Translate this page into:
Insecticidal and repellent activities of Solanum torvum (Sw.) leaf extract against stored grain Pest, Callosobruchus maculatus (F.) (Coleoptera: Bruchidae)
⁎Corresponding author. zookaleesh@gmail.com (B. Kaleeswaran)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Objectives
Pesticides are harmful to nature and therefore they are considered to be poisonous to the world. They have adverse effects on human health that include acute toxicity, cancer and endocrine systems, etc. Plants are a good alternative natural source for considering the negative impacts of conventional pesticides. Plant extracts are traditionally used to manage the insects.
Methods
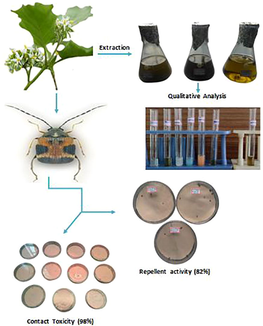
In the present study, the crude leaf extract of Solanum torvum (Sw.) was investigated for their preliminary phytochemical screening and their ability to protect the stored green grams from Callosobruchus maculatus (F.) adult infestation. The Solanum torvum (Sw.) ethyl acetate leaf extract was exhibited strong contact toxicity and repellent activity against Callosobruchus maculatus (F.) adult. The toxicity was significantly improved while extended treatment times and concentrations of Solanum torvum (Sw.).
Results
The mean percentage of ethyl acetate leaf extract repellent value was reached 82% at the dose of 1500 ppm/cm2 after 1 h, followed by methanol (52%) and hexane (28%) leaf extract. The mortality was reached over the ethyl acetate leaf extract nearly 98% at the dose of 900 µg/cm2 after 72 h, followed by methanol (70%) and hexane (48%) leaf extract. Contact toxicity value of Solanum torvum (Sw.) leaf extract LC50 at 72 h interval was observed at 393.271 µg/mL, 632.338 µg/mL and 894.333 µg/mL for ethyl acetate, methanolic and hexane extract respectively against Callosobruchus maculatus (F.) adult.
Conclusion
Thus the present study, Solanum torvum (Sw.) leaf extract could be useful for integrated pest management of Callosobruchus maculatus (F.) adult. The ethyl acetate leaf extract was shown good repellent and contact toxicity effect, followed by methanol and hexane extract. This method of natural plant extract can be used to control pests, alternate against the chemical insecticide.
Keywords
Agriculture
Solanum torvum
Callosobruchus
Contact toxicity
Pesticide
1 Introduction
The growing population of the world requires large quantities of food. Cultivated grains are the most common food for human. The stored grains become highly susceptible to pests and about one-third of the world's grain storage is attacked by pests. Nearly, 10–40% of grains are damaged by stored grain pestsevery year in developing countries. In the world stored grains, especially, green gram and black grams are economically damaged by the pulse beetle, Callosobruchus maculatus (Fab.) (Coleoptera: Bruchidae). Their seeds contain 92% of protein and carbohydrates and 8% of low levels of calcium, iron, vitamins and carotene (Olufunmilayo, 2012). Farmers cultivate cowpea grains, even in areas where other crops and grains are not grown. It helps them to improve their daily life and life skills (Oluwafemi et al., 2013). Ngamo et al. (2007) reported that 78% of farmers produce cowpea seeds in Northern Cameroon. The major cause of grain damages by C. maculatus, which causes more damages than other pests. It is a common pest in tropics and subtropics places of the world (Park et al., 2003). Due to damage of this pest, the world suffers from malnutrition. People living in poorer tropical countries suffer from protein deficiency in their daily lives (Oluwafemi et al., 2013).
Stored products, foodstuffs and harvestable crops have an important concern to prevent this insect. Many techniques prevent post-harvest losses against pests (Kamanula et al., 2010). Often the chemical pesticides are used to prevent or control pests in agricultural lands and stored areas. Stored product pests are controlled usually by methyl bromide and phosphine chemicals, especially against coleopteran pests (Mueller et al., 1990). These types of chemical pesticides control the pest and at the same time, many side effects are appeared directly and indirectly like, ozone depletion, toxicity on non-target species and pest resistance (Okonkwo and Okoye, 1996). Phosphine fumigant toxicity method is used to effectively manage the C. maculatus, which kills or poisonous to human (Garry et al., 1989). Abder-Rahman (1999) was reported that the aluminium phosphate used in the fumigant toxicity technique against the stored product insects. This chemical was seriously affected the internal organs of human, such as heart, blood vessels and lungs.
The pest control board makes a large number of efforts to control the pests with plant-derived compounds. Therefore, researchers are developing new methods that can have minimal side effects on the environment and organisms. Their first attempt was to use the compounds obtained by aromatic plants to control the pests of stored goods (Nerio et al., 2009). Essential oils of the plant are the best alternative insect control agent of stored grain pest (EzhilVendan et al., 2017).
