Translate this page into:
Insecticide residue analysis on vegetable crops through Rapid Bioassay of Pesticide Residue (RBPR) technique in Nepal
⁎Corresponding author. dipakbabu@hotmail.com (Dipak Khanal)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Insecticides applied on food crops and vegetables reduce the pest population and leave chemical residues that may result in serious health consequences. In Nepalese context, farmers use pesticides repeatedly to get rid of pests and also don’t consider the waiting period. The study was conducted to evaluate the amount of pesticide residue after application of different organophosphate and carbamate insecticides in vegetable crops. Five insecticides were used in seven different combinations (Dimethoate, Malathion, dichlorvos, Chlorpyriphos, Dimethoate + dichlorvos, Malathion + Chlorpyriphos and Carbofuran) and applied in mustard leaves and broccoli. The residues were assessed using the “Rapid Bioassay of Pesticide Residue technique”. Results exhibited that Chlorpyriphos treated mustard leaves were edible in 3 DAA (Days after application) and in 6 DAA when sticker was applied with treatment. With or without sticker Dimethoate followed by Malathion applied mustard leaves were edible in 6 DAA. With or without sticker Dichlorvos took longest (12 DAA) to reach the safe limits for mustard leaves. In contrary the Dichlorvos treated broccoli was edible in 3 DAA but took 6 DAA when sticker was applied. Malathion treated broccoli, with and without sticker, was edible in 6 DAA. For both crops Carbofuran exhibited anomalous nature showing lower residue level in the beginning and higher later. When both vegetables were applied treatments with stickers, they showed significantly higher residue and took longer time to reach edible limit. The applied insecticides took relatively longer to reach safe level in mustard leaf as compared to broccoli. The study suggests use of Chlorpyriphos for mustard leaves and Malathion for broccoli with at least 6 days of waiting period, with or without use of sticker.
Keywords
Carbamate
Organophosphate
Stickers
Waiting period
Safe limit
1 Introduction
Agriculture serves as the backbone of Nepalese economy as majority of the Nepalese directly involve in Agriculture as their primary occupation. Before 90s Nepalese agriculture was mainly cereal based but post 90 there has been tremendous rise in interest of people in commercial vegetable farming. With the introduction of green revolution to the world, Nepal also started the use of chemicals for warding off the pests. As vegetable being the good source of income and quicker way to get the return on investment, the use of chemicals is extremely high in case of vegetables Rai (2015). Pesticides are poisons, and unfortunately, they can harm more than just the “pests” at which they are targeted (Mahmood et al., 2016). In Nepalese context compared with other commercial food crops, even higher levels of pesticides were found in fruits and vegetables, which are considered the most valuable part of people’s diet. The market accessed areas have been found using pesticides intensively (Sharma, 2014). Improper use of pesticides hampers the health of both producers and consumers. However, farmers are less aware of the havoc these pesticides may impart as a slow poison. Many farmers due to mishandling of pesticides were suffering from acute health problems (Kafle et al., 2021). Pesticides are applied to food crops and vegetables to remove the infestation of different insect pests. As a result, not only do they reduce the pest population but also leave chemical residues that may cause serious health consequences (Kumar et al., 2012, Sookhtanlou et al., 2022). Some of the pollutants like Persistent Organic Pollutants (POPs) persist in nature and contaminate the natural bodies and thus have very long-term impacts (Al-Mamun, 2017).In Nepal most of the farmers are completely unaware that pesticides leave residue behind and specific waiting period is required for each pesticides (Khanal et al., 2022). Hence the need of assessing the persistence of insecticides in food crop after its application and the time it requires for them to be safe is very necessary.
Nepal imports over 600 tons of pesticides in a year (Prasain, 2020). The pesticide use is highest in Plains (Terai) (0.995 a.i. kg/ha) followed by valleys (0.47 a.i. kg/ha) and hill areas (0.314 a.i. kg/ha). Whereas high hill areas (0.085 a.i. kg/ha) makes the least use of chemical pesticides with the country average is 396 a.i./ha (Sharma, 2015). The trend of using pesticides is increasing by approximately 10–20 % every year, so does the pesticide expenses in vegetables and fruits in Nepal (Diwakar et al., 2008).Most of the farmers don’t measure the pesticides before application and apply haphazardly (Khanal and Singh, 2016). This increases the risks from pesticide use. In Southern Nepal, farmers generally use a higher dose of pesticides than the recommended dose (Bhandari et al., 2018). Such chemical pesticides could result in sub-chronic, chronic, or acute dietary exposure to pesticides (Essumang et al., 2008). Thus, it is crucial to apply insecticide as per the label and keep track of their residues after application till it reaches a safe level for harvesting and consumption.
