Translate this page into:
Inhibitory effects of cuminaldehyde on human liver cytochrome P450 enzymes
⁎Corresponding authors. aahad@ksu.edu.sa (Abdul Ahad), abdulahad20@yahoo.com (Abdul Ahad), aljenobi@ksu.edu.sa (Fahad I. Al-Jenoobi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
This work explored the influence of cuminaldehyde on the metabolic activity of four primary human cytochrome P450 (CYP) enzymes. Human liver microsomes (HLM) with and without cuminaldehyde were incubated with specific substrates of different CYP enzymes. The influence of cuminaldehyde on “CYP1A2, CYP2C9, CYP2D6 and CYP3A4” activities was examined. The formation of specific metabolites was analyzed by HPLC analytical methods. It was observed that cuminaldehyde appears to have a negligible influence on CYP1A2 activity. However, CYP2C9 activity was significantly and potently inhibited by cuminaldehyde at all investigated concentrations. It was found that cuminaldehyde (1 µM) inhibited the most CYP2C9 enzyme activity with 64.87 % inhibition followed by CYP3A4 (38.90 %), CYP2D6 (25.76 %) and CYP1A2 (4.99 %). Inhibition of both CYP2D6 and CYP3A4 enzyme activity was observed in response to cuminaldehyde concentrations. Findings of the current investigation indicate that herb-drug interactions are very possible when cuminaldehyde is concurrently taken with medicines predominantly metabolized by CYP2C9 and CYP3A4. Cuminaldehyde should be tested further to examine how it affects CYP2C9 and CYP3A4-metabolized drugs.
Keywords
Human liver microsomes
Cumin
Cuminaldehyde
Drug interaction
Cytochrome P450
1 Introduction
There is growing research indicating that interactions between drugs or herb-drug interactions lead to detrimental reactions, poor drugs' efficacy, or in some cases patients' death (Guengerich 2021). One of the primary causes of herb-drug metabolic interactions is the inhibition or induction of the cytochrome P450 (CYP) enzyme by various substances (Qin et al. 2014). It is reported that phase I reactions are believed to be involved in the metabolism of 60 %–70 % of clinical drugs. The majority of metabolism-related enzymes are CYP450 isoforms. CYP enzyme inhibition leads to drug accumulation and contributes to drug toxicity (Hakkola et al. 2020). A number of significant human CYP450 isoforms are suggested by the “Food and Drug Administration” to be investigated for drug-drug interactions, for instance, CYP1A2, CYP2C19, CYP2C8, CYP2C9, CYP2D6 and CYP3A. Most of the enzymatic metabolism of clinical drugs occurs with this group of CYP450 isoforms (Sudsakorn et al. 2020).
In our modern world, many consumers use herbal products for self-care or medical purposes every day. In cases where patients consume herbal remedies and drugs concurrently, consideration should be given to possible herb-drug interactions (van Breemen 2015). Over the last two decades, numerous studies have demonstrated potential CYP450 enzyme interactions among herbs and pharmaceutical drugs (Cheng et al. 2023; Zhang et al. 2022). In addition, patients are more likely to suffer unwanted and unexpected side effects. For example, recently it was reported that substantial herb-drug interactions might take place when thymoquinone or herbs containing thymoquinone are concurrently taken with phenytoin (Wang et al. 2022). In another study, an improved antihypertensive effect and enhanced oral bioavailability of amlodipine was observed when the drug was concurrently administered with cinnamon in hypertensive rats (Abdelrahman et al. 2023). In another finding, investigators revealed that thymoquinone exerts a considerable inhibitory influence on four primary CYP enzymes in human liver microsomes (HLM) (Albassam et al. 2018).
Cumin (Cuminum cyminum L.) is a popular herb that is a member of the Apiaceae family (Al Juhaimi and Ghafoor, 2013). This herb is primarily found in Egypt, India, and Iran. A large portion of the crop is grown in Asia, North Africa, South America, and Southern Russia (Tuncturk and Tuncturk 2006). There is a certain amount of fat (around 10 %) and approx. 1.5 % volatile oils, as well as phenolic compounds, sugars, and protein are present in these seeds. Foods flavored with cumin oil have a spicy flavor and a distinct essence (Huo et al. 2021; Sahoo et al. 2014). The potent antioxidant characteristics of cumin seeds contribute to a number of positive health welfares (Johri 2011).