The plant Solanum torvum (Sw.), family Solanaceae was used for our present research. It can be seen as a small shrub, distributed widely in India, Malaya, Pakistan, tropical America, Philippines. Solanum torvum has mainly steroids, saponins, alkaloids, and phenols as a chemical constituent and compounds derived from this plant can be used to treat variety of diseases. Pharmacological studies indicated that the stem and root of S. torvum have anti-microbial, anti- tumour, anti-inflammatory and other activities (Anonymous, 2000; Haritha et al., 2016). Methanolic extracts of S. torvum fruits and leaves were reported about their significant antimicrobial activities against human pathogen (Chah et al., 2000; Elango et al., 2016). Isoflavonoid sulfate and different steroidal glycosides were isolated from S. torvum fruits, which were used to antiviral and antioxidant activity (Abas et al., 2006; Glorybai et al., 2015). Recently, novel protein was isolated from the aqueous extract of S. torvum seed that has proved to be an effective antioxidant activity by Sivapriya and Srinivas (2007). Various parts of aqueous extract of the plant exhibit potential anti-inflammatory and analgesic properties (Ndebia et al., 2007; Fowsiya et al., 2016). Also, traditionally this plant is used for food and medicinal purposes by the local people to remove intestinal parasitic larvae and tooth related issues and from exhaustive literature survey, we couldn’t find any scientific report for the pest control or management. With this background interesting factors and information we were adapted this plant Solanum torvum (Sw.) for our research.
In pest management, repellent activity and contact toxic effect of different organic solvent Solanum torvum leaf extract not been studied against Callosobruchus maculatus (F.). There is no previous report worked against stored product insect’s C. maculatus. Hence, the present study was undertaken to investigate the contact toxicity and repellent activity of S. torvum leaf extract against C. maculatus.
2 Materials and methods
2.1 Callosobruchus maculatus
Suleiman et al. (2012) methods were adopted with slight changes of the rearing insect in our research. The laboratory subculture of C. maculatus insects were utilized to set up the experiment from the normally contaminated green gram seeds, which were gathered from the nearby markets of Thanjavur, Tamilnadu, India. The spotless green grams (uninfected 300 g) were set in five plastic containers and reared female and male C. maculatus in every plastic jar were released. Muslin fabric was used to cover the containers and wait for five days to permit oviposition of the C. maculatus. The subculture of C. maculatus has kept at 28 ± 3 °C & 81 ± 4 °C RH (Relative Humidity). The toxicity study and repellent activity were done in recently emerged adults.
2.2 Solanum torvum
The wholesome leaves of Solanum torvum (Sw.) were gathered from the Kovilvenni near to Thanjavur district (2018). It was taxonomically identified and authenticated by Rev Dr. S. John Britto SJ, Director, The Rapinat Herbarium and Centre for Molecular systematic, St. Joseph College (Autonomous), Tiruchirappalli, Tamilnadu, India. The voucher specimens are deposited at the Rapinat herbarium and the voucher number is ST 004. The leaves were dried and conceal with airtight container and powdered by blender for the experimental research.
3 Solvent extraction
The dehydrated S. torvum leaf powdered (5 kg) was extracted progressively with hexane, ethyl acetate and methanol solvent (Medox Biotech, India Pvt. Ltd.) in the Soxhlet apparatus. Then, excess of solvent was removed by a rotary vacuum evaporator under 60 °C temperature. Finally, the obtained extract (50 g) was collected and stored at 0 °C for the futuristic purposes.
3.1 Phytochemical screening
The extracts were subjected to analysis for various phytochemicals present in the dried leaves of S. torvum. Preliminary phytochemical screening was done by Harborne (1958). The tests were carried out for the presence and absence of alkaloids, saponins, tannins, sterols, flavonoids, phenols and anthraquinones. The chemicals and reagents were used for the above tests were freshly prepared in our laboratory.
4 Insecticidal activity studies
4.1 Repellent activity
Cosimi et al. (2009) method was adopted for the repellent activity area preference for the C. maculatus. Different concentration of methanol, ethyl acetate and hexane extracts of S. torvum (125, 250, 500, 1000 and 1500 ppm/cm2) were used in the experiment. Whatman No. 1 filter paper was cut into two half. One half applied with different extracts of organic extract in different petri dish and the other half was treated with methanol as a control treatment. After, 20 min for the evaporation of the solvent in both treated and control experiments, the well-matured adult C. maculatus (10 Nos) was released into each extract-treated filter paper fixed petri dish and then the petri dishes were airtight and closed. After 1, 3, 9, 12 and 24 h, the number of C. maculatus on treated and control portions of the filter papers were calculated. Five replicates were maintained for each experiment.
The Repellency test percent (PR) was calculated based on Nerio et al. (2009) method
Where Nc = Number of insect on the untreated area (Control)
Nt = Number of insects on treated half (Treatment)
The following classification based on the percent repellency was categorized by (Julianna and Su, 1983):
Class 0 = 0%–0.1% repellency, Class I = 0.1%–20%, Class II = 20.1%–40%, Class III = 40.1%–60%, Class IV = 60.1%–80%, Class V = 80.1%–100%.
4.2 Contact toxicity
Direct contact toxicity approaches were used for the insecticidal action of leaf extract of S. torvum against the C. maculatus (Rajashekar and Shivanandappa, 2010). Different concentrations (0.1, 0.3, 0.5, 0.7, and 0.9 mg/cm2) of 1 ml methanol, ethyl acetate and hexane extracts were sprayed on filter papers (Whatman No. 1 filter paper) separately and 1 ml methanol was used as control. The solvent becomes allowed to evaporate for 20 min and 10 unsexed adults of C. maculatus were released separately into each petri dish. The treatments of five replicates of each group were used for this experiment. Pest mortality was recorded at 24, 48 and 72 h of exposure.