The Rapid Bioassay of Pesticide Residues (RBPR) is a comparatively very cheap and fast way to analyze the pesticide residue in fruits and vegetables, which helps to protect consumers from consuming contaminated food (Chiu et al., 1991). The method came into practice especially in vegetable to rapid screening the chemical residue by GON (Government of Nepal) (ED 2019). This technique utilizes both the AChE (Acetylcholinesterase) test for insecticide assay and B.t.(Bacillus thuringiensis) test for fungicide assay to assess the residues of acetyl cholinesterase-inhibiting insecticides, EBDC's (Ethylene Bisdithio Carbamates, ETU-producing chemicals),and B.t. inhibiting fungicides. AChE blocks the neurotransmission inside the insects’ neurosystem leading to the death of insect (NERC, 2020).
The stickers are the chemical agents or oils that help pesticide solutions adhere to the leaves and other parts of the crops while protecting pesticides from rain, evaporation, and runoff (Kintl et al., 2022). Stickers are composed of mainly fatty acids, latex, aliphatic alcohols, or oils, thus increasing the effectiveness of the pesticides (Czarnota and Thomas, 2013). Due to the plant morphology, applied pesticides do not stick and hence the effect is limited. For better results, farmers apply more pesticides, which affect the environment and the health of producers as well as a consumer. The use of Stickers is found to be an effective means to increase the performance of pesticides. Adding stickers can overcome those hazardous impacts and meanwhile increase the performance. The main objective of this research is to identify the waiting period of different pesticides in broccoli and mustard leaf and the difference in waiting period due to application of stickers.
2 Materials and methods
2.1 Test crops, insecticides, and stickers
This study was conducted at the farm of Institute of Agriculture and Animal Science (IAAS) Paklihawa campus (Fig. 1), Tribhuvan University, Rupandehi, Nepal (270 41′ 0″ N latitude and 830 25′ 0″ E longitudes).
GIS map showing research site (Institute of Agriculture and Animal Science, Tribhuvan University).
The study plot was planned in a Completely Randomized Block Design with seven treatments and four replications. The T-42 variety of mustard (RP seeds, India) and Green sprouting variety of broccoli (SEEN seeds service Centre Pvt. ltd, Chandragiri, 10Thankot) were brought from Karki Agrovet Kalimati, Nepal. The crops were cultivated in the field with package of practices recommended by Nepal Agriculture Research Council (NARC) (NARC, 2022).
The insecticides used during this study are listed in Table 1.
Pesticide’s name
Trade Name
Recommended dose
Recommended dose (ppm of ai)
Category
WHO Class
Mode of application
Dimethoate (30%EC)
Roger
2 ml/ltr water
600 ppm
Organophosphate
II
Foliar spray
Malathion (50%EC)
Cythion
2 ml/ltr water
1000 ppm
Organophosphate
III
Foliar spray
Dichlorvos (76%EC)
Nuvan
2 ml/ltr water
1520 ppm
Organophosphate
Ib
Foliar spray
Chlorpyrifos (20%EC)
Lethal
2 ml/ltr water
400 ppm
Organophosphate
II
Foliar spray
Carbofuran (44% EC)
Furadan 4F
0.720 L/hectare
(Michaud et al., 2007)880 ppm
Carbamate
Ib
Soli drench
Dimethoate + Dichlorvos
Roger + Nuvan
2 ml/ltr water
1060 ppm
Organophosphate
II &Ib
Foliar spray
Malathion + Chlorpyriphos
Malathion + Lethal
2 ml/ltr water
700 ppm
Organophosphate
III & II
Foliar spray
For the improvement in the pesticide’s effectiveness, they were mixed with sticker (Bio-stick, an ecofriendly nonionic surfactant) manufactured by Kusmo Chemicals Pvt. Ltd Maharashtra, India and marketed by Idvans Innovations LLP, Maharashtra, India at the dose of 0.3 ml per liter.
On mustard leaves, hand sprayers (BLC-500) from KISAN Agromart, New Baneshwor, Kathmandu, Nepal with the dimension of 500 ml volume) was used to spray pesticides with and without stickers on the last week of December, and in broccoli, the spray was done with and without stickers on the third week of January.
2.2 Sample preparation
Mustard leaves and florets of broccoli were used for bioassay. The samples were randomly selected for the lab analysis and data was taken 3, 6,9,12, and 15 days after treatment (DAT).
2.3 RBPR bioassay
The RBPR bioassay was done at RBPR lab of Butwal. All the necessary chemicals as listed in Table 2 were prepared in respective amounts required for 200 tests. Standard guidelines provided by PPD (2017) was followed for the test as given below:
-
For preparing the control solution (Blank/standard solution), 3 ml Phosphate Buffer Solution buffer was taken in a cuvette (size 4 ml) and 20 µl AChE (Acetyl cholinesterase) solution was added, followed by the addition of 20 µl 95% ethanol.