Cuminaldehyde (Fig. 1) is a natural monoterpenoid that contains isopropyl and aldehyde groups at four positions. In addition to cumin oil, eucalyptus, myrrh, and cassia oils also contain cuminaldehyde.
Cuminaldehyde chemical structure.
It has been demonstrated that cumin oil has health-promoting properties owing to cuminaldehyde (Ansari et al. 2024). Further cumin oil demonstrated anti-inflammatory, antidiabetic, anticancer and neuroprotective properties (Ansari et al. 2024). Hence based on the above information, in this study, cuminaldehyde was assessed for its potential impact on four key CYPs namely CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in HLM.
2 Materials and methods
HLM was sourced from “Corning (Woburn, MA, USA)”. 6β-hydroxytestosterone, cuminaldehyde, testosterone, and tolbutamide were supplied by “Sigma-Aldrich (St. Louis, MO, USA)”. 4-hydroxytolbutamide was supplied by “Cayman Chemical Company (MI, USA)”. “Potassium dihydrogen phosphate was sourced from “Fisher Scientific (Leicestershire, UK)”. Acetonitrile for HPLC was provided by “Winlab laboratory and chemicals (Leicestershire, UK)”. Phenacetin, dextrorphan-D-tartrate, and dextromethorphan hydrobromide were acquired from “ICN Biomedicals, Inc. (Eschwege, Germany)”. “The NADPH was purchased from “Chem-Implex Int’l Inc. (Wood Dale, IL, USA)”. Caffeine was obtained from “Alfa Aesar (Ward Hill, MA, USA)”.
2.1 Phenacetin O-deethylation
Incubation of HLM was carried out at 37 °C as previously described (Eagling et al. 1998; Kobayashi et al. 1998). During this investigation, phenacetin served as a specific CYP1A2 substrate at a concentration of 100 µm. HLM was incubated with CYP substrate for 30 min in potassium phosphate buffer 0.1 M, pH 7.4 with or without cuminaldehyde at final concentrations (FC) of 0.01, 0.05, 0.1, 1, 10, and 100 µM. A total of 0.5 mg/ml of protein was present in the reaction mixture. Solution of NADPH, FC = 1 mM, was filled into the tube to initiate the incubation process. For the purpose of ceasing the metabolic processes, each sample was treated with perchloric acid (70 %). Later, 25 µl of caffeine (stock 10 µM) solution was transferred to each sample's tube as IS followed by centrifugation of each tube at 12000 rpm and injecting clear supernatant into HPLC for acetaminophen determination. As part of the analysis, a C18 (5.0 µm, 4.6 × 250 mm) HPLC column was employed for the separation. The mobile phase used was the combination of 15 % acetonitrile and 85 % phosphate buffer (0.05 M) pumped at 1.3 ml/min. Acetaminophen formation was detected by a UV detector at a wavelength of 245 nm (Kobayashi et al. 1998).
2.2 Tolbutamide 4-hydroxylation
The CYP2C9-mediated tolbutamide 4-hydroxylation reaction was assessed in this study. The formation of tolbutamide’s metabolite, 4-hydroxytolbutamide, was determined. A final volume of 500 µl incubation mixture contained tolbutamide (FC = 0.15 mM), HLM (0.5 mg protein), phosphate buffer (0.1 M, pH 7.4) and 1 mM NADPH with or without cuminaldehyde (FC = 0.01, 0.05, 0.1, 1, 10, and 100 µM). The incubation mixture was incubated for 30 min at 37 °C. In next step, in each sample, methanol (ice-cold, 250 µl) was used to inhibit the metabolic process. A 25 µl volume of nitrazepam [(IS) stock 1.0 µg/ml] was filled into each sample tube. After centrifugation for 10 min at 12,000 rpm, the clear supernatant from each sample was collected and placed in HPLC vials. This was followed by analysis of samples for the quantification of 4-hydroxytolbutamide formation by HPLC analytical method. In short, HPLC C18 (4.6 × 150 mm, 5.0 µm) column is used for analysis. A 75 % potassium phosphate buffer (0.02 M, pH 3.4) is combined with 25 % acetonitrile as the mobile phase. For 4-hydroxytolbutamide testing, a 1.5 ml/min flow rate of the mobile phase was used. Detection of the metabolite was performed with a UV-detector at 230 nm (Korashy et al. 2015).
