Translate this page into:
Inhibitory effect of Ginkgo biloba extract on vascular calcification in rats with chronic kidney disease by ROS-NF-κB signaling pathway
⁎Corresponding author at: Department of Renal Rheumatism, Shanghai Sixth People’s Hospital East Affiliated to Shanghai University of Medicine & Health Sciences, 222 3rd Huanhu Rd. (W) Pudong New District, Shanghai 201306, China. healthfirstgyp@yeah.net (Yongping Guo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In order to explore the mechanism of Extract Gingko biloba (EGb761) inhibiting vascular calcification in rats with chronic kidney disease (CKD) through ROS-NF-κB signaling pathway, adult male healthy SD (Sprague Dawley) rats were selected as the research objects. Animal models were constructed and randomly divided into control group, high phosphorus-induced residual renal vascular calcification model group (HPIAC group), and EGb761 intervention calcification group. Samples were collected from each group of rats, and the renal function, aortic calcification, and the expression of related proteins were detected by Western blot. The results showed that the contents of urea nitrogen, creatinine, and phosphorus in calcification model group increased significantly, with significant statistical difference (P < 0.05), but did not decrease significantly after intervention with EGb761. In the comparative analysis of calcium content in aorta of rats in each group, it was found that the calcium content of abdominal aorta in model group was significantly higher than that in control group. After the intervention of EGb761, the calcium content of abdominal aorta was significantly decreased, with significant statistical difference (P < 0.05). Western blot was used to detect the expression levels of NF-κB (nuclear factor-κB) P65, cbfα1 (core binding factor-α1), and α-SM (a-smooth muscle) actin in thoracic aorta of rats in each group. It was found that the expression levels of NF-κB p65 and cbfα1 in thoracic aorta of rats in HPIAC group increased significantly, while the expression of α-SM actin decreased significantly, with significant statistical difference (P < 0.05). After the intervention of EGb761, the change of the protein was alleviated obviously, with significant statistical difference (P < 0.05). Therefore, through this study, it was found that EGb761 can partially alleviate the calcification of chronic renal failure (CRF) caused by high phosphorus by interfering with the expression of NF-κB p65 protein in rat thoracic artery. Although there are some shortcomings in the experiment, it still provides experimental basis for the treatment of CRF in the later clinical stage.
Keywords
EGb761
Western blot
CKD
Vascular calcification
1 Introduction
Chronic renal failure (CRF) refers to the decrease of glomerular filtration rate (GFR) caused by chronic kidney disease (CKD) and the related metabolic disorders and clinical symptoms of the syndrome (Tajbakhsh et al., 2017). Some scholars have put forward the concept of CKD, that is, chronic kidney structure and dysfunction caused by various causes (kidney injury history >3 months), including normal and abnormal pathological damage of GFR, abnormal blood or urine composition, abnormal imaging examination, or GFR decrease with unknown causes (GFR < 60 mL/min-1.73 m2 more than 3 months), which is called CKD (Wang et al., 2016). Cardiovascular disease (CVD) is one of the main complications of patients with CKD and the main cause of death in patients with CKD. Vascular calcification is a common clinical manifestation in CKD and a powerful predictor of cardiovascular death (Yaguchi et al., 2017). Therefore, early treatment of vascular calcification in patients with CKD is of great significance.
The main components of Extract Gingko biloba (EGb761) are ginkgo flavonoids and ginkgolides. According to TCM (Traditional Chinese Medicine) theory, Gingko biloba leaves are sweet, bitter, and astringent. It has the functions of asthma-relieving, blood-activating, stasis-removing, and pain-relieving. EGb761 can reduce serum creatinine (Scr), blood urea nitrogen (BUN), and urinary protein in patients with CRF, and has a certain renal protective effect (Li et al., 2018; Ossani et al., 2017). At the same time, it was found that EGb761 had certain therapeutic effects on renal interstitial fibrosis, pulmonary interstitial fibrosis, and liver fibrosis. At present, it is believed that the mechanism of EGb761 in preventing and treating CRF is mainly to inhibit renal interstitial fibrosis. It may also play a certain role in renal protection by increasing SOD content, affecting the activity of Na+-K+ channel and Ca2+ channel (Vieira et al., 2017). Renin-angiotensin system (RAS) plays a key role in regulating renal blood pressure, renal function, and kidney diseases. In CRF, various causes lead to excessive activation of RAS, especially the increase of Angiotensin II (Ang II). Ang II is not only a vasoconstrictive substance, but also a proinflammatory cytokine, chemokine, and growth factor (Effect and Mechanism of Coptisine on the Rat Model of Chronic Renal Failure[J]. Chinese Journal of Modern Applied Pharmacy, 2017). Ang II can directly cause renal damage by altering glomerular hemodynamics, regulating water and electrolyte balance and vasoconstriction. It can also activate the secretion of cytokines, increase the synthesis of ECM, and stimulate cell proliferation and apoptosis.
