitoKATP channel blocker and/or L-NAME as an eNOS inhibitor. Of note, cardioprotection by combined therapy was completely reversed by 5HD. Therefore, greater cardioprotection by the combination of irisin and melatonin was achieved through the opening of mitoKATP channels. These channels regulate the matrix volume of mitochondria and maintain mitochondrial integrity, having a protective role in mitochondrial function and primary stages of apoptosis and inflammatory reactions (Ferdinandy et al., 2007). Thus, specific modulation of mitoKATP channels might be a reliable strategy to achieve cardioprotection in aged subjects with myocardial I/R injury.
NO increases the opening activity of mitoKATP channels (Gok et al., 2006). Our results indicated that combination therapy with irisin and melatonin significantly elevated the expression of eNOS and the levels of NO. Interestingly, inhibition of NO production by eNOS partially reversed the beneficial effects of this combined therapy. The findings indicated that combined conditioning exerted potent cardioprotective effects via the opening of mitoKATP channels, which are mediated in part by increasing the activity of the eNOS/NO signaling pathway. But besides eNOS/NO-dependent mechanism, other possible mechanisms may also cause the activation of mitoKATP channels by combined conditioning, which needs further studies. Here, other sources of NO production, as well as survival pathways like PI3K/Akt/GSK-3β and JAK2/STAT3, should be considered.
5 Conclusion
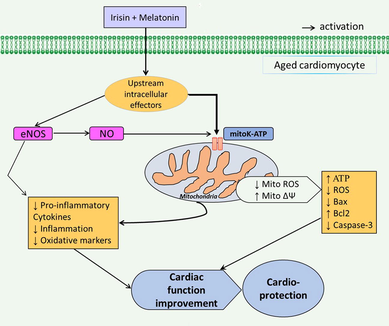
Combination of irisin and melatonin showed potent cardioprotective features in aged rats with I/R insult through improving mitochondrial function and suppressing inflammatory and apoptosis responses via activation of mitoKATP channels, which are mediated to some extent by activation of eNOS/NO signaling pathway (Fig. 8). However, additional efforts are needed for evaluating the impacts of this combined conditioning on the other key signaling pathways associated with mitoKATP channels-dependent heart protection under I/R damage in aged rats.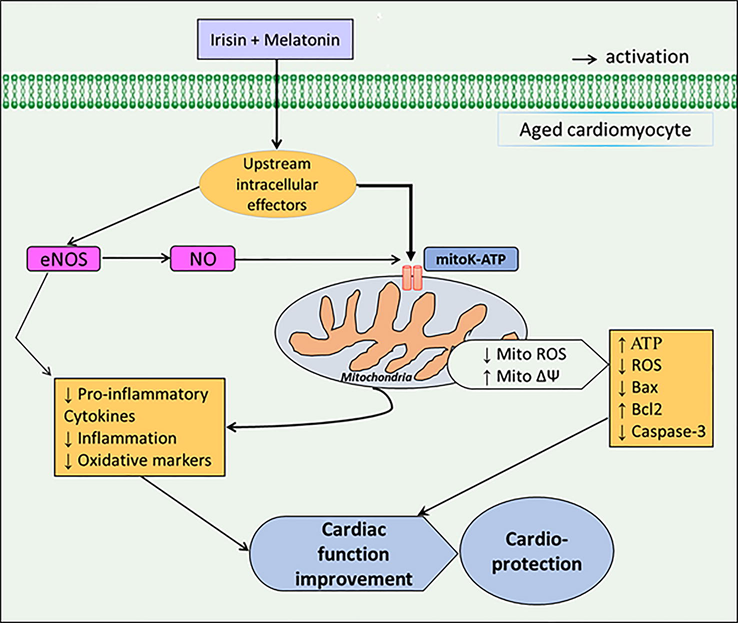
Graphical diagram of the study.
Availability of data and material
The authors confirm that the data supporting the findings of this study are available upon reasonable request.
Authors' contributions
All authors contributed to designing the project, performing the experimentations, analyzing and interpreting the data. ZX and GS were the major contributors in writing the manuscript. All authors read, revised, and approved the final manuscript.
Ethics approval
Animal care procedures, as well as all experimental protocols, were approved by the Institutional Animal Ethical Committee (ethical code: SDPT-2020112453).
Declarations of interest
None.