-
The solution was shaked for 5 s and left still for 2.5 min.
-
Then 100 µl DTNB (5, 5′-dithiobis (2-nitrobenzoic acid) Ellman's Reagent) solution was added and mixed with 20 µl ATCI solution. The solution so formed is termed as control solution. The absorbance for the control solution was measured with 412 nm spectrophotometer (220-240EC, UV-1280, Shimadzu company).
-
For preparing the test sample,1–2 gm of the finely chopped fresh mustard leaves and broccoli floret sample was taken separately in test tubes followed by the addition of 1 ml 95% ethanol to test tube containing carbamate (Carbofuran) and 2 ml for organophosphate and the combination of (Dimethoate, Malathion, Dichlorvos, Chlorpyriphos, Dimethoate + Dichlorvos, Malathion + Chlorpyriphos) samples respectively.
-
The treated sample was shaken (40 W, 2000 rev/minute) properly with the help of a vortex mixture (Touch-type, optics technology).
-
Further, 0.1 ml (100 µl) of 0.4% Bromine solution was added into organophosphate sample inside laminar airflow (Fume Hood Company. Dsidc Community Work Centre, Delhi).
-
The sample was left for 20 min to allow the evaporation of excess bromine water after which it was used for further tests.
-
For residue analysis, 3 ml PBS (Phosphate Buffer Solution) was added in the cuvette followed by 20 µl AChE solution and 20 µl sample extract and were mixed for 5 sec, left still for 2.5 min, and added with 100 µl DTNB solution.
-
Immediately 20 µl ATCI solution was added and mixed for 5 sec to start enzymatic reaction. Then it was tested in the spectrophotometer to obtain the inhibition percentage.
| SN | Reagents and Chemicals | The amount of distilled water used for 200 Tests |
|---|---|---|
| 1 | Acetyl cholinesterase, AChE | 4 ml |
| 2 | Acetyl thiocholine Iodine, ATCI | 4 ml |
| 3 | Color developing agent, DTNB | 20 ml |
| 4 | PBS Buffer | 600 ml |
| 5 | Bromine water (0.4%) | 20 ml |
2.4 Pesticide residue calculation
Pesticide residue was analyzed using the protocol provided by Taiwan agriculture Research Institute (Chiu et al., 1991).
Most of the pesticides exhibit toxicity by inhibiting AChE enzyme and thus the inhibition percentage is relevant for measuring the residue level (Pundir and Chauhan, 2012). When the enzyme inhibition percentage is 45% inhibition or above, the food is not considered safe to consume. For 35%-45% inhibition, decontamination by repetitive washing is suggested before consumption and for, 35% inhibition or below, it is considered safe for consumption (PPD, 2017). Higher inhibition % is proportionate with the higher residue level of the pesticide and is discussed below accordingly (Rajangam et al., 2018).
2.5 Statistical analysis
After the application of treatments on mustard leaves, the residue analysis was done 3 days after application followed by 6, 9, 12, and 15 days after the application. Data obtained from RBPR was tabulated in MS Excel 2019. The data were analyzed by separating means through DMRT (Duncan’s Multiple Range Test) at 5% level of significance using R studio 4.0.3 using Readxl and Agricolae packages. CV (Coefficient of Variation) and LSD (Least Significant Difference) were also calculated to exhibit the distribution of the data among the treatments. Paired t- Test was carried out to compare the performance with and without sticker, with the help of MS Excel 2019.