Abbot’s formula (1925) was used to calculate the mortality rate of C. maculatus,= Number of dead insects/Total number of insects × 100
4.3 Data analysis
The Abbot’s (1925) formula was used to calculate the mortality percentage of C. maculatus. The repeated measures analysis of variance using the percentage of repellency value at 1 h, 3 h, 6 h, 9 h 12 h, 24 h and contact toxicity mortality rate was calculated for 24 h, 48 h and 72 h of exposure of S. torvum extract against C. maculatus adults. The LC50 value was calculated using with Graphpad Prism 9.0.1 software.
5 Result
In the present study, the analysis of qualitative phytochemical screening was done in methanol, ethyl acetate and hexane leaves extract of S. torvum. The presence of alkaloids, saponins, tannins, sterols, flavonoids phenols and anthraquinones were shown in Table 1. (+) Presence (−) Absence.
S. No.
phytochemicals
Methanol
Ethyl Acetate
Hexane
1.
Alkaloid
+
+
+
2.
Saponins
+
+
+
3.
Tannins
+
+
–
4.
Steroids
+
+
–
5.
Flavonoids
+
+
+
6.
Phenol
+
–
–
7.
Anthraquinones
–
–
–
Phytochemical constitutes such as alkaloids, saponins, tannins, sterols, flavonoids phenols and anthraquinones were tested in S. torvum. In this study, alkaloids, saponins, tannins, sterols, flavonoids and phenols were observed in methanol leaf extract except anthraquinones. The phenol and anthraquinones were absent in ethyl acetate leaf extracts and alkaloids, saponins and flavonoids were found in hexane leaf extracts of S. torvum.
6 Repellence test
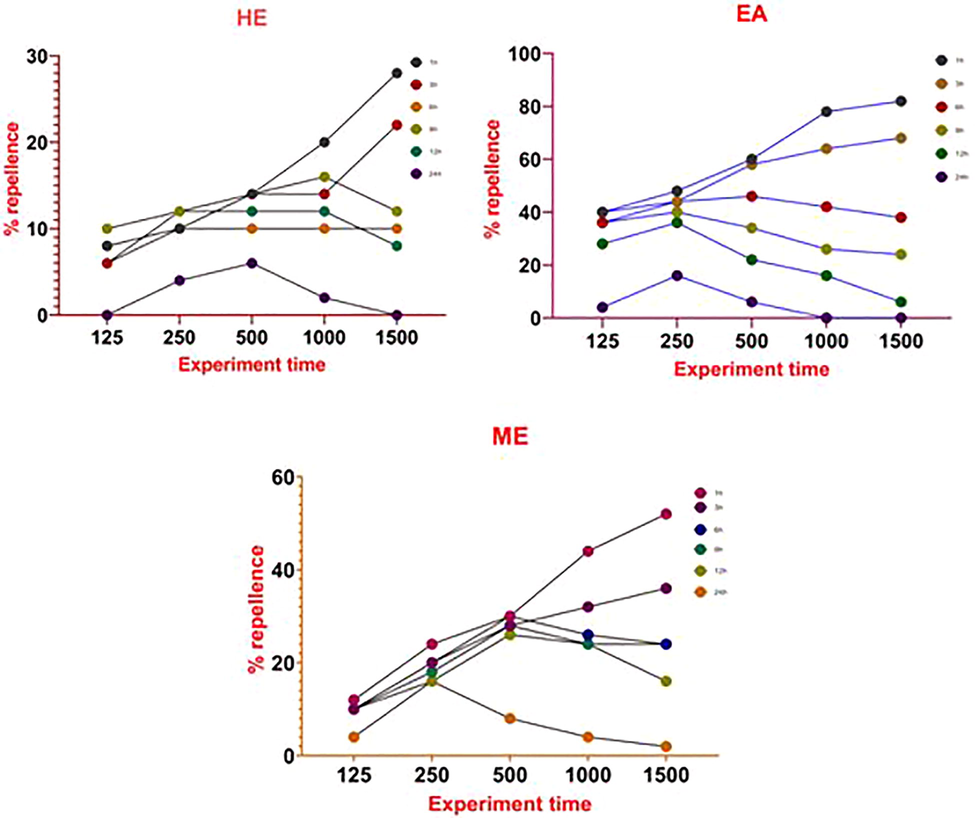
Table 2 were showed that repellence activity of methanol, ethyl acetate and hexane extracts of S. torvum. Highest repellency about (82% in RC V) was achieved at higher concentration (1500 ppm/cm2) of ethyl acetate extract of S. torvumafter1 h of treatment, followed by methanol extract (52% in RC III) and hexane extract (28% in RC II). Lowest repellency (00% in RC 0) was found in hexane extract of at the lowest treatment rate (125 ppm/cm2) after 24 h time intervals. The highest individual repellency activity was achieved at ethyl acetate extract of S. torvum against stored grain insect pests C. maculatus (Figs. 1 and 2). The each datum represents for five replicates (Mean ± SE, %), adults (n = 50) RC: Repellency.
