Translate this page into:
Insecticidal activity of Muntingia calabura extracts against larvae and pupae of diamondback, Plutella xylostella (Lepidoptera, Plutellidae)
*Corresponding author. Tel.: +1 604 822 2329; fax: +1 604 822 6394 Yasmin.akhtar@ubc.ca (Y. Akhtar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Available online 1 September 2012
Peer review under responsibility of King Saud University.
Abstract
The aim of the present study was to evaluate the insecticidal effects of hexane and ethanolic extracts of the flowers and fruits of Muntingia calabura against diamondback moth, Plutella xylostella. The leaf disc immersion methodology was carried out to assess the insecticidal effects on the larvae and pupae as well as duration of the larval phase following feeding of the first instar larvae of diamondback on the treated leaf discs for 72 h. All extracts were toxic to larvae and pupae of P. xylostella. The ethanolic extracts of the flowers and fruits of M. calabura were the most toxic against first instar P. xylostella larvae with LC50 values of 0.61 μg mL−1 and 1.63 μg mL−1 respectively, followed by the hexane extract of the fruits (LC50 = 5.5 μg mL−1) and flowers (LC50 = 18.9 μg mL−1). All extracts were more toxic to P. xylostella larvae compared with cordycepin, the positive control which produced 100% mortality at 500 μg mL−1 in 72 h. Fruit extracts were more active than the flowers in producing pupal mortality following 72 h of feeding of the first instar larvae on leaf discs treated with the extracts. Both the hexane and the ethanolic extracts of M. calabura fruits and flowers prolonged larval duration by ∼2 days in some cases as compared with the control (7.2 days). These results suggest that M. calabura has potential for development as commercial insecticide for controlling P. xylostella due to its insecticidal effects.
Keywords
Botanical insecticide
Muntingia calabura
Medicinal plants
Plutella xylostella
Larval toxicity
Pupal toxicity
Cordycepin
1 Introduction
Plutella xylostella (L.) (Lepidoptera, Plutellidae), commonly known as the diamond back moth, is one of the most important pests of cruciferous crops throughout the world, causing direct damage to cabbage, with losses as much as 100% (Castelo-Branco and Gatehouse, 2001). The synthetic insecticides have been the main control strategy for this pest (Sarfraz and Keddie, 2005). The indiscriminate use of synthetic pesticide is harmful to the ecosystem due to its high degree of toxicity and affects not only the target pest but also beneficial species. The use of these insecticides has facilitated the emergence of generations of more resistant insects and caused the contamination of crops with toxic residues, thereby placing human health at risk (Oliveira et al., 1999). Medicinal plants have been investigated as an alternative to conventional insecticides, with reports of toxicity to a number of arthropods long before the advent of synthetic insecticides (Martins et al., 1994).
The search for insecticidal properties in medicinal plants has increased significantly in recent years and has become a promising field of research. Recent studies involving organic extracts from plants with medicinal properties have reported insecticidal properties such as mortality (Rani et al., 1999; Boiça-Júnior et al., 2005; Li et al., 2008) and feeding deterrence (Zhang et al., 2003; Liu et al., 2007) in P. xylostella larvae as well as repellence (Hou et al., 2002) and infertility in adults (Gu et al., 2004).
The potential of P. xylostella to cause harm, together with emerging populations that are resistant to synthetic insecticides (Bhattacharya et al., 2002; Sayyed et al., 2008), has led researchers world-wide to search for botanical insecticides in different genera of plant species for the control of this pest. Muntingia calabura is one of the many species from the family Tiliaceae used in folk medicine throughout the world. It is commonly known as the Jamaican cherry, Panama berry or Singapore cherry in English and Pau-seda or calabura in Brazil. Muntingia calabura is a native plant from the Antilles that was introduced in Brazil by the Instituto Agronômico de Campinas in 1962 (Lopes-Moura et al., 1999). Although not native to South America, this plant has been widely cultivated and used in urban planning as a decorative tree on streets and public squares in different regions of Brazil. In folk medicine, infusions from the leaves were used to reduce gastric distress and prostate swelling (Morton, 1987). The flowers were used to make tea for headaches relief and early cold symptoms and have antiseptic and anti-spasm effects (Pio-Corrêa, 1987). The fruit is highly appreciated as an in natura food or used to make jams.
