Translate this page into:
Inhibitory effect of culinary herbs Za'atar (blend of thyme, sesame seeds and sumac) marinades on the formation of polar and non-polar heterocyclic amines carcinogen in fried beef patties: Determination by SPE/UPLC-MS/MS
⁎Corresponding author. mrkhan@ksu.edu.sa (Mohammad Rizwan Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract

Abstract
Objectives
In the present work, the inhibitory effect of culinary herbs Za'atar (blend of thyme, sesame seeds and sumac) marinades on the formation of polar (quinoxalines, quinolines and pyridines) and non-polar (α-carbolines, β-carbolines, γ-carbolines and δ-carbolines) heterocyclic amines (HCAs) in cooked beef patties were investigated.
Methods
The beef patties were subjected to pan-frying under controlled temperature and time, HCAs analysis was carried out by solid-phase extraction (SPE) and ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS).
Results
The amounts of HCAs quinoxalines (0.74–2.88 ng/g), quinolines (0.54–0.75 ng/g), pyridines (1.66–6.32 ng/g), α-carbolines (<limit of quantification), β-carbolines (3.62–7.89 ng/g), γ-carbolines (<limit of detection) and δ-carbolines (not detected) were found in cooked beef patties that contained no Za'atar (control sample). In the results achieved after marination with Za'atar at different periods (1, 2, 4, 6, 12 and 24 h), HCAs yields (except β-carbolines) were steadily decreased with time (59.38 to 100%, 2–24 h). On contrary, β-carbolines levels were found to be radually increased with time (44.76%, 24 h).
Conclusions
The variation of HCAs formation occurred due to the presence of natural antioxidants in Za'atar which usually stimulate the oxidative effect, and thus leading to the reduction and/or formation of HCAs in cooked meat. The achieved HCAs values from this work could be applied to estimate the HCAs human intake globally and add to Za'atar in such types of food products that diminish the risk of HCAs exposure, and thus to get healthier food quality and security.
Keywords
Heterocyclic amines
Fried beef patties
Za'atar
Marinades
β-carbolines
1 Introduction
Heterocyclic amines (HCAs) are mutagenic/carcinogenic compounds (Sugimura, 1992; Sugimura et al., 2004). The formation of these foodborne carcinogens occurred during traditional household cooking of protein-rich foods for instance meat and fish (Khan et al., 2009a; Khan and Azam, 2020; Khan et al., 2013; Khan et al., 2017a; Khan et al., 2015; Santacana, 2012; Sugimura, 1992; Sugimura et al., 2004; Zhang et al., 2020). To date, more than twenty-five HCAs have been identified in cooked meat and fish products (Alaejos et al., 2008; Khan and Naushad, 2014; Kizil et al., 2011). They are categorized into two major groups such as amino-imidazoazaarenes (IQ-type) and amino-carbolines (non IQ-type). The IQ-types are generated at normal cooking temperature by induced non-enzymatic browning Maillard reaction which involves reducing sugars, amino acid and creati(ni)ne while non-IQ-types are primarily produced by means of amino acids and proteins pyrolysis at cooking temperature greater than 300 °C (Murkovic, 2004; Pais et al., 1999). HCAs formation is greatly dependent on a variety of factors for instance meat compositions (meat types, sugar, free amino acids, creatinine, moisture and fat levels), cooking method, temperature and time (Murkovic, 2004; Oz et al., 2007; Pais et al., 1999; Skog et al., 1995). Most of the HCAs are known to be more lethal than a polycyclic aromatic hydrocarbon (benzopyrene) carcinogen formed in coal tar and cigarette smoke (Hecht, 1999). According to the Ames test, HCAs quinolones (IQ and MeIQ) and quinoxalines (MeIQx) were found to be the most potent carcinogens (Turesky, 2007) even hundred times more potent than pyridines (PhIP) (Ito et al., 1997; Turesky, 2007). HCAs initiate the occurrence of cancer disease via gene transformations and produce new cells to grow in an unrestrained way which cause a cancerous growth (Sugimura, 1992). Epidemiological studies have demonstrated the links between consumptions of HCAs via diets particularly intake of cooked meats and increase the threat of various cancers such as prostate, colon, pancreas, lungs, rectum and breast (Abu-Ghazaleh et al., 2020; Budhathoki et al., 2020; Butler et al., 2003; Lo et al., 2020; Deoula et al., 2020). The World Cancer Research Fund had also revealed that the consumption of cooked red meat is a substantial risk factor of certain cancers (Marmot et al., 2007). Based on animal feeding experiments, the International Agency for Research on Cancer (IARC) has classified numerous HCAs (PhIP, MeIQ, MeIQx, Glu-P-1, Trp-P-1, Trp-P-2, AαC and MeAαC) as possible (class 2B) and (IQ) as probable human carcinogens (class 2A) (Program, 2019). Later on, the National Toxicology Program (NTP) has categorized IQ, MeIQ, PhIP and MeIQx as reasonably anticipated to be human carcinogens (Humans et al., 1993), and also specified that IQ has been allied with breast cancer, MeIQ with colon and rectal cancer, PhIP with breast and stomach cancer, and MeIQx with lung cancer (Humans et al., 1993). Nevertheless, no existing recommendations emphasis on the suggested HCAs intake limit in cooked meat products (https://www.cancer.gov/about-cancer/causes-prevention/risk/diet/cooked-meats-fact-sheet#r19).