Dosage ppm/cm2
Ethyl acetate
Methanol
Hexane
1 h
3 h
6 h
9 h
12 h
24 h
1 h
3 hrs
6 hrs
9 hrs
12 hrs
24 hrs
1 hrs
3 hrs
6 hrs
9 hrs
12 hrs
24 hrs
125
40.00 ± 0.632 (RC II)
40.00 ± 0.632 (RC II)
36.00 ± 0.748 (RC II)
36.00 ± 0.400 (RC II)
28.00 ± 0.489 (RC II)
04.00 ± 0.244 (RC I)
12.00 ± 0.200 (RC I)
10.00 ± 0.000 (RC I)
10.00 ± 0.000 (RC I)
10.00 ± 0.316 (RC I)
10.00 ± 0.000 (RC I)
04.00 ± 0.244 (RC I)
08.00 ± 0.200 (RC III)
06.00 ± 0.244 (RC III)
08.00 ± 0.200 (RC III)
10.00 ± 0.000 (RC III)
06.00 ± 0.244 (RC III)
00.00 ± 0.000 (RC III)
250
48.00 ± 0.800 (RC III)
44.00 ± 0.400 (RC III)
44.00 ± 0.505 (RC III)
40.00 ± 0.707 (RC II)
36.00 ± 0.244 (RC II)
16.00 ± 0.400 (RC I)
24.00 ± 0.400 (RC II)
20.00 ± 0.316 (RC I)
20.00 ± 0.000 (RC I)
18.00 ± 0.200 (RC I)
16.00 ± 0.509 (RC I)
10.00 ± 0.316 (RC I)
10.00 ± 0.000 (RC III)
10.00 ± 0.000 (RC III)
10.00 ± 0.000 (RC III)
12.00 ± 0.200 (RC III)
12.00 ± 0.200 (RC III)
04.00 ± 0.244 (RC III)
500
60.00 ± 0.632 (RC III)
58.00 ± 0.374 (RC III)
46.00 ± 0.743 (RC III)
34.00 ± 0.400 (RC II)
22.00 ± 0.374 (RC II)
06.00 ± 0.244 (RC I)
30.00 ± 0.316 (RC II)
28.00 ± 0.200 (RC II)
30.00 ± 1.048 (RC II)
28.00 ± 0.583 (RC II)
26.00 ± 0.244 (RC II)
08.00 ± 0.374 (RC I)
14.00 ± 0.244 (RC III)
14.00 ± 0.244 (RC III)
10.00 ± 0.447 (RC III)
14.00 ± 0.400 (RC III)
12.00 ± 0.200 (RC III)
06.00 ± 0.244 (RC III)
1000
78.00 ± 0.374 (RC IV)
64.00 ± 0.244 (RC IV)
42.00 ± 0.663 (RC III)
26.00 ± 0.400 (RC II)
16.00 ± 1.000 (RC I)
00.00 ± 00.00 (RC 0)
44.00 ± 0.244 (RC III)
32.00 ± 0.374 (RC II)
26.00 ± 0.509 (RC II)
24.00 ± 0.400 (RC II)
24.00 ± 00.00 (RC II)
04.00 ± 0.244 (RC I)
20.00 ± 0.316 (RC III)
14.00 ± 0.244 (RC III)
10.00 ± 0.316 (RC III)
16.00 ± 0.244 (RC III)
12.00 ± 0.200 (RC III)
02.00 ± 0.000 (RC III)
1500
82.00 ± 0.374 (RC V)
68.00 ± 0.200 (RC IV)
38.00 ± 0.374 (RC II)
24.00 ± 0.244 (RC II)
06.00 ± 0.400 (RC I)
00.00 ± 00.00 (RC 0)
52.00 ± 0.374 (RC III)
36.00 ± 0.678 (RC II)
24.00 ± 0.244 (RC II)
24.00 ± 0.244 (RC II)
16.00 ± 0.244 (RC I)
02.00 ± 0.200 (RC I)
28.00 ± 0.374 (RC III)
22.00 ± 0.374 (RC III)
10.00 ± 0.316 (RC III)
12.00 ± 0.374 (RC III)
08.00 ± 0.374 (RC III)
00.00 ± 0.000 (RC III)
Repellence activity of S. torvum against C. maculatus at different concentrations (ppm/cm2) with different time interval. (ME) – Methanol, (EA) – Ethyl acetate and (H) – Hexane.
Repellence activity (%) of (ME) – Methanol, (EA) – Ethyl acetate and (H) – Hexane leaf extracts of S. torvum against C. maculatus at different concentrations (ppm/cm2) individual replicate with mean value.
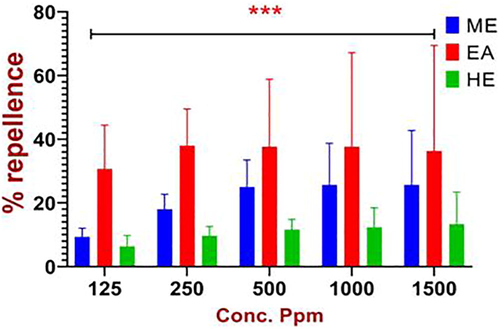
The individual replicate with mean value was showed that highest repellence activity in ethyl acetate extract at 1 h interval. The repeated measure analysis of S. torvum against C. maculatus between various doses of 125, 250, 500, 1000 and 1500 ppm/cm2 after 1, 3, 6, 9, 12 and 24 h respectively were significant at p < 0.05 level (Table 3).