There are literature reports on chemical and biological studies of the leaves and roots of M. calabura. Kaneda et al. (1991) and Su et al. (2003) studied the anti-tumour properties of organic extracts from the roots and leaves, respectively. The leaf extract also has antinociceptive properties against chemically or thermally induced noxious stimuli (Zakaria et al., 2006, 2007). Despite the studies published on the proven effectiveness of organic and/or aqueous extracts from the leaves and flowers of M. calabura, reports about the biological action of organic extracts from the fruits and flowers against P. xylostella are lacking. The chemical composition of M. calabura plant is already known and the leaves have been shown to contain flavonoids, chalcones, terpenoids and phenolic compounds (Preethi et al., 2012).
As part of a systematic study to evaluate the insecticidal potential of the medicinal flora that grows in Pernambuco (Brito et al., 2006; Pereira et al., 2008, 2009), the aim of the present study was to assess the insecticidal effect of hexane and ethanolic extracts from the fruits and flowers of M. calabura against P. xylostella larvae and pupae. Since P. xylostella larvae are very difficult pests to control it is important to control them at an early stage to minimize damage to the crucifer production in many parts of the world.
2 Material and methods
2.1 Plant material
Flowers and fruits were collected from the M. calabura tree located in the campus of the Universidade Rural Federal de Pernambuco (UFRPE), Recife – Brazil in July 2008. The plant was identified by Dr. Carmen Zickel from Biology Department, UFRPE and a voucher sample was deposited in the Vasconcelos Sobrinho Herbarium, UFRPE (#2816).
2.2 Hexane and ethanolic extract preparation
Hexane and ethanolic extracts were prepared from the fruits and flowers of M. calabura. Fruits and flowers after collection were washed and oven-dried at 40 °C for 48 h and were ground in a mill and weighed separately. They were then transferred to glass beakers and hexane was added. Cold maceration was carried out for 72 h with 24 h intervals to ensure the extraction of a larger number of substances from the plant. The extract was filtered and evaporated at reduced pressure to minimize possible degradation of the chemical constituents at high temperatures. After removal of the solvent, crude hexane extract was obtained. The same procedure was repeated using ethanol as a maceration solvent to obtain crude ethanolic extract.
2.3 Insects rearing and colony maintenance
A colony of P. xylostella was maintained at the Laboratory of Insect Biology of UFRPE. Plutella xylostella was raised based on the method described by Barros and Vendramim (1999). The newly-emerged adults were sexed and placed in plastic cages with a sponge soaked in water to maintain proper humidity inside the cage. On the sponge was placed a disc of filter paper (8.0 cm diameter) and a leaf-disc of Brassica oleracea (8.0 cm diameter) to stimulate the oviposition of P. xylostella. Adults were fed with a 10% honey solution provided in polyurethane foam attached to a circular hole at the top of the cage.
Discs of kale leaves with the eggs were transferred to Petri dishes daily, where they remained until the hatching of the P. xylostella. The discs and the larvae were kept in rectangular plastic containers (60 × 30 cm) with organic kale leaves used as food. The larvae remained in these containers until pupation and the kale leaves were replaced daily with the fresh ones. These pupae were collected in test tubes sealed with plastic containing PVC (polyvinyl chloride) for air circulation. The pupae were kept under room temperature until emergence of adults, which were transferred to new cages (rectangular plastic containers; 60 × 30 cm).
2.4 Screening of insecticidal activity
A one-gram aliquot of hexane and ethanolic extract from the flowers and fruits of M. calabura was diluted in 19.9 mL of distilled water and 0.1 mL of Tween 80 as an emulsifier. The solution was mixed and filtered through a Whatman No. 1 filter paper and served as a stock solution. Stock solution was diluted to the desired concentrations ranging from 0.25 to 30 μg mL−1. All experiments were conducted in the Laboratory of Insect Biology/Plant Resistance to Insects, Agronomy Department, UFRPE, between October 2008 and January 2009, at temperature = 30 ± 0.5 °C, Relative humidity = 67 ± 2.0% and photoperiod = 12 h.