3 Results
Results exhibited that the percentage inhibition level of Dichlorvos was significantly higher than other treatments at 3 DAA (Fig. 2), followed by Dimethoate and the combination of Malathion and Chlorpyriphos. On 6 DAA, the percentage inhibition of Dichlorvos still exhibited a significantly higher level than the rest of the treatments followed by the combination of Malathion and Chlorpyriphos and the combination of Dimethoate and Dichlorvos. Even on 9 DAA, Dichlorvos exhibited significantly high percentage inhibition followed by Carbofuran and Malathion. After 12 days, Carbofuran showed a significantly high percentage inhibition followed by Dichlorvos and on 15 DAA, Carbofuran was on top followed by Dichlorvos. In the case of Carbofuran, the percentage kept increasing until it reached the highest point on 12 DAA and started to reduce thereafter. The percentage inhibition of Carbofuran was in the acceptable range after 15 DAA. Some treatments like Dimethoate and Chlorpyriphos showed least percentage of inhibition after 6 DAA after which the percentage further, decreased on the following days. After 9 DAA except for Dichlorvos and Carbofuran, the rest of the treatments showed a safe level of inhibition percentage.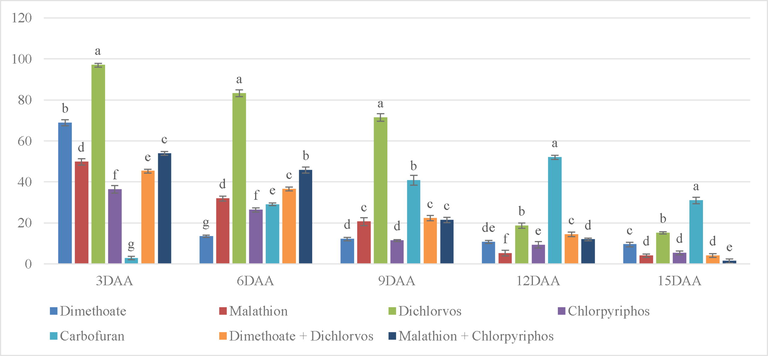
% inhibition on mustard leaf due to different treatments application with stickers. (Note. The bars on top of graphs are produced from standard error. Mean in column with same superscript is not significant at 5% level of significance (p ≥ o5).’***’0.001 ‘**’0.01 ‘*’0.05; DAA = Days after application.).
At 3 DAA, a maximum percentage inhibition was found with Dichlorvos followed by Dimethoate and Malathion (Fig. 3), whereas the lowest percentage was observed in Carbofuran followed by Chlorpyriphos. On 6 DAA, Dichlorvos still had the highest percentage of inhibition followed by the combination of Malathion + Chlorpyriphos and Dimethoate + Dichlorvos. Pesticides except for Dichlorvos and the combination of Malathion + Chlorpyriphos, presented inhibition lower than safe limits. On 9 DAA, Dichlorvos still exhibited the highest inhibition percentage followed by Carbofuran. The inhibition observed in Dichlorvos and Carbofuran were more than safe limit whereas in Dimethoate and Chlorpyriphos treated samples was least among all treatments. After 12 DAA, Carbofuran showed significantly highest inhibition followed by Dichlorvos. The percentage inhibition of Dichlorvos finally falls within a safe range. The inhibition by Carbofuran was still on top on 15 DAA followed by Dichlorvos while in others the percentage was extremely lower. Chlorpyrifos was found to be least inhibited, after Carbofuran, as compared to other treatments from the beginning. After 9 DAA, every treatment was at a safe level except Carbofuran as its inhibition kept increasing. After 15 DAA, all the pesticides are showing the safer percentage of inhibition.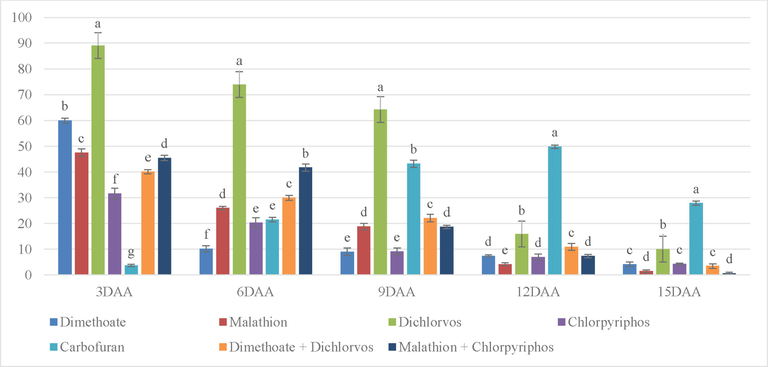
% inhibition on mustard leaves due to different treatments without stickers. (Note. The bars on top of graphs are produced from standard error. Mean in column with same superscript is not significant at 5% level of significance (p ≥ o5).’***’0.001 ‘**’0.01 ‘*’0.05; DAA = Days after application.).