In conclusion, in order to better understand the effect of EGb761 on vascular calcification in rats with CKD, SD rats were selected as the research object, and the animal models were grouped. After that, the samples were collected and the indexes of renal function, aortic calcification, and related protein expression levels were detected and analysed, to provide new ideas for the treatment of CRF in later clinical stage.
2 Materials and methods
2.1 Experimental object
24 healthy adult male SD rats (SPF grade) were purchased from Kaixue Biotechnology (Shanghai) Co., Ltd. with an individual mass of 150–180 g, raising in normal day-night alternating environment at 20–25C, keeping humidity around 70%, ensuring 12 h of light every day, sufficient water and food for feeding. In order to avoid the influence of environmental changes on rats, a formal experiment was conducted one week after the rats entered the feeding room to adapt to the environment. Animal disposal and experimental procedures are in line with the national standards for laboratory animals. All animal experiment programs have been approved by the Animal Management Committee of Shanghai Sixth People's Hospital East Affiliated to Shanghai University of Medicine & Health Sciences.
2.2 Animal grouping and model preparation
In this study, all the subjects were randomly divided into control group, high phosphorus-induced aortic calcification group (HPIAC), and EGb761 intervention group, with 8 rats in each group. The control group was treated with sham operation, and the diet was normal phosphorus feed (phosphorus (0.9%) and calcium (1.2%); the HPIAC group was treated with nephrectomy, and the diet was high phosphorus feed (phosphorus (1.2%) and calcium (1.6%)); the EGb761 intervention group was treated with nephrectomy, and its diet was original high phosphorus feed added with EGb761 (65 mg EGb761 was added to every 100 g high phosphorus feed). The rats in each group were continuously intervened in diet for 16 weeks. Rats in each group were fed in cages and chambers, so that they could freely take drinking water, keep the animal room at constant temperature and humidity, and disinfect the air regularly.
In the nephrectomy operation, the experimental animals received two stages of operation. Firstly, all animals were fasting for 12 h before operation, but water was not allowed. 10% chloral hydrate (0.3 mL/100 g) was used for abdominal anesthesia. In the first stage of operation, the prone position of the remnant kidney model group was fixed on the rat platform, exposed the left and right kidney areas, felt the position of both kidneys under the costal ridge angle with the hand, sheared hair, and sterilized the skin with 75% alcohol cotton ball. According to the location of the kidney, oblique incision was made from 1.5 cm to the left rib, muscular layer was bluntly separated and entered the abdominal cavity to expose the left kidney. The upper, middle and inferior arteries of the left kidney were separated from the perirenal fat sac. The branches of the arteries were carefully clamped with micro-forceps to observe the blood supply range. 6-0 needle suture threaded through the connective tissue between the vessels and ligated about one third of the upper and lower poles of the arteries, leaving only one third of the blood supply of the kidneys. Hemorrhage was obviously stopped by pressing hemostasis with gelatin sponge for a moment, repositioning kidney, closely suturing muscle layer by layer, suturing skin, disinfecting wound with alcohol cotton ball, and wiping blood stains. One week later, two-stage operation was performed, anesthesia, fixation, skin preparation and disinfection were performed at the same stage. The right rib was obliquely cut outward and downward at 1.5 cm. The muscular layer was bluntly separated and entered the abdominal cavity. The right kidney was exposed and the fat sac around the kidney was separated. The renal capsule was carefully peeled off from the lower pole of the right kidney to the upper pole. The pedicle was ligated with 2-0 silk thread. The right kidney was completely resected. The muscular layer was closely sutured layer by layer and the skin was sutured. In the sham-operated group, the left kidney was separated from the renal artery without ligation, while the right kidney was separated from the renal capsule without removal, preserving the integrity of both kidneys.