2.5 Larval toxicity
The leaf disc immersion method was used to determine toxicity of larvae. Discs of kale leaf (8 cm diameter), were immersed for 10 s in 50 μl of the different concentrations of the extract solutions: MCHFL (M. calabura-hexane extract of flowers), MCHFR (M. calabura-hexane extract of fruits), MCEFL (M. calabura-ethanolic extract of flowers), and MCEFR (M. calabura-ethanolic extract of fruits). Leaf-discs were air dried and transferred individually to Petri dishes (9 cm diameter) containing a filter paper disc (8 cm diameter) soaked in distilled water. Ten first instar larvae (<12 h old) were introduced on the leaf discs. After placing lids on the Petri dishes they were wrapped in a Parafilm to avoid larval escaping. Concentrations varied from 0.25 to 30 μg mL−1 for the extracts. The experimental design was entirely randomized, with five treatments including the control and four replications per treatment. Experiment was repeated twice. Mortality was assessed after 72 h of being feeding on the treated/control leaf discs. Cordycepin (500 μg mL−1) was used as a positive control as it produced 94% mortality in third instar P. xylostella (72 h after treatment) at a concentration of 500 mg L−1 (Kim et al., 2002). It is described as an insecticidal constituent of the fungus, Cordyceps militaris fruiting body (Kim et al., 2002). Although the exact mode of action of cordycepin is not known, it has been shown to act as a stomach poison rather than having a direct inhibitory effect on chitin synthesis (Kim et al., 2002).
2.6 Pupal toxicity
Mortality of the pupae was based on the method described by Park et al. (2002) with modifications. First instar larvae were reared on kale leaf discs treated with different concentrations of the extract solutions for 72 h after which the non-treated kale leaf discs were introduced into the cages and replaced with fresh ones every 48 h as described earlier until pupation. Control group was treated with water alone. We started off with approximately 35 larvae for each treatment to have at least 20 healthy larvae pupated/treatment, due to toxicity at higher concentrations.
Concentrations varied from 0.25 to 30 μg mL−1 for the extracts. Experiment was repeated twice. The larvae that had transformed into the pupae were individually transferred to dishes to determine mortality within 72 h of being in pupation.
2.7 Larval duration
Larval duration was determined by rearing first instar larvae on kale leaf discs treated with different concentrations of the extract solutions for 72 h after which the non-treated kale leaf discs were introduced into the cages and replaced with fresh ones every 48 h until pupation as described earlier. Control group was treated with water alone. We started off with approximately 35 larvae for each treatment to have at least 20 healthy larvae/treatment until pupation as the extracts were toxic at higher concentrations. Experiment was repeated twice. Larval duration was described as the number of days from hatching of the larvae to the pupal stage. Concentrations of the extracts varied from 0.25 to 30 μg mL−1.
2.8 Statistical analysis
Data obtained in these experiments were subjected to a One-Way Analysis of Variance using statistics software (Statistix 8, 2008). Where significant F values were found, a Tukey’s multiple comparison test was used to test for significant differences between individual treatments. LC50 (lethal concentration producing 50% mortality) values with 95% confidence level were calculated with the Probit model using Polo-PC Software program (LeOra Software, 1987).
3 Results
3.1 Larval toxicity
The toxic effects of the M. calabura extracts varied depending on the part of the plant and type of solvent. The ethanolic extract of the flowers (MCEFL) was the most active followed by the ethanolic extract of the fruits (MCEFR), hexane extract of the fruit (MCHFR) and hexane extract of the flower (MCEFL) (Table 1). n = Number of insects tested, df = Degrees of freedom, χ2 = Chisquare, CI = Confidence Interval, TR = Toxicity ratio calculated according to Robertson and Presley (1992). MCEFL = ethanolic extract from flowers, MCEFR = ethanolic extract from fruits, MCHFL = hexane extract from flowers, MCHEFR = hexane extract from fruits of M. calabura. Cordycepin, the positive control, produced 100% mortality at 500 μg mL−1 in 72 h.
Extracts
n
df
Slope (CI 95%)
LC50 (μg mL−1) (CI 95%)
χ2
TR50
Plutella xyolstella larvae
MCEFL
600
4
2.76 (2.57–2.95)
0.61 (0.45–0.79)
9.34
–
MCEFR
600
4
2.43 (2.27–2.58)
2.61 (2.09–3.21)
6.03
4.23
MCHFL
600
4
1.45 (1.30–1.59)
18.94 (13.23–33.92)
6.27
30.72
MCHFR
700
4
2.27 (2.12–2.42)
5.50 (4.40–6.81)
10.60
8.91
LC50 values (concentrations causing 50% mortality compared with the control) and the corresponding confidence intervals of the M. calabura extracts for larval mortality are displayed in Table 1. Cordycepin, the positive control, produced 100% mortality at 500 μg mL−1 in 72 h.