Fig. 4 shows that on 3 DAA, the inhibition percentage of Chlorpyriphos was found to be significantly higher followed by the combination of Malathion and Chlorpyriphos. On 6 DAA, the percentage of inhibition by Chlorpyriphos still exhibited significantly highest residue level followed by the combination of Malathion and Chlorpyriphos and Carbofuran. On 9 DAA, Carbofuran exhibited significantly highest percentage inhibition followed by Chlorpyriphos and a combination of Malathion and Chlorpyriphos. Even on 12 days, Carbofuran showed significantly highest inhibition level followed by Chlorpyriphos and Dichlorvos. On 15 DAA, the inhibition by the combination of Malathion and Chlorpyriphos was on top. In the case of Carbofuran, the inhibition level kept increasing until it reached the highest on 9 DAA and started falling onwards. For broccoli, Dichlorvos was detected with a low inhibition level and appeared safe within 6 DAA.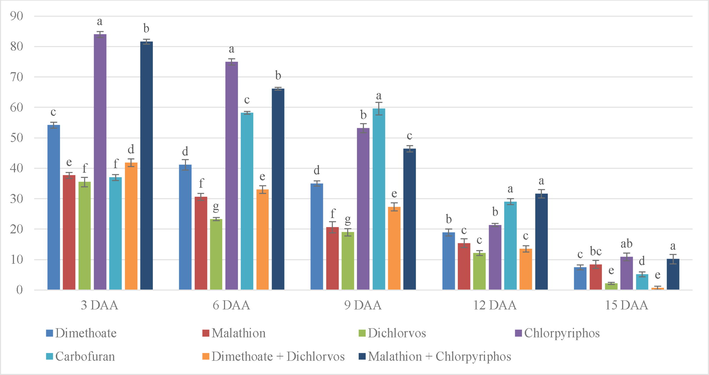
% inhibition on broccoli due to different treatments application with stickers. (Note. The bars on top of graphs are produced from standard error. Mean in column with same superscript is not significant at 5% level of significance (p ≥ o5).’***’0.001 ‘**’0.01 ‘*’0.05; DAA = Days after application.).
The result (Fig. 5) shows that on 3 DAA, the inhibition level of Chlorpyriphos is highest and most significant followed by the combination of Malathion and Chlorpyriphos. On 6 DAA, Carbofuran exhibited significantly highest percentage of inhibition followed by the combination of Malathion and Chlorpyriphos and Carbofuran. On 9 DAA, Carbofuran still showed significantly higher percentage followed by the combination of Malathion and Chlorpyriphos. Even on 12 days, Carbofuran showed significantly highest inhibition followed by the combination of Malathion and Chlorpyriphos. On 15 DAA combination of Malathion and Chlorpyriphos was found to contain the highest inhibition percentage among all treatments.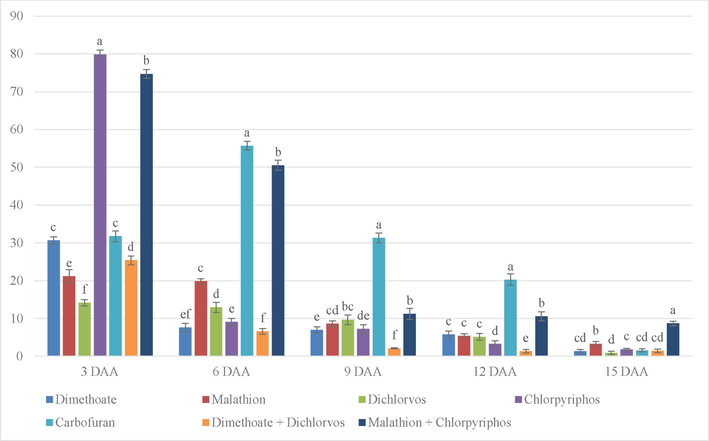
% inhibition on Broccoli due to different treatments without the sticker. (Note. The bars on top of graphs are produced from standard error. Mean in column with same superscript is not significant at 5% level of significance (p ≥ o5).’***’0.001 ‘**’0.01 ‘*’0.05; DAA = Days after application.).
The results in Fig. 6 reflects the difference in inhibition percentage due to the use of sticker in mustard leaves. For 3DAA the average inhibition exhibited with sticker is significantly higher than without sticker. Similarly on 6 DAA, 12 DAA and 15 DAA the difference is highly significant whereas on 9 DAA the difference is just significant enough.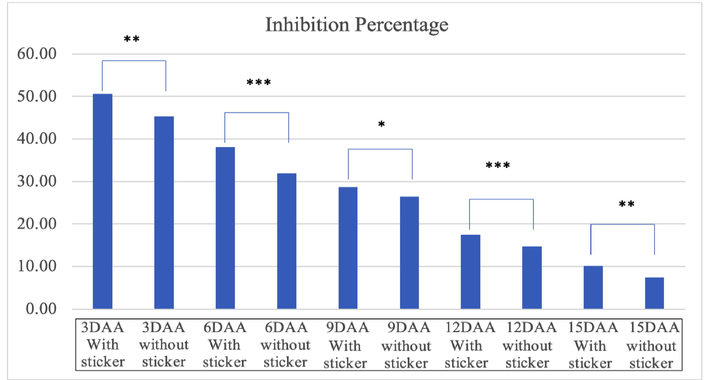
Comparison of % inhibition on Mustard leaves due to different treatments with and without the sticker. (Note. The * label on the top of bars represents there is significant difference at 5% level of significance (p ≥ o5).’***’0.001 ‘**’0.01 ‘*’0.05; DAA = Days after application.).