2.3 Dextromethorphan O-demethylation
In this study, the CYP2D6 mediated “Dextromethorphan O-demethylation” metabolic reaction was investigated using CYP2D6 substrate dextromethorphan in accordance with earlier reported protocols (Al-Jenoobi et al. 2014). The incubation mixture contained HLM (FC = 0.5 mg/ml), magnesium chloride (FC = 6 mM), dextromethorphan (FC = 25 µM), and NADPH (FC = 1 mM) in a 0.1 M phosphate buffer pH 7.4 with and without cuminaldehyde (FC = 0.01, 0.05, 0.1, 1, 10, and 100 µM). At 37 °C, the mixture was incubated for 30 min. A solution of perchloric acid (70 %) was added after 30 min to stop the reaction. In order to analyze the samples, the clear supernatant was collected after centrifuging them for 10 min at 12,000 rpm. The analysis of dextromethorphan’s metabolite was conducted with C18 Nucleodur, 5.0 µm, 4.6 × 250 mm. The mobile phase employed was a combination of 25 % acetonitrile and 75 % HPLC-grade water (pH 3.0) with 1.5 % glacial acetic acid and 0.1 % trimethylamine; which was pumped at 1 ml/min. For determining dextrorphan formation, a fluorescence detector set λEX at 280 nm and λEM at 330 nm was used (Bendriss et al. 2001).
2.4 Testosterone 6β-hydroxylation
“Testosterone 6β-hydroxylation” reaction was assessed with CYP3A4 substrate testosterone. In the microcentrifuge tube HLM with a protein FC of 0.25 mg/ml was incubated with testosterone FC = 50 µm with and without cuminaldehyde (0.01, 0.05, 0.1, 1, 10, and 100 µM) in phosphate buffered 0.1 M pH 7.4. 1 mM NADPH solution was incorporated to the tube to initiate incubation (Baati et al. 2012; Wang and Yeung, 2011). For 30 min, the sample was incubated at 37 °C. In order to terminate the reaction, 250 μl of methanol (cold) was transfer to each sample. Eight microliters of phenacetin as IS were transferred to each sample tube; IS stock 50 μg/ml was prepared in methanol. In order to determine the level of 6β-hydroxytestosterone, the samples were centrifuged for a period of 10 min at 12,000 rpm. The clear supernatant was transferred to vials for HPLC analysis. HPLC analysis was performed to determine testosterone metabolite formation in the HLM. The mobile phase used was 70 % methanol. The HPLC column “C18 Nucleodur (5.0 µm, 4.6 × 250 mm)” was used. In this experiment, the mobile phase was flowed at a rate of 0.5 ml/min. A 242 nm wavelength was chosen to measure the metabolite formation. (Pan et al. 2012).
2.5 Data analysis
The findings are presented in percentages of the control group. “Dunnett's Multiple Comparison test” was applied to compare the mean of the different treated groups with control and p < 0.05 was taken to be significant. The statistical evaluation was carried out with “GraphPad Instat (v3.06, San Diego, CA, USA)”.
3 Results
3.1 Phenacetin O-deethylation
During this experiment, CYP1A2′s potential to metabolize phenacetin to its metabolite acetaminophen in the HLM was examined. As a result, cuminaldehyde appears to have a negligible influence on CYP1A2 expression.
Cuminaldehyde inhibited CYP1A2 activity by 4.99 %, 9.84 %, and 12.76 % (p > 0.05) at 1, 10 and 100 μM level respectively (Fig. 2). While at 0.01, 0.05 and 0.1 μM level the CYP1A2 activity was inhibited by 11.96 %, 14.58 % and 12.51 % respectively. The CYP1A2 activity was not significantly affected by any of the cuminaldehyde concentrations investigated. In conclusion, cuminaldehyde seems to have no effect on CYP1A2 activity.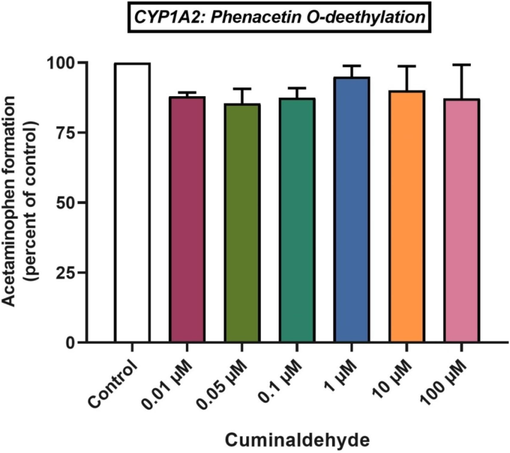
Effects of cuminaldehyde on CYP1A2-mediated conversion of phenacetin to acetaminophen in HLM, mean ± SEM.