Toxicity of the hexane and ethanolic extracts of the flower and fruit of M. calabura against first instar P. xylostella larvae, differed significantly from each other based on their non-overlapping confidence intervals of the LC50 values (Table 1). The ethanolic extracts were significantly more active than hexane extracts. Within the same extracts, the MCEFL (LC50 = 0.61 μg mL−1) was 4.3-fold more toxic than MCEFR (LC50 = 2.61 μg mL−1), while MCHFR (LC50 = 5.50 μg mL−1) was 3.4-fold more toxic than MCHFL (LC50 = 18.94 μg mL−1). Comparing the relative toxicities between them, MCEFL was 31.0-fold more toxic than MCHFL and 4.2 and 8.9 folds more toxic than MCEFR and MCHFR, respectively (Table 1).
3.2 Pupal toxicity
The results of pupal toxicity following prior exposure of larvae to the extracts are displayed in Fig. 1. Treatment of the first instar larvae for 72 h with the hexane and the ethanolic extracts of M. calabura fruit (MCHFR) produced greater mortality of the subsequent pupae compared with the flower extracts. Pupal mortalities were 39% and 77% following prior exposure of the larvae to 9 and 20 μg mL−1 of the ethanolic and the hexane fruit extracts respectively. Pupal mortalities were 11% and 18% following prior exposure of the larvae to 3.5 and 30 μg mL−1 of ethanolic and hexane extracts of the flower respectively. Mortality of the flower extracts was <8% at the lowest concentrations. A One-Way ANOVA on pupal mortality following feeding of the first instar larvae on the leaf discs treated with the extracts for 72 h showed significant F values (F7,23 = 88.3; p < 0.05 for MCEFL, F7,23 = 1128; p < 0.05 for MCHFL, F7,23 = 1834; p < 0.05 for MCEFR, F7,23 = 7848; p < 0.05 for MCHFR). Mortality in the subsequent pupae following feeding on the extracts was significantly greater than the control pupae (Tukey’s test, P < 0.05) (Fig. 1) at all concentrations.
Pupal mortality of P. xylostella following larval feeding on kale leaf discs treated with different concentrations of M. calabura extracts. Mortality of the pupae was assessed within 72 h in the pupal stage. Means (±SE) followed by the same letters are not significantly different from each other (Tukey’s test; P = 0.05).