Fig. 7 shows the difference in inhibition percentage with and without sticker in case of Broccoli. The inhibition difference on 3 DAA is highly significant. The difference gradually decreases on 6 DAA as the difference between them however the difference is significant enough. Whereas on 9 DAA and 12 DAA the difference between the inhibition is very high and the difference gap narrows on 15 DAA.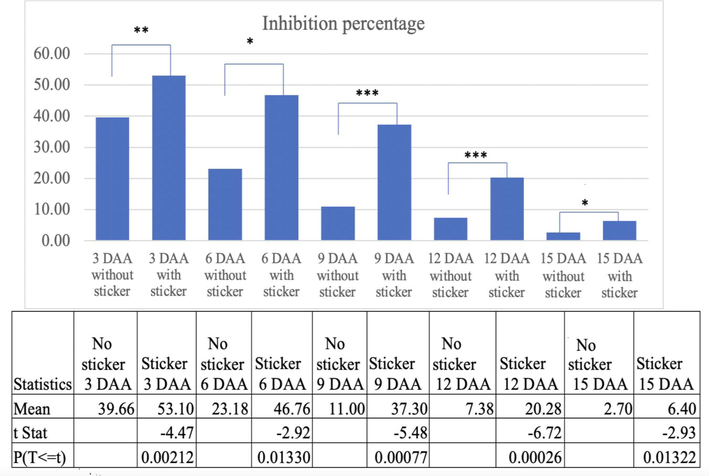
Comparison of % inhibition on Broccoli due to different treatments with and without the sticker. (Note. The * label on the top of bars represents there is significant difference at 5% level of significance (p ≥ o5).’***’0.001 ‘**’0.01 ‘*’0.05; DAA = Days after application.).
4 Discussion
In case of mustard leaf Dimethoate treated sample was found to have safe level of residue at 6 DAA. This means whether or not sticker is applied along with Dimethoate, mustard leaves were found to be edible after 6 days. A study by Gopalakrishnan et al., (2018) in the waiting period of Dimethoate 30 EC was found to be 7 DAA in spinach. However, with the sticker in Broccoli, the fall in Dimethoate residue was significantly slower as compared to without sticker that reached a safer limit only after 9 DAA. However, without the sticker, the waiting period of 6 days was found in both mustard leaf and broccoli for Dimethoate. A similar result was observed in a study by Sharma and Choudhury (2018), that reported the waiting period of Dimethoate 30 EC in cauliflower and cabbage to be 6 days.
When Dichlorvos was applied with the sticker in the mustard leaf, the residue level remained extremely high, and the degradation process was also very slow for 9 days. In 12 days, the residue declined to an incredibly low level and well below the safer limit. Similar results were seen by Sinyangwe et al., (2016), where Dichlorvos exhibited a significantly high residue level above WHO recommended limits. When Dichlorvos was applied without sticker the results were quite the same, but the residue level was relatively low. In both cases, with or without the sticker, the waiting period was found to be 12 days. A study conducted by Jyothi et al., (2013) to assess the waiting period of pesticides on mulberry leaves shows the waiting period of 9 days. In the case of Broccoli, pesticide residue was initially low and gradually kept decreasing, reaching a safer limit in 6 days with the sticker. However, without the sticker, the residue was already very lower than a safe critical limit in 3 days after application, and the level fell slowly onwards. A waiting period of 3 days was suggested for Dichlorvos by Paramasivam et al., (2012) in the case of mulberry leaf for feeding silkworms.
When both Dimethoate and Dichlorvos were mixed and used in mustard leaves, the residue level was found lesser as compared to individual application in 3 DAA and reached a safe limit in 9 days. A similar result was found where the residue of Dichlorvos was not detected after 9 days in cauliflower (Abdel-Wali et al., 2006). However, in the case of the mustard leaf without the sticker, the waiting period of the combination was found to be 6 DAA. For broccoli, the combination exhibited a safer residue level in 3 days after application, and after 6 days, the residue level was incredibly low, which gradually decreased onwards. However, with the sticker, it took 6 DAA to become safe for consumption.
When Malathion was sprayed in mustard leaf, the safer residue level was achieved in 6 days when applied with or without the sticker. When the sticker was applied, the reduction in residue level was slower as compared to that without the sticker. While in Broccoli, it reached a safer limit in 3 days after application but with sticker, it took 6 days to reach a consumable limit. Thus, the waiting period of Malathion can range from 3 to 6 days. Related results were obtained by Fouche et al. (2000) in which the waiting period of Malathion was suggested to be 1–7 DAA of pesticide in general.