3.2 Tolbutamide 4-hydroxylation
Tolbutamide was utilized to investigate CYP2C9 activity in the present study. A CYP2C9 enzyme was assessed for its ability to transform tolbutamide into the active metabolite 4-hydroxytolbutamide in HLM. This study noticed that CYP2C9 metabolic activity was significantly inhibited by cuminaldehyde at all investigated cuminaldehyde concentrations.
Cuminaldehyde demonstrated potent inhibition of CYP2C9 activity by 63.06 ± 1.21 %, 65.12 ± 1.82 % and 65.88 ± 2.69 % at 0.01 μM, 0.05 μM and 0.1 μM cuminaldehyde levels (Fig. 3). Comparing the experimental group with the control group, the inhibition of CYP2C9 activity was statistically significant (P < 0.05). Further, cuminaldehyde inhibited CYP2C9 at 1 μM, 10 μM and 100 μM (P < 0.05) with inhibitions of 64.87 ± 1.06 %, 66.48 ± 0.72 % and 67.05 ± 1.63 %, respectively. In this study, it was discovered that cuminaldehyde is a strong CYP2C9 inhibitor (Fig. 3).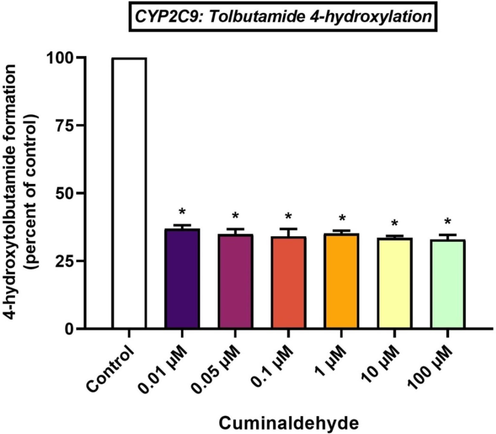
Effects of cuminaldehyde on CYP2C9-mediated conversion of tolbutamide to 4-hydroxytolbutamide in HLM, *p < 0.05 versus control, mean ± SEM.
3.3 Dextromethorphan O-demethylation
Dextromethorphan was used as a probe to measure the impact of cuminaldehyde on CYP2D6 activity in HLM. Dextrorphan (dextromethorphan metabolite) was significantly suppressed by cuminaldehyde at all concentrations that were observed.
Cuminaldehyde reduced CYP2D6 activity by 19.59 ± 3.17 %, 19.07 ± 0.81 % and 16.43 ± 1.07 % at 0.1 μM, 0.05 μM and 0.01 μM respectively. It is interesting to note that the inhibitory effect appears to be influenced by cuminaldehyde concentration (Fig. 4). Additional increases in cuminaldehyde levels at 1 μM, 10 μM and 100 μM resulted in further reductions in dextrorphan formation significantly (p < 0.05) by 25.76 ± 5.90 %, 29.62 ± 1.87 % and 30.37 ± 1.39 % respectively (Fig. 4).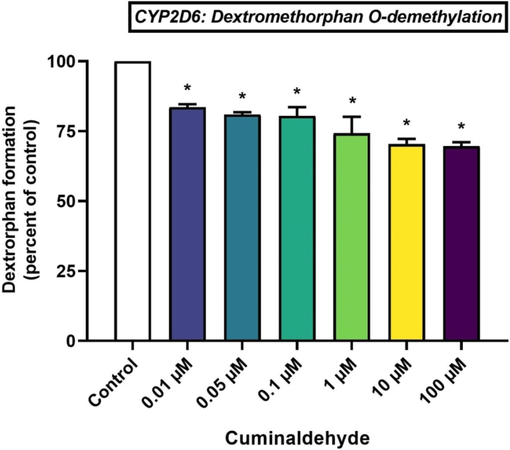
Effects of cuminaldehyde on CYP2D6-mediated conversion of dextromethorphan to dextrorphan in HLM, *p < 0.05 versus control, mean ± SEM.
3.4 Testosterone 6β-hydroxylation
Furthermore, an investigation of the influence of cuminaldehyde on CYP3A4 metabolic activity was carried out in the current investigation.