SS
DF
MS
F (DFn, DFd)
P value
Treatment (between columns)
1636
2
818.0
F (1.254, 5.016) = 101.7
P = 0.0001
Individual (between rows)
208.7
4
52.18
F (4, 8) = 6.486
P = 0.0125
6.1 Contact toxicity
Highest contact toxicity against C. maculatus was achieved 98% at higher concentration (900 µg/cm2) of ethyl acetate extract after 72 h of treatment, followed by 70% at methanol extract of and 48% at hexane extract of S. torvum. Lowest contact toxicity 00% was observed inhexane extract at a lowest treatment rate of 100 µg/cm2 after 48 h time intervals (Table 4).
Dosage µg/cm2
Ethyl acetate
Methanol
Hexane
24 h
48 h
72 h
24 h
48 h
72 h
24 h
48 h
72 h
100
0.000 ± 0.000
14.00 ± 0.244
18.00 ± 0.374
0.000 ± 0.000
0.000 ± 0.000
10.00 ± 00.00
0.000 ± 00.00
0.000 ± 0.000
10.00 ± 0.316
300
18.00 ± 0.200
20.00 ± 0.374
42.00 ± 0.374
10.00 ± 0.316
12.00 ± 0.200
20.00 ± 0.000
10.00 ± 00.00
10.00 ± 0.000
16.00 ± 0.244
500
36.00 ± 0.244
46.00 ± 0.244
54.00 ± 0.400
24.00 ± 0.244
34.00 ± 0.244
34.00 ± 0.4
20.00 ± 0.400
26.00 ± 0.400
24.00 ± 0.224
700
44.00 ± 0.244
62.00 ± 0.200
78.00 ± 0.200
36.00 ± 0.244
46.00 ± 0.244
58.00 ± 0.374
26.00 ± 0.244
32.00 ± 0.200
36.00 ± 0.224
900
50.00 ± 0.316
86.00 ± 0.509
98.00 ± 0.200
44.00 ± 0.244
54.00 ± 0.509
70.00 ± 0.547
30.00 ± 0.509
40.00 ± 0.316
48.00 ± 0.200
The S. torvum ethyl acetate leaf extracts were expressed the most toxic contact toxicity effect against C. maculatus followed by methanol and hexane extract (Fig. 3). LC50 analysis of the S. torvum ethyl acetate extract was the most effective control agent against C. maculates (LC50 = 393.271 µg/mL) followed by methanol and hexane extract with respective LC50 values were 632.338 and 894.333 µg/mL (Table 5). When the experiment time was increased the low concentration of S. torvum extracts were also showed the good results. Time was the main factor to determine the mortality rate at C. maculatus was reflected in Fig. 4. The contact toxicity repeated measures analysis of S. torvum against C. maculatus variance exposure between the doses of 100, 300, 500, 700 and 900 µg/cm2 after 24, 48 and 24 h respectively significant at p < 0.05 level (Table 6).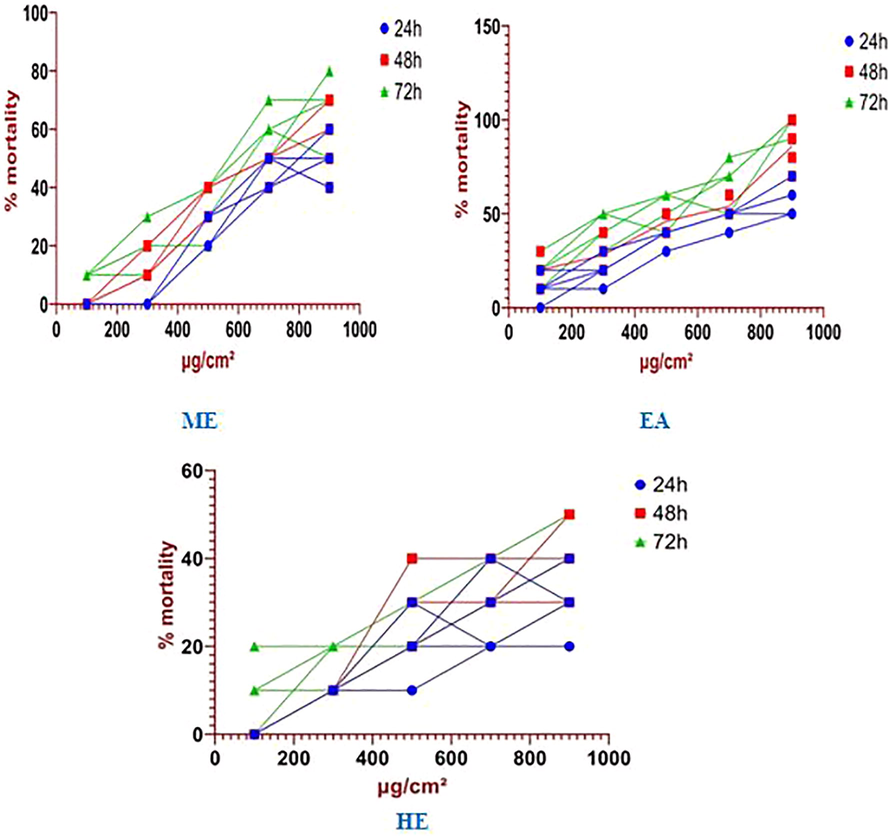
Mortality rate (%) of S. torvum against stored grain insect pests C. maculatus at different concentrations (µg/cm2). (ME) – Methanol, (EA) – Ethyl acetate and (H) – Hexane.