When Chlorpyriphos was applied to mustard leaves, after 6 DAA, the residue level was within a safer limit with sticker, and without the sticker, it was found safe by 3 days after application. In broccoli, the initial residue was significantly the highest in DAA, but by 6 days after application, the residue was lower than the safe critical limit. The safe period for Chlorpyriphos without sticker was found to be 6 DAA. However, when the sticker was applied along with, the fall in the residue was slow which took 12 days to reach a consumable residue range. However, according to European Food Safety Authority (2012), a waiting period of 21 DAA was recommended for Broccoli.
When both Chlorpyriphos and Malathion were mixed and applied on the mustard leaf, the residue level was higher than the safe residue level for 6 days, after which the residue level reached drastically below the acceptable limit in 9 DAA. While in Broccoli, it took 12 days with sticker and 9 days without sticker to reach the safe residue level.
Unlike other pesticides, the residue level of Carbofuran was initially found extremely low which gradually increased in both mustard leaf and broccoli. It was applied as soil drench rather than foliar application and thus the absorption rate by the crop was considerably low initially. However, in mustard leaf, for 12 DAA, the residue level kept increasing sharply, and on 15 DAA, the residue level was found just below the critical point. The waiting period of Carbofuran was observed 15 days (Morais et al., 2012). In the case of Broccoli, the residue level kept increasing for 9 DAA when it was applied with stickers, and on 12 DAA, the vegetable was safe to consume. Without sticker, the residue level kept increasing for 6 days and reached a safe limit on 9 days after application.
It’s a myth that the leafy vegetables are very risky to use chemical pesticides as all the shoot parts are edible compared to that of other crops. However, the results show mean residue levels show that the reduction in residue level was comparatively faster in case of mustard leaves to that of broccoli. The possible reason behind this is the leafy vegetables offer higher surface area for transpiration and transpire more pesticides and the level of chemical in it falls quickly. Use of pesticide should not be encouraged in developing countries like Nepal where farmers lack adequate knowledge on waiting period and harmful impact of chemical pesticides. They don’t even have proper access to agriculture technician and depend on Agrovets for advice (Rijal et al., 2018). IPM should be the first resort of pest management and the chemical means of control as final option (Paudel et al., 2020).
Pesticides like Dichlorvos and Carbofuran were recently banned from use in Nepal (PPS, 2023). The study justifies the ban as the degradation of Dichlorvos in mustard leaves was very slow and the degradation of Carbofuran was very anomalous. However, when used in combination the degradation was relatively faster.
5 Conclusion
Among the applied insecticide combination Chlorpyriphos was found to be the quickest to reach the safe limits. If applied with sticker it could be consumed within 6 DAA and without sticker in just 3 DAA. For Broccoli Dichlorvos was the safest insecticide with residue level very low below the ceiling of safe limits in just 3 DAA, whereas took 6 DAA with sticker. However, Dichlorvos exhibited the highest residue level in mustard leaves and Chlorpyriphos in case of Broccoli. The safer insecticide for a crop doesn’t necessarily exhibits safe nature for other crops. Farmers in Nepal consider the pesticides in general way and rank the hazard only on the basis of label behind the packet. Some insecticides like Carbofuran which seemed to be safer early on 3 DAA was later found have unsafe level of residue later on for both crops. More study should be done with different dose to assess this nature of Carbofuran. Addition of sticker was found to increase the level of residue and also increased the waiting period for consumption. So, more studies should be done regarding the waiting period of pesticides with stickers. If possible, the waiting period of pesticides with and without sticker should be clearly mentioned in the bottle of pesticide. For Nepalese context agriculture extension agents and Agrovets should work closely with researchers and share updated information with farmers in regular basis. The study suggests farmers to practice IPM and keep chemical management of pest as last resort. Farmers are also recommended to use pesticides with stickers rather than using pesticides frequently and wait accordingly. More research should be carried on regarding the waiting period and the varying residue level for different DAA with varying dose of pesticides.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abdel-Wali, M., Bahdousheh, M., Al-Awamleh, A., Shaderma, A., Arabyat, S., Ananbieh, K., Ayassreh, M., Frehat, A., Romiah, N., Alawneh, Y., 2006. Determining Pesticides Waiting Periods and Residues on Vegetables under Jordan Valley Conditions. In: I International Symposium on Fresh Food Quality Standards: Better Food by Quality and Assurance. 741.
- Pesticide degradations, residues and environmental concerns. Pesticide Residue in Foods, Springer 2017:87-102.
- [Google Scholar]
- Modification of the existing MRLs for chlorpyrifos in various crops and in products of animal origin. EFSA J.. 2012;10(1):2510.
- [Google Scholar]
- Factors affecting pesticide safety behaviour: the perceptions of Nepalese farmers and retailers. Sci. Total Environ.. 2018;631:1560-1571.
- [Google Scholar]
- Rapid bioassay of pesticide residues (RBPR) on fruits and vegetables. 中華農業研究.. 1991;40(2):188-203.