The author(s) declared no potential conflicts of interest concerning the research, authorship, and/or publication of this article.
References
- Melatonin: therapeutic and clinical utilization. Int. J. Clin. Pract.. 2007;61:835-845.
- [CrossRef] [Google Scholar]
- A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468.
- [Google Scholar]
- Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J. Am. Coll. Cardiol.. 2019;73(1):89-99.
- [Google Scholar]
- Decreased level of melatonin in serum predicts left ventricular remodelling after acute myocardial infarction. J. Pineal. Res.. 2012;53(3):319-323.
- [Google Scholar]
- Interaction of cardiovascular risk factors with myocardial ischemia/reperfusion injury, preconditioning, and postconditioning. Pharmacol. Rev.. 2007;59:418-458.
- [CrossRef] [Google Scholar]
- The role of ATP sensitive K+ channels and of nitric oxide synthase on myocardial ischemia/reperfusion-induced apoptosis. Acta. Histochem.. 2006;108(2):95-104.
- [Google Scholar]
- The Role of Mitochondria in the Mechanisms of Cardiac Ischemia-Reperfusion Injury. Antioxidants (Basel).. 2019;8:454.
- [CrossRef] [Google Scholar]
- Protective effects of melatonin on myocardial ischemia/reperfusion injury in vivo. J. Pineal. Res.. 2002;33:72-80.
- [CrossRef] [Google Scholar]
- Aging might increase myocardial ischemia/reperfusion-induced apoptosis in humans and rats. Age. 2012;34(3):621-632.
- [Google Scholar]
- Pifithrin-alpha attenuates p53-mediated apoptosis and improves cardiac function in response to myocardial ischemia/reperfusion in aged rats. Shock. 2006;26:608-614.
- [CrossRef] [Google Scholar]
- Irisin attenuates myocardial ischemia/reperfusion-induced cardiac dysfunction by regulating ER-mitochondria interaction through a mitochondrial ubiquitin ligase-dependent mechanism. Clin. Transl. Med.. 2020;10(5):e166
- [CrossRef] [Google Scholar]
- Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc. Res.. 2002;55:429-437.
- [CrossRef] [Google Scholar]
- Melatonin protects against heart ischemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Am. J. Physiol. Heart. Circ. Physiol.. 2009;297(4):H1487-H1493.
- [Google Scholar]
- Protective effect of melatonin against mitochondrial dysfunction associated with cardiac ischemia- reperfusion: role of cardiolipin. FASEB. J.. 2006;20(2):269-276.
- [Google Scholar]
- Mitochondrial dysfunction associated with cardiac ischemia/reperfusion can be attenuated by oxygen tension control. Role of oxygen-free radicals and cardiolipin. Biochim. Biophys. Acta.. 2005;1710(2-3):78-86.
- [Google Scholar]
- Protective effects of melatonin on myocardial ischemia/reperfusion induced infarct size and oxidative changes. Physiol. Res.. 2005;54:491-495. PMID: 15641932
- [Google Scholar]
- Role of tumour necrosis factor-alpha and other cytokines in ischemia-reperfusion-induced injury in the heart. Exp. Clin. Cardiol.. 2005;10:213-222. PMID: 19641672
- [Google Scholar]
- Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am. J. Physiol. Heart. Circ. Physiol.. 2001;281:H1630-H1636.
- [CrossRef] [Google Scholar]
- Mitochondrial dysfunction in cardiac aging. Biochim. Biophys. Acta.. 2017;1847:1424-1433.
- [CrossRef] [Google Scholar]
- Irisin plays a pivotal role to protect the heart against ischemia and reperfusion injury. J. Cell. Physiol.. 2017;232(12):3775-3785.
- [Google Scholar]
- Irisin protects heart against ischemia-reperfusion injury through a SOD2-dependent mitochondria mechanism. J. Cardiovasc. Pharmacol.. 2018;72(6):259-269.
- [Google Scholar]
- Zhai, M., Li, B., Duan, W., 2017. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal. Res. 63, e12419. https://doi.org/10.1111/jpi.12419.
- Hydrogen sulfide contributes to cardioprotection during ischemia-reperfusion injury by opening K ATP channels. Can. J. Physiol. Pharmacol.. 2007;85:1248-1253.
- [CrossRef] [Google Scholar]