3.3 Duration of larval phase
Larval duration varied with the extracts. A One-Way ANOVA on larval duration following feeding of the first instar larvae on the leaf discs treated with the extracts for 72 h produced significant F values (F7,23 = 7.68; p < 0.05 for MCEFL, F7,23 = 42.6; p < 0.05 for MCHFL, F7,23 = 10.6; p < 0.05 for MCEFR, F7,23 = 29.8; p < 0.05 for MCHFR). Larval duration of the treated larvae was significantly greater than the control for most of the concentrations of ethanolic and hexane extracts of the fruits and the flowers (Tukey’s test; P = 0.05). Comparing the effects of the different concentrations of M. calabura ethanolic extracts on larval duration, although there was a lack of dose response, larval duration of the treated larvae was significantly greater than the control at some concentrations (2.5 and 3.5 μg mL−1 of MCEFL, 2.0, 4.0, 8.0 and 9.0 μg mL−1 of MCEFR) (Fig. 2). Comparing M. calabura hexane extracts, both MCHFL (larval duration 7.8–9.3 days) and MCHFR (larval duration 8.7–9.4 days) produced a significant increase in larval duration at all concentrations (Tukey’s test; P = 0.05) except for the two lowest concentrations of hexane extract of fruits (MCHFR) compared with the control (larval duration 7.2 days) (Fig. 2).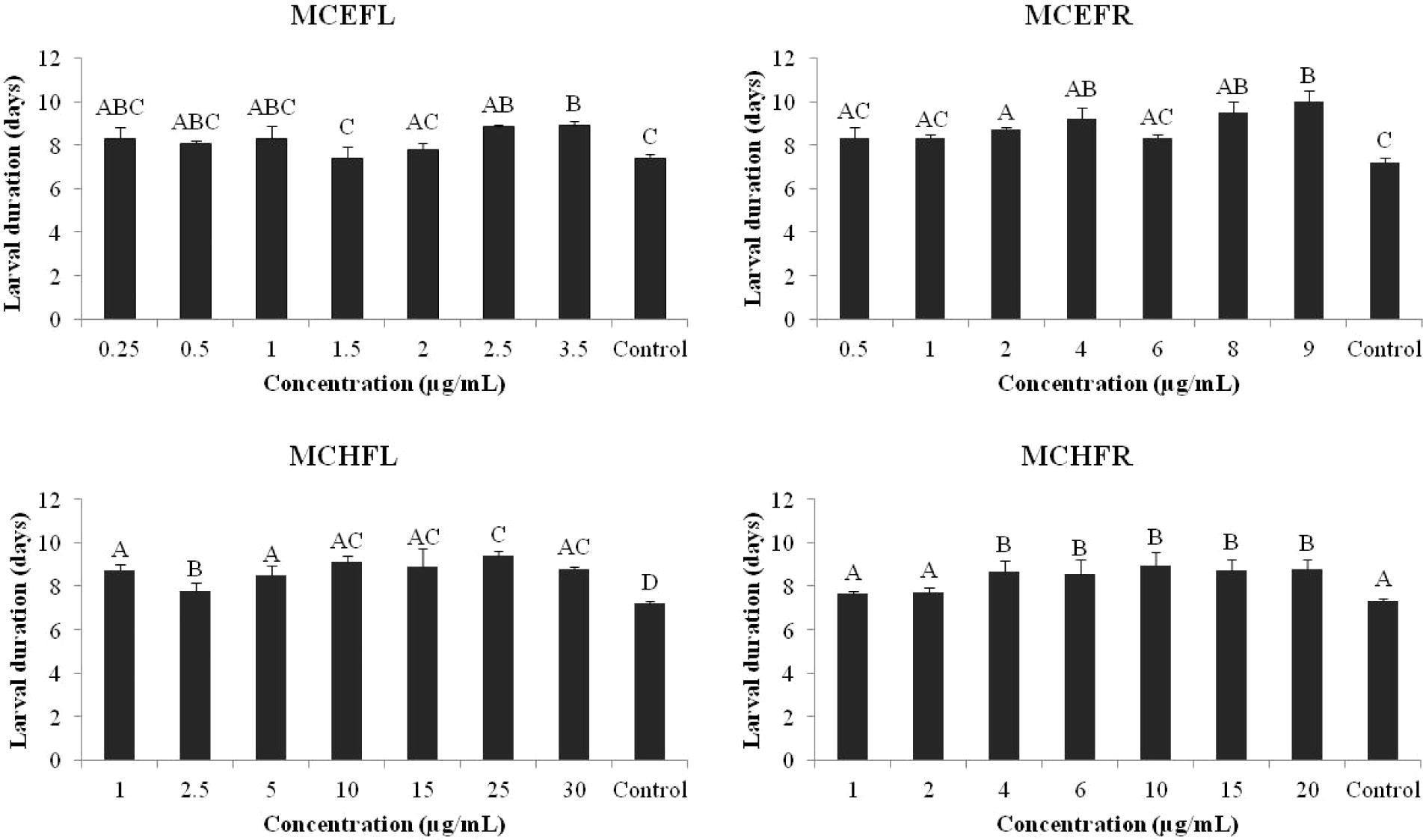
Larval duration of P. xylostella following larval feeding of kale leaf discs treated with the Calabura extracts at different concentrations (n = 20). Means followed by the same letters are not significantly different from each other (Tukey’s test; P = 0.05).
4 Discussion
All extracts including the hexane and ethanolic fruit and flower extracts of M. callabura were toxic to P. xylostella larvae. Prior feeding of the first instar larvae on extracts treated leaf discs for 72 h also had an effect on subsequent pupae as well as prolongation of the larval duration. Our results have demonstrated that M. calabura extracts are more toxic than other organic and aqueous extracts from different plant species that have been evaluated for toxicity against P. xylostella larvae (Kodjo et al., 2011; Boiça-Júnior et al., 2005; Li et al., 2008; Trindade et al., 2011).