2.3 Collection and preservation of specimens
The experimental rats were killed after 16 weeks of intervention. Blood was taken from the hearts of rats in each group and placed in a coagulation promoting tube. Serum was centrifuged after blood coagulation and frozen in a refrigerator at −20 °C for reserve (for determination of serum creatinine, urea nitrogen, calcium, phosphorus and other indicators). The upper thoracic aorta of rats was quickly taken for dissolution with 10% formalin. The lower segment of thoracic aorta was placed in cryopreservation tube and preserved in liquid nitrogen tank for Western blot analysis. The whole segment of abdominal aorta was dried in oven at 80 C until the quality of the aorta did not change and then stored at room temperature to measure calcium content. The remaining artery specimens were stored in a refrigerator at −80 °C.
2.4 Detection of renal function indicators
The serum samples of rats in each group were taken out from the refrigerator at − 20 °C. The serum urea nitrogen, creatinine, calcium and phosphorus were detected by automatic biochemical analyzer to judge whether the rat model of CRF was successfully established and to observe the effect of EGb761 on calcium and phosphorus metabolism.
2.5 Detection of aortic calcification
Von Kossa calcification staining was used to detect the vascular calcification of thoracic aorta in all groups. The specific steps of calcification staining are: fixing vascular tissue to 10% neutral formalin and routine dehydration and embedding. The slice thickness was 5 m, and the wax was dewaxed to water routinely. After washing with distilled water for 1 min, slices were irradiated by strong light in Von Kossa silver nitrate solution. Van Gieson stained the nucleus. Conventional dehydration is transparent and neutral gum is sealed. Microscopic observation, photography and image analysis were performed to observe whether the staining was successful. The abdominal aorta was dried until the weight of the aorta was no longer changed. The aorta was digested in 1 mmol/L hydrochloric acid at 37C for 24 h. Absorb supernatant decalcification solution, collect supernatant after centrifugation for 10 min at 8000 rpm, and use calcium content determination kit (o-cresol phthalocarboxylic ketone colorimetric method). The concentration of calcium ion in vascular tissue was detected with reference to manual operation, and the calcium content/dry tissue weight was calculated.
2.6 Western blot testing
Firstly, the lower thoracic aorta vessel walls of rats preserved in liquid nitrogen tanks were ground into powder for protein extraction. The blood vessel (about 100 mg) tissue of each group of rats was packed into Ep (Eppendorf) tube. The protein lysate containing PMSF (Phenylmethanesulfonyl fluoride) was added according to the volume of l mL/100 mg. The tissue was cut up as much as possible with clean scissors, and the tissue was decomposed in ice bath for 30 min. Then centrifuged, 15 000 rpm, 4 °C, centrifuged for 10 min. The supernatant was taken and added with loading buffer, blue. 1/4 of the supernatant, can be added before running glue. Water bath at 99 for 10 min, heat to de-naturate it. Then it is packed separately and frozen at − 20 °C for reserve to prevent repeated freezing and thawing. Then, the concentration of protein sample was measured by BCA (bicinchoninic acid) method. The volume of each pore supernatant sample was calculated by the protein concentration measured by BCA protein concentration detection method. The total protein content of each pore supernatant sample was equal. Sample proteins in each pore supernatant were boiled after adding sample buffer, and then electrophoresis in SDS (sodium dodecyl sulfate)-polyacrylamide gel was carried out. The electrophoresis conditions were as follows: electrophoresis at 80 V for about 0.5 h, then electrophoresis at 110 V for about 1 h. After electrophoresis, the protein in the supernatant was transferred to the membrane by wet transfer method, and the protein on the polyacrylamide gel was transferred to the nitrocellulose membrane at constant pressure 100 V for 1.5 h. The membrane was washed with TBST (Tris-Buffered Saline Tween-20) buffer and soaked in utensils. After the protein transmembrane of the supernatant sample was completed, the membrane was sealed at room temperature for 60 min with a sealing solution. The closed membrane was diluted with fresh 1% skimmed milk powder according to the instructions; the PLDL1 antibody was diluted at 1:500, the beta-actin was diluted at 1:3000, and stored in the refrigerator at 4 °C; the PVDF film and a suitable amount of primary antibody were added into the antibody incubator, and it was preferable to soak the PDVF film completely and shake the bed at 4 °C for overnight. The next morning, absorb the first antibody, add TBST and shake the PVDF film on the horizontal shaking bed at room temperature for 3 times, about 1 0 min each time. HRP-labelled antibodies were diluted with fresh 1% skimmed milk powder in incubation. The second anti-rat antibody was 1:3000 and the second anti-rabbit antibody was 1:2000. The operation was the same as incubation, shaking bed at room temperature for 1 h. After these operations were successfully completed, the bands of cellulose nitrate membrane specimens were analyzed by image pro plus (BIO-RAD, USA). The gray value of NC film (BIO-RAD, USA) was compared with the gray value of beta-actin protein band, and then the ratio of the two was compared. The mean and standard deviation of the ratio were calculated after repeated three times.