CYP3A4 metabolic activity was inhibited non-significantly (P > 0.05) by cuminaldehyde at 0.01 µM and 0.05 µM respectively by 13.90 ± 9.07 % and 14.10 ± 12.07 % (Fig. 5). Furthermore, cuminaldehyde at 0.1 µM showed considerable inhibition of CYP3A4 activity by 25.16 ± 4.22 %. Nevertheless, the two groups do not differ significantly (p > 0.05). The suppressive action of cuminaldehyde on CYP3A4 enzyme activity was dose-dependent (Fig. 5). Further, at concentrations of 1 µM and 10 µM, CYP3A4 metabolic activity was diminished by 38.90 ± 1.64 % and 40.28 ± 2.64 %, respectively. Furthermore, the maximum inhibitory effect occurred at a 100 µM cuminaldehyde concentration, which reduced CYP3A4 enzyme activity by 45.64 ± 2.04 % (P < 0.05).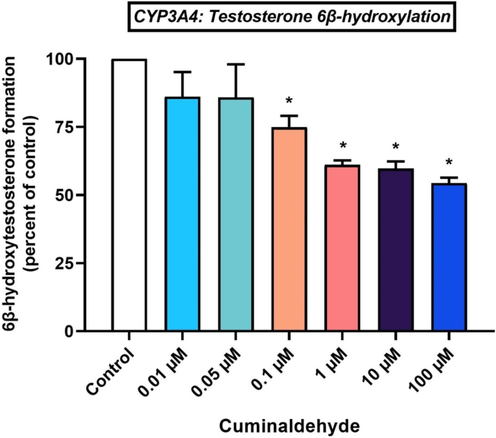
Effects of cuminaldehyde on CYP3A4-mediated conversion of testosterone to 6β-hydroxytestosterone in HLM, *p < 0.05 versus control, mean ± SEM.
4 Discussions
Herbal medicine has been utilized since ancient times (Choudhury et al. 2023). Complementary treatments are becoming increasingly popular among patients. A worldwide trend has been developing towards herbal alternatives to pharmaceutical therapies, with the perception that herbal medicines provide more cost-effective, affordable, and side-effect-free treatments (Falzon and Balabanova 2017; Gunjan et al. 2015). In addition to this, there is a growing use of herbal medicine alongside modern medications to lessen undesirable reactions, improve efficacy, or achieve synergistic effects. This has led to a growth in the popularity of natural remedies. (Du et al. 2015; Remirez et al. 2009). Saudi Arabia is also a very common user of herbal medicines (Abdelmola et al. 2021; Aljofan and Alkhamaiseh 2020; Koshak 2019). It was revealed in a recent study that high rates of herbal supplements consumption were observed in patients suffering from chronic health conditions in Saudi Arabia. In a recent study, 63 % of participants used herbal supplements, with ginger being the most common used (74.7 %) followed by mint (72.0 %), and the third most commonly consumed herb is cumin which is consumed by 66.7 % of participants. Investigators concluded that it is imperative to discuss herbal supplements' concurrent use with medical professionals in order to prevent drug interactions and adverse events. This is particularly relevant for chronically ill patients (Albassam et al. 2024). Further, in another report, the prevalence of herb consumption by women during pregnancy in Saudi Arabia was investigated. As per the study, cumin was the second most frequently used herb during pregnancy (Al Essa et al. 2019). It was reported that pharmacodynamics and pharmacokinetic interactions are likely to be observed if natural products are taken concurrently with mainstream pharmaceuticals (Ahad et al. 2022). A variety of biologically active components are commonly found in herbal medicines. This has resulted in herb–drug interactions becoming more prominent in clinical practice (Izzo and Ernst 2009). Studies have revealed that herbal medicines and conventional drugs interact primarily by affecting the activity of metabolic enzymes (Hakkola et al. 2020). CYP induction is associated with increased elimination of concurrently administered drugs leading to a reduction in drug effects (Pelkonen et al. 2008). Meanwhile, CYP inhibition results in elevated plasma concentrations of drugs, which can lead to drug toxicity (Izzo and Ernst, 2001, 2009). CYP contributes vital roles in the metabolism of numerous endogenous substances that include a variety of drugs. A large percentage of CYP activity in the human liver is attributed to the 30 % CYP3A, 20 % CYP2C and 13 % CYP1A2 subfamilies. The remaining enzymes are 13 % CYP1A2, 7 % CYP2E1, 4 % CYP2A6, 2 % CYP2D6, and 1 % CYP2B6 [26]. In light of these findings, herb–drug interactions involving herbal medicines are worth studying. Hence in this study, the effect of cuminaldehyde (a major component of cumin) on four major CYPs enzymes namely CYP1A2, CYP2C9, CYP2D6 and CYP3A4 in HLM was investigated.