In the present study, ethanolic extract of flowers (MCEFL) of M. callabura was the most active against P. xylostella larvae with a LC50 of 0.61 μg mL−1. Our extracts seem to be more active than some of the other extracts reported previously against P. xylostella. Kodjo et al. (2011) tested aqueous extracts of the leaf, roots and seed as well as the oil emulsion of Ricinus communis (castor bean) against diamondback moth. A 10% oil emulsion of R. communis produced 100% mortality in third instar diamondback moth larvae using contact and ingestion bioassays. Whereas, aqueous extracts were less active producing 71%, 52% and 50% mortalities following feeding on cabbage leaf discs treated with 20% of seed, leaf and root extracts of R. communis respectively (Kodjo et al., 2011). Dursban, a positive control used in the same study produced 88% mortality at 5%. This shows that our extracts were more active than R. communis extracts, oil emulsion as well as dursban (positive control). Uma et al. (2009) tested the insecticidal effects of stem and leaf extracts of 4 Euphorbia species against diamondback moth. Mortality rates were 7% and 43% for second instar P. xylostella larvae following 96 h feeding on mustard leaf discs treated with 20% alcoholic leaf extracts of Euphorbia nivula and E. pulcherrima respectively. Mortality rates were 7% and 10% following 96 h feeding on mustard leaf discs treated with 20% alcoholic stem extracts of E. antiquorum and E. tirucalli respectively (Uma et al., 2009). Boiça-Júnior et al. (2005) used 10% aqueous extracts of Enterolobium contortisillidium (fruits), Nicotiana tabacum (leaves), Sapindus saponaria (fruits) and Trichilia pallida (twigs) to achieve a 100% P. xylostella larval mortality following feeding on treated kale (B. oleracea var. acephala) leaves. Mortality was 100% in P. xylostella larvae following feeding on kale leaf discs treated with crude ethanolic extract of Annona muricata at 5 mg mL−1 for 12 days. Larval duration was also increased by 2.6 days at this concentration (Trindade et al., 2011).
Torres et al. (2001) obtained the same mortality rate for P. xylostella larvae by using 10% aqueous extracts from the seeds and bark of Azadirachta indica A. Juss. and Aspidosperma pyrifolium Mart. Recently, Li et al. (2008) demonstrated 91.7% larval mortality rate by using acetone fraction of the chloroform extract of Xanhtium sibiricum at a concentration of 50 g/L, making this extract 15-fold less toxic than the MCEFL extract. Rani et al. (1999) achieved a 100% mortality rate of P. xylostella larvae with an ethanolic extract from the twigs of Melia azaderach at concentrations >7.5%, which are much higher than the concentrations used in our study.
Plant extracts are mixtures of several compounds. Blends are more effective than individual compounds in terms of forestalling and diluting resistance (Feng et al., 1995) and habituation (Akhtar et al., 2003) for long-term use. A possible explanation is that the activity of extracts may be attributed to more than one compound resulting either from the synergistic interaction between major compounds or their interaction with minor compounds (Akhtar et al., 2012a).
All extracts produced a significant increase in larval duration at most of the concentrations compared with the control. The mean number of days on M. calabura ethanolic fruit extract was 8.9 days, followed by the ethanolic extract of the flowers (8.6 days) and the hexane extracts of the fruits (8.5 days) and the flowers (8.3 days). This shows that both the ethanolic extracts were more active than the hexane extracts in prolongation of the larval period. This is consistent with the toxicity data against P. xylostella larvae. Similar findings have been described by Boiça-Júnior et al. (2005) and Torres et al. (2001). A plausible explanation for prolonged larval phase may be reduction in larval feeding possibly due to phagocytosis-inhibiting substances in the extracts (Hernandez and Vendramim, 1997).
Our experiments have shown that extracts were less toxic to the pupae following feeding of the larvae on leaf discs treated with the extracts for 72 h. Prior treatment of the larvae with the hexane and the ethanolic fruit extracts produced greater pupal mortality than flower extracts. Although, MCEFL was the most active extract against larvae, it was not very active against subsequent pupae. Larval duration was also significantly prolonged following prior feeding of the larvae on leaf discs treated with the extracts compared with the control. Increased larval phase had practically no influence over mortality in the pupal phase in our study conducted in the laboratory. However, increased larval phase in the field will expose the insects to predators, parasitoids and diseases which will ultimately result in an increase in their mortality rate before reaching the pupal stage (Akhtar et al., 2010, 2012b). Some of the treated larvae that would survive the larval phase may also die as pupae due to the long lasting effects of the extracts. Although the exact mode of action is not known, it seems like the extracts are acting as stomach poison. The results of the present study suggest that the M. callabura extracts can be used to protect crops against the extensive damage caused by P. xylostella larval feeding. Since the plant is easily grown and widespread, resource availability should not be an issue and the crude extract of M. callabura can be used as a crop protectant for local use in Brazil and many other regions of the world.