LC50 at different time intervals
Plant Extract
24 h
48 h
72 h
Methanol
723.506 ± 47.412
667.291 ± 17.694
632.338 ± 75.128
Ethyl acetate
676.382 ± 18.539
625.381 ± 69.925
393.271 ± 98.484
Hexane
–
–
894.333 ± 5.0136
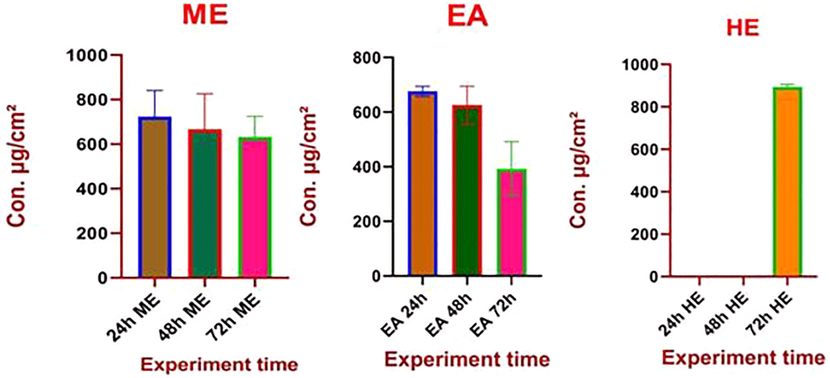
LC50 (µg/mL) value of S. torvum extracts against adult C. maculatus at different concentrations (µg/mL). (ME) – Methanol, (EA) – Ethyl acetate and (H) – Hexane.
SS
DF
MS
F (DFn, DFd)
P value
Treatment (between columns)
6771
8
846.4
F (1.436, 5.746) = 15.96
P = 0.0058
Individual (between rows)
15,935
4
3984
F (4, 32) = 75.11
P < 0.0001
7 Discussion
Number of research articles were supported the plant product is good for the repellent and contact toxicity study of stored product insects. The Solanace family plants were most useful for economically and ecologically important to the world. The important genera Solanum, Lycopersico Capsicum and Nicotiana were affected various insect pests in lethal and sublethal effects (Szymon et al., 2016). There is no previous report found for the S. torvum contact and repellent activity of stored product insects previously, this plant mostly used for immunomodulatory and nephroprotective activity.
The aim of the research S. torvum leaf extract was used as eco friendly manner for the integrated pest management of C. maculatus. Moreover, the plant compounds are possess the significant potential insecticide. Which were used to manage the stored grain pest C. maculatus. The observed result of S. torvum was provided for the repellent and contact toxicity study and it could be naturally used pest control for the future.
The ethyl acetate leaf extract of S. torvum was shown the higher result than other extracts like methanol and hexane. The different types of stored grain pests a large number of plants extract and essential oils were used in the ovicidal activity, repellent activity and contact toxicity study (EzhilVendan et al., 2017). The present study, the mean percentage of ethyl acetate leaf extract were showed the highest repellency value reached upto82% at the dose of 1500 ppm/cm2 after 1 h treatment, followed by methanol (52%) and hexane (28%) extract.
The present study was close to Habib-ur-Rehman et al. (2018); who was used the methanol extract of C. paradise 83.15% of repellency and it was reached at 15% concentration against T. castaneum. It may be a little different because of the use of a different plant and insect pest. The 1415 μg/cm2 at 7 h reached 87% repellency was observed in methanol extract of turmeric plant and maximum repellencyupto79% at 1415 μg/cm2 in 5 h after exposure in hexane extract of peacock ginger plant (Dewi et al., 2016). Similarly Sidra-Tul et al. (2017) was proved that Azadirachta indica expressed 85.33% repellency in 24 h treatment, 86.67% in 48 h and 93.33% was observed in 72 h exposure. Nearly 20% Meliaaza dirachwas shown 77.33% repellency at 24 h experiment, 81.33% at 48 h and 90.67%at 72 h. Pegnum hermala 82.67% repellency was observed at 72 h exposure treatment at 20% concentration. Emblica officinalis extract expressed 88.66% and Datura alba extract 77.58% repellency was observed (Dwivedi et al., 2004). Mohiuddin et al., (1987) was proved that 75% repellency in Momordica charantia extract. The repellent method mostly used to control the pests in packing materials (JianhuaLü, 2015).