All investigated CYP enzymes other than CYP1A2 were markedly affected by cuminaldehyde at 0.1 µm. CYP2C9, CYP3A4, CYP2D6 and CYP1A2 metabolic activity was decreased by 65.88 %, 25.16 %, 19.59 % and 12.51 % respectively. This study found that cuminaldehyde at 1 µm significantly (p < 0.05) decreased the metabolic activity of all investigated CYP enzymes except CYP1A2 (4.99 %). Cuminaldehyde inhibited CYP2C9 enzyme activity the most with a 64.87 % inhibition at 1 µm. This was followed by CYP3A4 (38.90 %), and CYP2D6 (25.76 %). CYP2C9 activity was suppressed by 66.48 % when the cuminaldehyde concentration was increased to 10 µm. This was followed by CYP3A4 (40.28 %) > CYP2D6 (29.62 %) > CYP1A2 (9.84 %). The CYP activity of all examined CYPs decreased significantly (P < 0.05) at 10 µm concentrations of cuminaldehyde except CYP1A2. A maximum 67.05 % reduction in CYP2C9 activity occurred when the cuminaldehyde concentration was increased from 10 µm to 100 µm. This was followed by a relative reduction in metabolic activity of CYP3A4 (45.64 %), CYP2D6 (30.37 %) and CYP1A2 (12.76 %). All CYP enzyme activities were significantly inhibited by 100 µM cuminaldehyde except CYP1A2. Our findings indicated that CYP2C9 efficiency was most strongly suppressed by 63 % at 0.01 µM cuminaldehyde concentration. Nevertheless, comparable decreases in CYP activity were also observed at 1, 10 and 100 µM level (67 %), which reflected that CYP2C9 inhibition does not appear to be concentration-dependent. However, the influence of cuminaldehyde on CYP2C9 metabolic activity was found to be extremely potent. It was observed that both the CYP namely CYP2D6 and CYP2C9 are inhibited in a concentration-dependent fashion. Further, cuminaldehyde significantly inhibited CYP2D6 activity at all investigated cuminaldehyde concentration. However, the inhibition was not potent. At 100 µM, 1 µM and 0.01 µM the CYP2D6 activity was decreased by 30 %, 25 % and 16 % respectively. In CYP2D6 case, concentration dependent inhibition was observed, although the maximum inhibition was found approx. 30 %.
The research revealed that CYP3A4 inhibition by cuminaldehyde was concentration-dependent, with a significant effect was noted at 0.1 µM, 1 µM, 10 µM and 100 µM concentrations. CYP3A4 metabolic activity was markedly (P < 0.01) reduced by cuminaldehyde at 0.1 µM level by 25.16 %. By increasing cuminaldehyde from 0.1 µM to 1 µM and 10 µM, CYP3A4 activity was further hindered by 38.90 % and 40.28 %. A maximum of 45.64 % CYP3A4 inhibition was achieved at 100 µM cuminaldehyde concentration. Cuminaldehyde is a benzaldehyde with a substituted isopropyl group at the 4-position. A benzene ring links the aldehyde group, contributing to its characteristic chemical structure. As a result of its structure and properties, cuminaldehyde is able to inhibit human liver P450s. Cuminaldehyde is a small molecule with a molecular weight and logP of about 148.20, and 3.170 respectively (see: https://hmdb.ca/metabolites/HMDB0002214, accessed on 13 Sept 2024) (Monteiro-Neto et al. 2020). Over the years, physicochemical attributes have been employed to predict CYP inhibition potential. It has been shown that the cytochrome P450 enzymes prefer substrates that are lipophilic due to their inherent lipophilicity. A majority of major cytochrome inhibitors are lipophilic in nature. Many CYP inhibitors, specifically those for CYP3A4 and CYP2C9, possess a larger molecule and high lipophilicity. In the current study, both CYP2C9 and CYP3A4 are substantially inhibited by cuminaldehyde. The CYP2C9 was inhibited by 67.05 % while CYP3A4 was weakened by 45.64 % at 100 µM cuminaldehyde level. It has been demonstrated that both CYP2C9 and CYP3A4 have strong selectivity for cuminadehyde, which is a lipophilic compound. It has been reported that CYP2D6 inhibitors are larger molecules (Beck et al. 2021). According to the current study, 100 µM cuminaldehyde inhibits CYP2D6 by 30 %. It is likely that CYD26 will not be very selective for CYP2D6 due to cuminaldehyde's small size.