2.7 Statistical analysis
The results of this study were expressed as mean ± standard deviation (x ± s). SPSS22.0 statistical software was used for statistical analysis. Rank sum test was used for comparison between groups. T test was used for comparison of results between two groups. One-way ANOVA was used for statistical analysis in multiple groups. P < 0.05 showed statistical difference, and P < 0.01 showed significant statistical difference.
3 Results
3.1 Detection and analysis of renal function indexes in rats of each group
Fig. 1 shows that after 16 weeks of intervention, the contents of urea nitrogen, creatinine and phosphorus in the HPIAC group increased significantly (P < 0.05), while there was no significant slowdown trend after Egb761 intervention. Calcium content is gradually decreasing, but the change is not obvious. Therefore, this indicates that the HPIAC group can successfully construct the rat model of CRF after nephrectomy and high phosphorus diet induction, but oral Egb761 cannot prevent the decrease of glomerular filtration rate in the residual kidney rats with high phosphorus diet.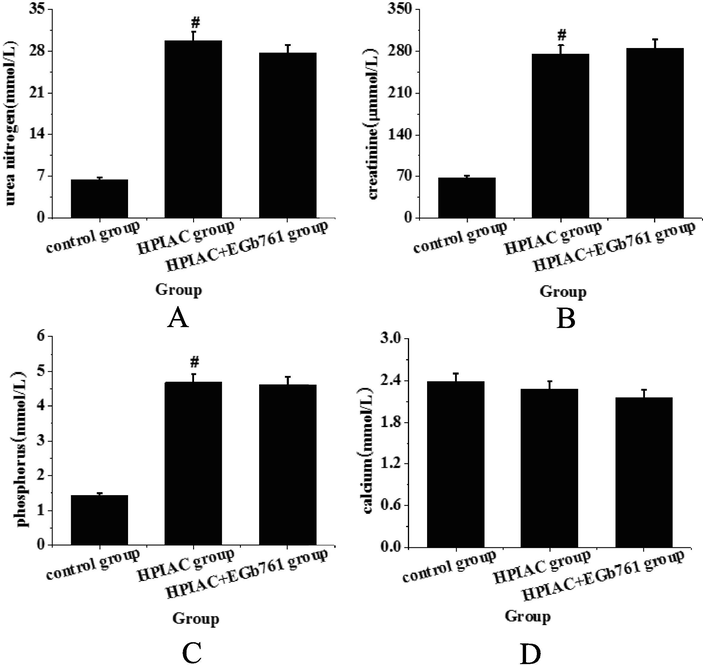
Detection and analysis of renal function indexes of rats in each group (A. BUN; B. Scr; C. phosphorus; D. calcium) (compared with control group, #P < 0.05 has significant statistical significance).
3.2 Comparative analysis of calcium ion content in aorta of rats in each group
As shown in Fig. 2, the calcium content of abdominal aorta in the control group was the lowest, that is to say, the calcium content of abdominal aorta in the HPIAC group was significantly higher than that in the control group, with significant statistical difference (P < 0.05). After the intervention of Egb761, the calcium content of abdominal aorta was significantly decreased, which was significantly different from that of model group (P < 0.05). Therefore, it can be presumed that Egb761 can significantly reduce arterial calcification in rats with CRF symptoms (see Fig. 3).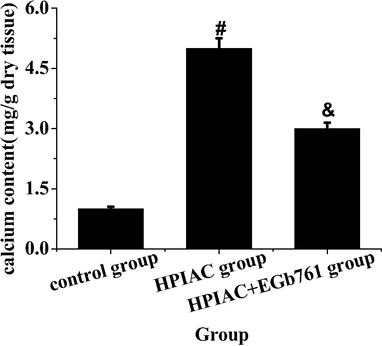
The comparative analysis of calcium content in aorta of rats in each group (compared with the control group, #P < 0.05 has significant statistical significance; compared with the HPIAC group, & P < 0.05 has significant statistical significance).