In the present study, more over 98% mortality was observed in S. torvum leaf extract against C. maculatus 72 h treatment. The present study was close to 3.5 mg/cm2 concentration of Acorus calamusvar, Acorus gramineus rhizome, Foeniculum vulgare fruit, Angustatus rhizome and Illicium verum fruit extracts in 3 or 4 days treatment at 90% mortality rate was observed (Kima et al., 2003). The Cucumis sativus, Tamarindus indica, Azadirachta indica and Psidium guajava hexane extracts at 24, 48 and 72 h after the treatment, 80% mortality was observed in 1571.83 μg/cm2concentration (Mostafa et al., 2012).
Resistance and contact toxicity studies were showed the most important result against to stored grain pest. The worldwide awareness on safe environment has led scientists to seek less dangerous or environmentally friendly alternative pest management practices. In future, based on this laboratory study, we will be evaluated the pesticide formulation under field condition for the societal purposes.
8 Conclusion
Pesticides are the efficient weapons to manage the insect pests in crop and food storage fields. Indiscriminate use and ill effects of synthetic chemical pesticides, alternative natural pesticides are needed to manage the insect pests in eco-friendly manner. At present, alternative strategies and adequate methods are required for plant based insect pest control. The worldwide awareness on safe environment has led scientists to seek less dangerous or environment friendly alternative pest management practices. The mean percentage of ethyl acetate leaf extract repellent value was reached 82% at the dose of 1500 ppm/cm2 after 1 h and the mortality was reached over the ethyl acetate leaf extract nearly 98% at the dose of 900 µg/cm2 after 72 h against C. maculatus (F.) adult. Solanum torvum leaf extract could be useful for the integrated pest management of C..maculatus; it was used to analysis the repellent and contact toxicity study. The ethyl acetate leaf extract was shown good repellent and contact toxicity effect, followed by methanol and hexane extract. This method of natural plant extract can be used to control pests, alternate against the chemical insecticide. Insect pests are the major destroyers causing severe damages to agricultural food crops and stored food commodities. Based on the laboratory study results, pesticide formulation will be prepared and evaluated under field condition and we will be assessed the effect of active fractionated compound from the Solanum torvum leaf extract against Callosobruchus maculates.
Acknowledgements
The authors are grateful to A.V.V.M Sri Pushpam College (Autonomous), Poondi, Thanjavur district, Tamil Nadu for providing the necessary facilities. The authors gratefully acknowledge the Deanship of Scientific Research Deanship at King Saud University for funding this work through research group no. RG-1441-329. Also the authors are very thankful to Dr. S. Ezhil Vendan, Scientist, & AcSIR Faculty, Food Protectants & Infestation Control, CSIR-Central Food Technological Research Institute, Mysore, India for his timely suggestion,
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant and nitric oxide activities of selected Malay traditional vegetables. Food Chem.. 2006;95:566-573.
- [Google Scholar]
- Effect of aluminium phosphide on blood glucose level. Vet Hum. Toxicol.. 1999;41:31-32.
- [Google Scholar]
- The State Pharmacopoeia Commission of People’s Republic of China. Chem. Industry Press.. 2000;107
- [Google Scholar]
- Antimicrobial activity of methanolic extract of Solanumtorvumfruit. Fitoterapia.. 2000;71:187-189.
- [Google Scholar]
- Bioactivity and qualitative analysis of some essential oils from Mediterranean plants against stored-product pests: evaluation of repellency against SitophiluszeamaisMotschulsky, Cryptolestesferrugineus (Stephens) and Tenebriomolitor (L.) J. Stored Prod.. 2009;45:125-132.
- [Google Scholar]
- Sartika Dewi Aryani., and Wanida, Auamcharoen. Repellency and contact toxicity of crude extracts from three Thai plants (Zingiberaceae) against maize grain weevil, Sitophiluszeamais (Motschlusky) (Coleoptera: Curculionidae) JBiopest 9 1 2016 52 62
- S. Dwivedi C., and N, B, Shekhawat., Repellent effect of some indigenous plant extracts against Trogodermagranarium (Everts) Asian J. Exp. Sci. 18 2004 47 51
- G. Elango S.M. Roopan K.I. Dhamodaran K. Elumalai N.A. Al-Dhabi M.V. Arasu Spectroscopic investigation of biosynthesized nickel nanoparticles and its larvicidal, pesticidal activities Journal of Photochemistry & Photobiology, B: Biology. 2016b 162 167
- S. EzhilVendan S. Manivannan M. Sunny Anila R. Murugesan Phytochemical residue profiles in rice grains fumigated with essential oils for the control of rice weevil PLoS ONE.12(10) 2017 e0186020.https://doi.org/10.1371/journal. pone.0186020
- Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J. Photochem. Photobiol., B: Biol.. 2016;162:395-401.
- [Google Scholar]
- Glorybai L, Barathi K.K, Arasu MV, Al-Dhabi NA, Agastian P. 2015. Some biological activities of Epaltes divaricata L. - an in vitro study. Annals of Clinical Microbiology and Antimicrobials. 2015, 14:18.
- Habib-ur-Rehman., SaimaMirza., Mansoor-ul-Hasan., Qurban Ali3., Hafiz Abdullah Shakir., Muhammad Yasir., 2018. Repellent Potential of Three Medicinal Plant Extracts against Triboliumcastaneum (Coleoptera: Tenebrionidae). Punjab Univ. J. Zool. 33(2), 121-126. http:dx.doi.org/10.17582/pujz/.