The outcomes of the current assessment reveal that the possibility of herb-drug interactions is very possible when cuminaldehyde is concomitantly consumed together with medicines that are predominantly metabolized by CYP2C9 and CYP3A4. The alteration of CYP enzyme activity by cuminaldehyde might influence the pharmacokinetics of concurrently administered medications, particularly narrow-spectrum medications, and, in turn, could increase the possibility of adverse drug reactions.
5 Conclusion
It was concluded that the influence of cuminaldehyde on the metabolic activity of CYP1A2 is negligible. Further, cuminaldehyde was found to be an extremely potent inhibitor of CYP2C9. Cuminaldehyde significantly affects CYP2C9 activity, exhibiting a higher potency against this enzyme compared to other investigated enzymes. Potent and statistically significant inhibition was seen even at the lowest used concentration of the compound. Therefore, the investigated compound is expected to interact with CYP2C9 drug substrates. The suppressive actions of cuminaldehyde on CYP2D6 and CYP3A4 appear to follow a concentration-dependent manner. Cuminaldehyde demonstrates a significant reduction in CYP3A4 activity at higher concentrations, while its inhibitory effects are unremarkable at lower concentrations. In light of this, clinical studies should be performed to determine if cuminaldehyde affects CYP2C9 and CYP3A4-metabolized drugs.
Ethical Statement: “This article does not contain any studies with human participants or animals performed by any of the authors.”
CRediT authorship contribution statement
Naif Fahad M. Alharbi: Writing – review & editing, Writing – original draft, Formal analysis. Abdul Ahad: Writing – review & editing, Writing – original draft, Supervision, Software. Yousef A. Bin Jardan: Writing – review & editing, Statistical analysis, Supervision, Software. Fahad I. Al-Jenoobi: Writing – review & editing, Supervision, Conceptualization, Methodology.
Acknowledgment
“The authors are thankful to the Researchers Supporting Project number (RSPD2024R959), King Saud University, Riyadh, Saudi Arabia, for funding this project”.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Prevalence, knowledge, and perception about the use of herbal medicines jazan - Saudi Arabia. J Family Med Prim Care.. 2021;10:2386-2393.
- [Google Scholar]
- Cinnamon modulates the pharmacodynamic & pharmacokinetic of amlodipine in hypertensive rats. Saudi Pharm J.. 2023;31:101737.
- [CrossRef] [Google Scholar]
- Changes in pharmacokinetics and pharmacodynamics of losartan in experimental diseased rats treated with Curcuma longa and Lepidium sativum. Pharmaceuticals (basel).. 2022;16:33.
- [CrossRef] [Google Scholar]
- Pregnant women's use and attitude toward herbal, vitamin, and mineral supplements in an academic tertiary care center, Riyadh. Saudi Arabia. Saudi Pharm J.. 2019;27:138-144.
- [Google Scholar]
- Extraction optimization and in vitro antioxidant properties of phenolic compounds from Cumin (Cuminum cyminum l.) seed. Int Food Res J.. 2013;20:1669-1675.
- [Google Scholar]
- Inhibition of cytochrome P450 enzymes by thymoquinone in human liver microsomes. Saudi Pharm J.. 2018;26:673-677.
- [Google Scholar]
- Beliefs, awareness, use, and factors associated with herbal supplements usage among patients with chronic diseases-A cross-sectional insight from Alkharj. Saudi Arabia. Plos One.. 2024;19:e0295116.
- [Google Scholar]
- Potential inhibitory effect of herbal medicines on rat hepatic cytochrome P450 2D gene expression and metabolic activity. Pharmazie.. 2014;69:799-803.
- [Google Scholar]
- Prevalence and factors influencing use of herbal medicines during pregnancy in Hail, Saudi Arabia: A cross-sectional study. Sultan Qaboos Univ Med J.. 2020;20:e71-e76.
- [Google Scholar]
- Pharmacological basis for the antidiarrheal and antispasmodic effects of cuminaldehyde in experimental animals: In silico, ex vivo and in vivo studies. Front Biosci (landmark Ed).. 2024;29:43.
- [CrossRef] [Google Scholar]
- In vitro determination of the CYP 3A4 activity in rat hepatic microsomes by liquid-phase extraction and HPLC-photodiode array detection. J Pharmacol Toxicol Methods.. 2012;66:29-34.