5 Conclusions
Our study demonstrates that efficacy of the extract varies with different parts of the plant and the type of organic solvent used in the preparation of the extract. The ethanolic extract of the flowers (MCEFL) was the most active toxicant followed by the ethanolic extract of the fruits (MCEFR), hexane extract of the fruit (MCHFR) and hexane extract of the flower (MCEFL) to first instar larvae of P. xylostella. Prior feeding of the larvae with the extracts had some long term toxic effects on subsequent pupae. Fruit extracts were more active than the flower extracts in this respect. All extracts produced a significant increase in larval duration at most of the concentrations compared with the control. These organic extracts have potential for development as botanical insecticides. However further studies are needed, especially to evaluate the cost/benefit ratio regarding its use in the integrated pest management schemes and its effects on non-target organisms.
Acknowledgements
The authors would like to thank CAPES for the Master’s scholarship to the first author and CNPq for financial support.
References
- Decreased response to feeding deterrents following prolonged exposure in the larvae of a generalist herbivore, Trichoplusia ni (Lepidoptera: Noctuidae) J. Insect. Behav.. 2003;16:811-831.
- [Google Scholar]
- Dialkoxybenzene and dialkoxyallylbenzene feeding and oviposition deterrents against the cabbage looper, Trichoplusia ni: potential insect behavior control agents. J. Agric. Food Chem.. 2010;58:4983-4991.
- [Google Scholar]
- Effect of chemical complexity of essential oils on feeding deterrence in larvae of the cabbage looper. Physiol. Entomol.. 2012;37:81-91.
- [Google Scholar]
- Antifeedant and toxic effects of naturally occurring and synthetic quinones to the cabbage looper, Trichoplusia ni. Crop Prot.. 2012;31:8-14.
- [Google Scholar]
- Efeito de Cultivares de Repolho, Utilizadas para Criação de Plutella xylostella (L.) (Lepidoptera, Plutellidae), no Desenvolvimento de Trichogramma pretiosum riley (Hymenoptera, Trichogrammatidae) An. Soc. Entomol. Bras.. 1999;28:469-476.
- [Google Scholar]
- Development of insect-resistant transgenic cabbage plants expressing a synthetic cryia (B) gene from Bacillus Thuringiensis. Curr. Sci.. 2002;83:146-150.
- [Google Scholar]
- Efeito de extratos aquosos de plantas no desenvolvimento de Plutella xylostella (L.) (Lepidoptera, Plutellidae) em couve. Arq. Inst. Biol.. 2005;72:45-50.
- [Google Scholar]
- Toxicidade de natuneem sobre Tetranychus urticae Koch (Acari, Tetranychidae) e ácaros predadores da família Phytoseiidae. Ciênc. Agrotec.. 2006;30:685-691.
- [Google Scholar]
- Survey of insecticide susceptibility in Plutella xylostella (L) (Lep., Yponomeutidae) in the Federal District. Brazil. Neotrop. Entomol.. 2001;30:327-332.
- [Google Scholar]
- Synergism of malathion and inhibition of midgut esterase activities by an extract from Melia toosendan (Meliaceae) Pestic. Biochem. Physiol.. 1995;53:34-41.
- [Google Scholar]
- Bioactivity of Myoporum bontioides extracts to Plutella xylostella. Yingyong Shengtai Xuebao. 2004;15:1171-1173.
- [Google Scholar]
- Avaliação da Bioatividade de Extratos Aquosos de Meliaceae sobre Spodoptera frugiperda (J. E. Smith) Rev. Agric.. 1997;72:305-318.
- [Google Scholar]
- Effect of azadirachtin against diamondback moth, Plutella xylostella. Kunchong Xuebao. 2002;45:47-52.
- [Google Scholar]
- Plant anticancer agents 48 new cytotoxic flavonoids from Muntingia Calabura roots. J. Nat. Prod.. 1991;54:196-206.
- [Google Scholar]
- Larvicidal activity against Plutella xylostella of cordycepin from the fruiting body of Cordyceps militaris. Pest Manag. Sci.. 2002;58:713-717.
- [Google Scholar]
- Bio-insecticidal effects of plant extracts and oil emulsions of R. communis on the diamondback moth, Plutella xylostella L. (Lepidoptera: Plutellidae) under laboratory and semi-field conditions. J. Appl. Biosci.. 2011;43:2899-2914.