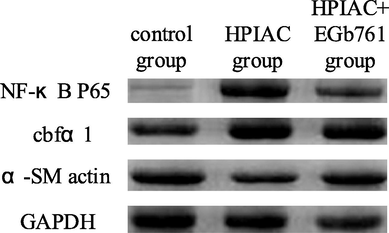
The levels of related proteins in thoracic aorta of rats in each group detected by Western blot.
3.3 Western blot analysis of expression levels of NF-κB P65, cbfα1 and α-SM actin in thoracic aorta of rats in each group
Western blot was used to detect the expression levels of NF-κB P65, cbfα1 and α-SM actin in thoracic aorta of rats in each group. Quantitative analysis of protein expression in each group of rats was performed as shown in Fig. 4. From Fig. 4, it was found that the expression of NF-κB P65 and cbfα1 in the thoracic aorta of the high Phosphorus-induced residual renal vascular calcification model group was significantly higher than that of the control group, while the expression of α-SM actin protein was significantly lower (P < 0.05), while the expression of NF-κB P65 and cbfα1 in the EGb761 intervention group was significantly higher than that in the residual renal vascular calcification model group (P < 0.05). The expression of cbfα1 decreased, while the expression of α-SM actin increased significantly (P < 0.05). Compared with the control group, the expression of α-SM actin in the calcified thoracic aorta of rats decreased significantly, which indicated that the vascular smooth muscle cells transformed from contractile type to synthetic type and osteoblasts during vascular calcification.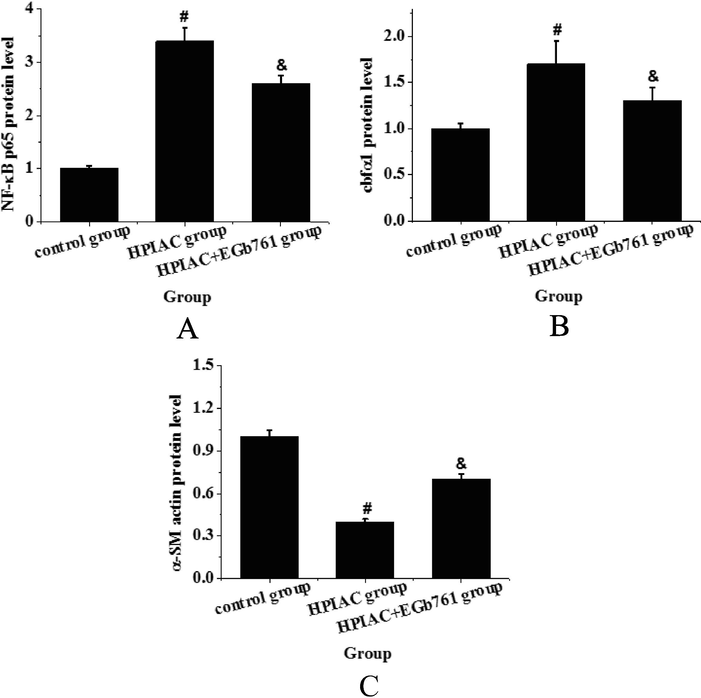
The expression levels of related proteins in thoracic aorta of rats in each group (A. NF-κB P65 expression level change analysis; B. cbfα1 expression level change analysis; C. α-SM actin expression level change analysis (compared with control group, #P < 0.05 had significant difference; compared with HPIAC group, & P < 0.05 had significant statistical significance).