- [Google Scholar]
- Descriptors of Cytochrome Inhibitors and Useful Machine Learning Based Methods for the Design of Safer Drugs. Pharmaceuticals (basel).. 2021;14
- [Google Scholar]
- High-performance liquid chromatography assay for simultaneous determination of dextromethorphan and its main metabolites in urine and in microsomal preparations. J Chromatogr B Biomed Sci Appl.. 2001;754:209-215.
- [Google Scholar]
- Herb-drug interactions and their impact on pharmacokinetics: An update. Curr Drug Metab.. 2023;24:28-69.
- [Google Scholar]
- Pharmacovigilance of herbal medicines: Concerns and future prospects. J Ethnopharmacol.. 2023;309:116383.
- [CrossRef] [Google Scholar]
- Differential selectivity of cytochrome P450 inhibitors against probe substrates in human and rat liver microsomes. Br J Clin Pharmacol.. 1998;45:107-114.
- [Google Scholar]
- A history of the roles of cytochrome P450 enzymes in the toxicity of drugs. Toxicol Res.. 2021;37:1-23.
- [CrossRef] [Google Scholar]
- Marketing trends & future prospects of herbal medicine in the treatment of various disease. World J Pharm Res.. 2015;4:132-155.
- [Google Scholar]
- Inhibition and induction of CYP enzymes in humans: an update. Arch Toxicol.. 2020;94:3671-3722.
- [Google Scholar]
- Chemical Constituents of the Essential oil from Cuminum cyminum L. and Its Antifungal Activity against Panax notoginseng Pathogens. Chem Biodivers.. 2021;18:e2100638.
- [Google Scholar]
- Interactions between herbal medicines and prescribed drugs: a systematic review. Drugs. 2001;61:2163-2175.
- [Google Scholar]
- Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs. 2009;69:1777-1798.
- [Google Scholar]
- Inhibitory effects of antiarrhythmic drugs on phenacetin O-deethylation catalysed by human CYP1A2. Br J Clin Pharmacol.. 1998;45:361-368.
- [Google Scholar]
- Impact of Herbal Medicines like Nigella sativa, Trigonella foenum-graecum, and Ferula asafoetida, on Cytochrome P450 2C11 Gene Expression in Rat Liver. Drug Res (stuttg).. 2015;65:366-372.
- [Google Scholar]
- Prevalence of herbal medicines in patients with chronic allergic disorders in Western Saudi Arabia. Saudi Med J.. 2019;40:391-396.
- [Google Scholar]
- Cuminaldehyde potentiates the antimicrobial actions of ciprofloxacin against Staphylococcus aureus and Escherichia coli. PLoS One.. 2020;15:e0232987.
- [Google Scholar]
- Development and validation of HPLC methods for the determination of CYP2D6 and CYP3A4 activities. Curr Pharm Anal.. 2012;8:219-224.
- [Google Scholar]
- Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol.. 2008;82:667-715.
- [Google Scholar]
- A high-throughput inhibition screening of major human cytochrome P450 enzymes using an in vitro cocktail and liquid chromatography-tandem mass spectrometry. Biomed Chromatogr.. 2014;28:197-203.
- [Google Scholar]
- Interactions between herbal remedies and medicinal drugs–considerations about Cuba. Drug Metabol Drug Interact.. 2009;24:183-194.
- [Google Scholar]
- Anti-diarrhoeal investigation from aqueous extract of Cuminum cyminum Linn. Seed in Albino Rats. Pharmacognosy Res.. 2014;6:204-209.
- [Google Scholar]
- 2020 FDA Drug-drug Interaction Guidance: A Comparison Analysis and Action Plan by Pharmaceutical Industrial Scientists. Curr Drug Metab.. 2020;21:403-426.
- [Google Scholar]
- Effects of Different Phosphorus Levels on the Yield and Quality Components of Cumin (Cuminum cyminum L.) Res J Agric Biol Sci.. 2006;2:336-340.
- [Google Scholar]
- Development of Safe and Effective Botanical Dietary Supplements. J Med Chem.. 2015;58:8360-8372.
- [Google Scholar]
- Potential herb-drug interaction risk of thymoquinone and phenytoin. Chem Biol Interact.. 2022;353:109801.
- [CrossRef] [Google Scholar]
- Effects of Salvia miltiorrhiza extract on the liver CYP3A activity in humans and rats. Phytother Res.. 2011;25:1653-1659.
- [Google Scholar]
- Overview of current herb-drug interaction databases. Drug Metab Dispos.. 2022;50:86-94.
- [Google Scholar]