- Phytochemical methods. London: Chapman and Hall Ltd.; 1958. p. :125.
- Green chemical approach towards the synthesis of SnO2 NPs in argument with photocatalytic degradation of diazo dye and its kinetic studies. J. Photochem. Photobiol., B: Biol.. 2016;162:441-447.
- [Google Scholar]
- JianhuaLü, Dan Ma., 2015. Repellent and Contact Toxicity of Alpiniaofficinarum Rhizome Extract against Lasiodermaserricorne Adults.PLoS ONE.10(8), e0135631. doi:10.1371/journal.pone.0135631.
- Laboratory studies on several plant materials as insect repellents for protection of cereal grains. J. Econ. Entomol.. 1983;76:154-157.
- [Google Scholar]
- Farmers’ insect pest management practices and pesticidal plant use in the protection of stored maize and beans in Southern Africa. Int. J. Pest Manage.. 2010;57:41-49.
- [Google Scholar]
- M. Mostafa Hemayet Hossain., M. Anwar Hossain., PizushKantiBiswas., M. ZahurulHaque. Insecticidal activity of plant extracts against Triboliumcastaneum Herbst J AdvSci Res. 3 3 2012 80 84
- Laboratory investigations on the repellency of some plant oils to red flour beetle. Triboliumcastenum. 1987
- [Google Scholar]
- Practical applications of pheromone traps in food and tobacco industry. J. Kansas Entomol. Soc.. 1990;63:548-553.
- [Google Scholar]
- Analgesic and anti-inflammtory properties of aqueous extract from the leaves of Solanumtorvum. (Solanaceae) Afr. J. Trad. Complim. Altern.. 2007;42:240-244.
- [Google Scholar]
- Repellent activity of essential oils from seven aromatic plants grown in Colombia against SitophiluszeamaisMotschulsky (Coleoptera) J. Stored Prod.. 2009;45:212-214.
- [Google Scholar]
- Potential of Anisopteromaluscalandrae (Hymenoptera: Pteromalidae) as biocontrol agent of Callosobruchusmaculatus (F.) (Coleopetera: Bruchidae) Afr. J. Agric. Res.. 2007;2:168-172.
- [Google Scholar]
- The efficacy of four seed powders and the essential oils as protectants of cow-pea and maize grain against infestation by Callosobruchusmaculatus (Fabricius) (Coleoptera: Bruchidae) and Sitophiluszeamais (Motschulsky) (Coleoptera: Curculionidae) in Nigeria. Intl. J. Pest Manag.. 1996;42:143-146.
- [Google Scholar]
- Bioactivity of the leaf extracts of Morinda Lucida (Benth.) against cowpea bruchid, CallosobruchusMaculatus (F.) (Coleoptera: Chrysomelidae) Exp. Agric. Hortic.. 2012;1:1-7.
- [Google Scholar]
- Evaluation of four cowpea lines for bruchidCallosobruchusmaculatus) tolerance. J. Nat. Sci. Res.. 2013;3:46-51.
- [Google Scholar]
- Insecticidal activity of asarones identified in Acorusgramineusrhizome against three Coleopteran stored - product insects. J. Stored Product Res.. 2003;39:332-342.
- [Google Scholar]
- novel natural insecticide molecule for grain protection. Julius-Kühn-Archiv.. 2010.A;425:913-917.
- [Google Scholar]
- Muntaha Sidra-Tul Sagheer Muhammad Hasan Mansoor-ul Talib Shahbaz Sahi., Repellent and Growth Inhibitory Impact of Plant Extracts and Synthetic Pyrethroids on Three Strains of Callosobruchuschinensis L Pakistan Journal of Zoology. 49 2017 581
- Isolation and purification of a novel antioxidant protein from the water extract of sundakai (Solanumtorvum) seeds. Food Chem.. 2007;104:510-517.
- [Google Scholar]
- Soon-Il Kima Jung-YeonRoha., Do-HyoungKima., Han-SeungLeeb., Young-JoonAhn., Insecticidal activities of aromatic plant extracts and essential oils against Sitophilusoryzae and Callosobruchuschinensis Journal of Stored Products Research. 39 2003 293 303
- Control of Sitophiluszeamais (Motsch) (Coleoptera: Curculionidae) on Sorghum using some plant powders. Int. J. Agric. Forestry. 2012;2(1):53-57.
- [Google Scholar]
- Szymon, Chowa, nski., Zbigniew, Adamski., Paweł, Marciniak., Grzegorz, Rosi., nski., Ender Büyükgüzel., Kemal Büyükgüzel., PatriziaFalabella., Laura Scrano., EmanuelaVentrella., FilomenaLelario., and Sabino, A. Bufo., 2016. A Review of Bioinsecticidal Activity of Solanaceae Alkaloids Toxins.8, 60.
- V.F. Garry J. Griffith T.J. Danzl R.L. Nelson E.B. Whoston L.A. Krueger Human genotoxicity: pesticide applicators and Phosphine, Science. 246 1989 251 255