4 Discussion
Vascular calcification is one of the important causes of cardiovascular disease in patients with CKD. Studies have shown that vascular calcification is an active and highly adjustable process, similar to bone formation (Dumas and Canet, 2016). EGb761, as a standard extract of Ginkgo biloba leaves obtained by modern technology, has obvious effects on improving myocardial ischemia, endothelial cell injury, and atherosclerotic plaque formation through multiple signal pathways such as antioxidant, anti-inflammatory, anti-platelet aggregation, anti-apoptosis, and regulation of angiogenesis (Rahyussalim et al., 2017). In this study, through the detection and analysis of renal function indexes of rats in each group, it was found that the contents of urea nitrogen, creatinine and phosphorus in calcification model group increased significantly, with significant statistical difference (P < 0.05), but there was no significant slowdown trend after the intervention of EGb761. This indicates that the model of renal failure has been successfully established, but oral administration of EGb761 does not prevent the decline of GFR in residual kidney rats fed with high phosphorus diet. However, there was no significant difference in the calcium content of each group, indicating that CKD may not affect the content of calcium in the kidney.
In physiological conditions, there are some factors inhibiting calcification in blood vessels, such as serum pyrophosphate, matrix Gla protein, osteopontin, and osteoprotegerin. Under pathological conditions, all kinds of factors activate the inducing factors of vascular calcification and destroy the inhibitory factors of vascular calcification, and ultimately promote the process of vascular osteoid calcification (Wang et al., 2018). In this study, through comparative analysis of calcium ion content in aorta of rats in each group, it was found that calcium ion content in abdominal aorta of model group was significantly higher than that of control group, but after the intervention of EGb761, calcium ion content in abdominal aorta was significantly decreased, with significant statistical difference (P < 0.05); and in the thoracic aorta of rats in each group, Western blot analysis showed that the expression levels of NF-κB P65, cbfα1 and α-SM actin in the thoracic aorta of the model group were significantly increased, while the expression of α-SM actin was significantly decreased, with significant statistical difference (P < 0.05). The prognosis showed that the change degree of obvious protein was alleviated with significant statistical difference (P < 0.05). Therefore, it can be inferred that EGb761 can inhibit calcification in patients with renal failure.
In conclusion, through this study, it is found that EGb761 can partially alleviate the vascular calcification of CRF caused by high phosphorus, and provide experimental basis for the treatment of CRF in later clinical stage. However, there are still some shortcomings in the experimental process. For instance, the experimental design is still in the animal experimental stage. Further research should be carried out focusing on applying it in practice, so as to provide a more reliable basis for the clinical treatment of human CRF calcification in the later stage.
Acknowledgement
This work was supported by National Natural Science Foundation of China (81703751).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Effect of hemodialysis on oxidants and antioxidant factors in chronic renal failure. Saudi J. Kidney Diseases Transplant.. 2017;28(3):507.
- [Google Scholar]
- Effect of Fushengong decoction on the expression of nephrin mRNA in kidney of rats with chronic renal failure. J. Sichuan Univ.. 2016;47(3):342-346.
- [Google Scholar]
- PA21, a novel phosphate binder, improves renal osteodystrophy in rats with chronic renal failure. PLoS ONE. 2017;12(7):e0180430
- [Google Scholar]
- Impact of vitamin D receptor gene polymorphism on chronic renal failure susceptibility. Therapeutic Apheresis and Dialysis. 2018;22(6)
- [Google Scholar]
- Chronic kidney disease and renal failure due to lithium treatment. Vertex. 2017;XXVII I(135):325-329.
- [Google Scholar]
- Skeletal muscle metaboreflex in patients with chronic renal failure. Clin. Physiol. Funct. Imaging. 2017;37(2):229-234.
- [Google Scholar]
- Effect and mechanism of coptisine on the rat model of chronic renal failure. Chin. J. Modern Appl. Pharmacy, 2017, 34(1), 30–33.
- Effets cardiovasculaires graves des chimiothérapies, thérapies ciblées et des traitements immunosuppresseurs. Réanimation. 2016;25(3):1-14.
- [Google Scholar]
- Improvement of renal function after human umbilical cord mesenchymal stem cell treatment on chronic renal failure and thoracic spinal cord entrapment: a case report. J. Med. Case Rep.. 2017;11(1):334.
- [Google Scholar]
- Change of peripheral blood Treg/Thl7 in cognitive impairment with chronic renal failure patients. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol.. 2018;45(1):281.
- [Google Scholar]