- [Google Scholar]
- LeOra Software, 1987. POLO-PC. A user’s Guide to Probit or Logit analysis. LeOra Software Inc., Berkeley, California, pp. 22.
- Insecticidal activity of extracts from forty-eight plants including Xanthium sibiricum Patrin. Huanjing Xuebao Jinan. 2008;17:33-37.
- [Google Scholar]
- Preliminary study on bioactivity of two plants extracts gainst three kinds of pests. Xiandai Nongyao, Shenyang. 2007;6:27-29.
- [Google Scholar]
- Manual of the Flowering Plants of South America. Santiago: University of Chile Press; 1999.
- Plantas Medicinais. Viçosa: Universidade Federal de Viçosa; 1994.
- Fruits of Warm Climates. Florida, USA: AgScience, Inc.; 1987. p. 505
- Bioatividade de pós vegetais sobre o caruncho do feijão em grãos armazenados. Rev. Agric.. 1999;74:217-224.
- [Google Scholar]
- Insecticidal and acaricidal activity of pipernonaline and piperoctadecalidine derived from dried fruits of Piper longum L. Crop. Prot.. 2002;21:249-251.
- [Google Scholar]
- Atividade inseticida de óleos essenciais e fixos sobre Callosobruchus maculatus (FABR., 1775) (Coleoptera, Bruchidae) em grãos de caupi [Vigna unguiculata (L.) WALP.)] Ciênc. Agrotec.. 2008;32:717-724.
- [Google Scholar]
- Influência do período de armazenamento do caupi [Vigna unguiculata (L.) Walp.], tratado com óleos essenciais e fixos, no controle de Callosobruchus maculatus (Fabricius, 1775) (Coleoptera, Chrysomelidae, Bruchinae) Ciênc. Agrotec.. 2009;33:319-325.
- [Google Scholar]
- Dicionário das Plantas úteis do Brasil e das Exóticas Cultivadas. Rio de Janeiro: Imprensa Nacional; 1987.
- Anti-inflammatory activity of Muntingia calabura fruits. Pharmacognosy J. 2012 doi:105530pj20123010
- [Google Scholar]
- Chemical components and biological efficacy of Melia azedarach Stems. J. Med. Arom. Plant Sci.. 1999;21:1043-1047.
- [Google Scholar]
- Pesticide Bioassays with Arthropods. Boca Raton, FL: CRC Press; 1992.
- Conserving the efficacy of insecticides against Plutella xylostella (L.) (Lep., Plutellidae) J. Appl. Entomol.. 2005;129:149-157.
- [Google Scholar]
- Genetic, biochemical, and physiological characterization of spinosad resistance in Plutella xylostella (Lepidoptera: Plutellidae) J. Econ. Entomol.. 2008;101:1658-1666.
- [Google Scholar]
- Statistix 8. 2008. Analytical Software, Statistix 8 for Windows 95, 98, NT. Analytical Software, Tallahassee, Fl, USA.
- Activity-guided isolation of the chemical constituents of Muntingia calabura using a quinone reductase induction assay. Phytochemistry. 2003;63:335-341.
- [Google Scholar]
- Efeito de extratos aquosos de plantas do desenvolvimento de Plutella xylostella (L.) (Lepidoptera, Plutellidae) Neotrop. Entomol.. 2001;30:151-156.
- [Google Scholar]
- Larvicidal activity and seasonal variation of Annona muricata (Annonaceae) extract on Plutella xylostella (Lepidoptera: Plutellidae) Rev. Colomb. Entomol.. 2011;37(2):0120-0488.
- [Google Scholar]
- Efficacy of some euphorbiacaeae plant extracts against cabbage diamondback moth, plutella xylostella L. Karnataka J. Agric. Sci.. 2009;22:688-689.
- [Google Scholar]
- The antinociceptive activity of Muntingia calabura aqueous extract and the involvement of L-arginine/nitric oxide/cyclic guanosine monophosphate pathway in its observed activity in mice. Fundam. Clin. Pharmacol.. 2006;20:365-372.
- [Google Scholar]
- The antinociceptive action of aqueous extract from Muntingia calabura Leaves, the role of opioid receptors. Med. Prin. Pract.. 2007;16:130-136.
- [Google Scholar]
- Chemical components of volatile oil from Mikania micrantha and its biological activity on insects. Yingyong Shengtai Xuebao, Guangzhou. 2003;14:93-96.
- [Google Scholar]