- [Google Scholar]
- Study on major pesticides and fertilizers used in Nepal. Sci. World. 2008;6(6):76-80.
- [Google Scholar]
- Annual Report, 2075/76 (2018/19). NARC, Khumaltar, Lalitpur, Nepal: Entomology Division; 2019. p. :156.
- Analysis of some pesticide residues in tomatoes in Ghana. Hum. Ecol. Risk Assess.. 2008;14(4):796-806.
- [Google Scholar]
- Fouche, C., Molinar, R., Canevari, M., Joshel, C., Mullen, B., Weber, J., 2000. Pesticides for specialty crops.
- Persistence and dissipation pattern of dimethoate 30 EC in/on foxtail amaranthus and spinach. Madras Agric. J.. 2018;105(march (1–3)):1.
- [Google Scholar]
- Waiting period for insecticides and a botanical used in control of Mulberry Thrips and there safety to silkworm. Annual Plant Protection Sci.. 2013;21(1):42-45.
- [Google Scholar]
- Factors associated with practice of chemical pesticide use and acute poisoning experienced by farmers in Chitwan district, Nepal. Int. J. Environ. Res. Public Health. 2021;18(8):4194.
- [Google Scholar]
- Khanal, D., Neupane, A., Dhital, A., Paudel, K., Shrestha, M., Upadhyaya, N., 2022. Knowledge, skills, and behavior towards chemical pesticide among vegetable growers, vegetable sellers, and consumers of Rupandehi District, Nepal.
- Patterns of pesticide use and associated factors among the commercial farmers of Chitwan, Nepal. Environ. Health Insights. 2016;10 EHI. S40973
- [Google Scholar]
- Potential effect of wetting agents added to agricultural sprays on the stability of soil aggregates. Soil. 2022;8(1):349-372.
- [Google Scholar]
- Harmful effects of pesticides on human health. Annals Agri-Bio Res.. 2012;17(2):125-127.
- [Google Scholar]
- Effects of pesticides on environment. Plant, soil and microbes, Springer 2016:253-269.
- [Google Scholar]
- Impact of the stem borer, Dectes texanus, on yield of the cultivated sunflower, Helianthus annuus. J. Insect Sci.. 2007;7(1)
- [Google Scholar]
- Nepal Agriculture Research Council. Singhadurbar Plazza, Kathmandu: NARC; 2022.
- Annual Report, 2076/77 (2019/20). Nepal.: National Entomology Research Centre, NARC, Khumaltar, Lalitpur; 2020. p. :164.
- Persistence and dissipation of dichlorvos and profenofos on mulberry leaves. Madras Agric. J.. 2012;99(7–9):583-585.
- [Google Scholar]
- Conservation agriculture and integrated pest management practices improve yield and income while reducing labor, pests, diseases and chemical pesticide use in smallholder vegetable farms in Nepal. Sustainability. 2020;12(16):6418.
- [Google Scholar]
- Standard Operating Procedures for Rapid Bioassay of Pesticide Residues Laboratory. Lalitpur, Nepal Plant Protection Directorate: D. o. P. Protection; 2017. p. :23.
- Banned pesticide list in Nepal. Hariharbhawan: Kathmandu, Plant Protection Society, Nepal; 2023.
- Nepal continues to import pesticides at an alarming rate, government’s own data shows. Kathmandu: The Kathmandu Post; 2020.
- Acetylcholinesterase inhibition-based biosensors for pesticide determination: a review. Anal. Biochem.. 2012;429(1):19-31.
- [Google Scholar]
- Integrated pest management for vegetable crops. Improv Prod. Technol. Veg Crop.. 2015;59:150-169.
- [Google Scholar]
- Progress in enzyme inhibition based detection of pesticides. Eng. Life Sci.. 2018;18(1):4-19.
- [Google Scholar]
- Farmers’ knowledge on pesticide safety and pest management practices: a case study of vegetable growers in Chitwan, Nepal. Agriculture. 2018;8(1):16.
- [Google Scholar]
- Use of pesticides and its residue on vegetable crops in Nepal. J. Agric. Environ.. 2015;16:33-42.
- [Google Scholar]
- Sharma, D., Choudhury, P. P., 2018. Pesticide use and their residue management in vegetables.
- Sharma, D.R., 2014. Practical aspects of pesticide risk assessment and phasing out of Highly Hazardous Pesticides (HHPs) in Nepal.
- Determination of dichlorvos residue levels in vegetables sold in Lusaka, Zambia. Pan African Medical J.. 2016;23(1)
- [Google Scholar]
- Health risk of potato farmers exposed to overuse of chemical pesticides in Iran. Saf. Health Work. 2022;13(1):23-31.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102671.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