In order to assess the HCAs intake and its exposure to human, it is highly essential to study the presence of these carcinogens in a variety of cooked meat products. HCAs levels and types are usually measured by the physicochemical methods (types of cooking method, temperature and time, level of meat precursors, amount of water present in the meat and meat pH) and chemicals involved in inhibiting or increasing impacts (Alaejos and Afonso, 2011; Oz et al., 2007; Skog et al., 1995). Many studies have illustrated that the use of either synthetic or natural antioxidants during meat processing can inhibit the HCAs formation. The radicals are produced in Maillard reaction which might be scavenged via antioxidants and thus decrease the HCAs formation. Kikugawa (1999) has studied the antioxidants scavenging influence on cation radicals (pyrazine), found that it prevent the HCAs formation (Kikugawa, 1999). In earlier studies, the authors have revealed the inhibitory influence of synthetic and natural products on the formation of HCAs in cooked meat, for instance use of ethanolic extracts of rosemary (Puangsombat and Smith, 2010), antioxidants (Vitaglione and Fogliano, 2004), rosemary extracts (Tsen et al., 2006), carotenoids from tomatoes (Vitaglione et al., 2002), elaborate recipes (Khan et al., 2019), amino acids (Linghu et al., 2020), hydrocolloids (Yang et al., 2020), natural food condiments (Khan, 2015; Khan et al., 2021; Khan et al., 2017b; Lee et al., 2020), black pepper (Oz and Kaya, 2011), chili pepper and capsaicin (Zeng et al., 2017). In the meantime, there is increasing concern in the prospective health benefits of natural products especially comprising polyphenols. Za'atar is known to be a culinary herb comprising a mixture of thyme, sesame seeds and sumac. They are chemically characterized by the presence of great quantities of polyphenolic compounds and demonstrate high antioxidants activities (El-Guendouz et al., 2019; Kosar et al., 2007; Shahidi et al., 2006). Za'atar is primarily used as a seasoning of meat and vegetable products, and preparation of bread. Za'atar has been popularized all through the Arab countries and also spread to other parts of the world. The objective of this study was to study the inhibitory effect of Za'atar marinades on the formation of polar and non-polar HCAs in fried beef patties. Besides, the present study also aims to identify the optimal marination periods that can minimize the HCAs exposure and offer a healthier food, and find novel food safety threats.
2 Experimental
2.1 Materials
HPLC grade dichloromethane, acetonitrile and methanol were obtained from Merck (Darmstadt, Germany). Milli–Q ultra-pure water was purified from Millipore purification system (Bedford, USA). Formic acid (HCOOH, ≥98%), sodium hydroxide (NaOH, pellets, ≥97.0%), ammonium acetate and ammonium formate (NH₄HCO₂) were purchased from Merck (Darmstad, Germany). Ammonia (25%) was obtained from Panreac Quimica (Barcelona, Spain). Hydromatrix (dispersing material) was purchased from Agilent Technologies (Apple Valley, USA). Empty extraction cartridges (Extrelut NT20) were obtained Merck (Darmstad, Germany). Extraction cartridges (bond elut propylsulfonyl silica, PRS, 500 mg and octadecylsilane, C18, 100 mg), manifolds with stopcocks and coupling pieces were supplied from Varian (Harbor City, USA).
The polar ((2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (4,8-DiMeIQx), 2-amino-3,7,8-trimethyl-imidazo[4,5-f]quinoxaline (7,8-DiMeIQx), 2-amino-3,4,7,8-tetramethylimidazo[4,5-f]quinoxaline (4,7,8-TriMeIQx, internal standard) 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx), 2-amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-amino-3,4-dimethylimidazo[4,5-f]quinoline (MeIQ), 2-amino-1,6-dimethyl-imidazo[4,5-b]pyridine (DMIP) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP)) and non-polar HCAs ((2-amino-9H-pyrido[2,3-b]indole (AαC), 2-amino-3-methyl-9H-pyrido[2,3-b]indole (MeAαC), 2-amino-6-methyldipyrido[1,2-α:3́,2́-d]imidazole (Glu-P-1) and 2-aminodipyrido[1,2-α:3́,2́-d]imidazole (Glu-P-2), 3-amino-1,4-dimethyl-5H-pyrido[4,3-b]indole (Trp-P-1) and 3-amino-1-methyl-5H-pyrido[4,3-b]indole (Trp-P-2)) were purchased from Toronto Research Chemicals (Toronto, Canada) except non-polar HCAs 1-methyl-9H-pyrido[3,4-b]indole (harman) and 9H-pyrido[3,4-b]indole (norharman) were purchased from Sigma-Aldrich (St. Louis, USA). The HCAs structures and their abbreviation have been displayed in Fig. 1.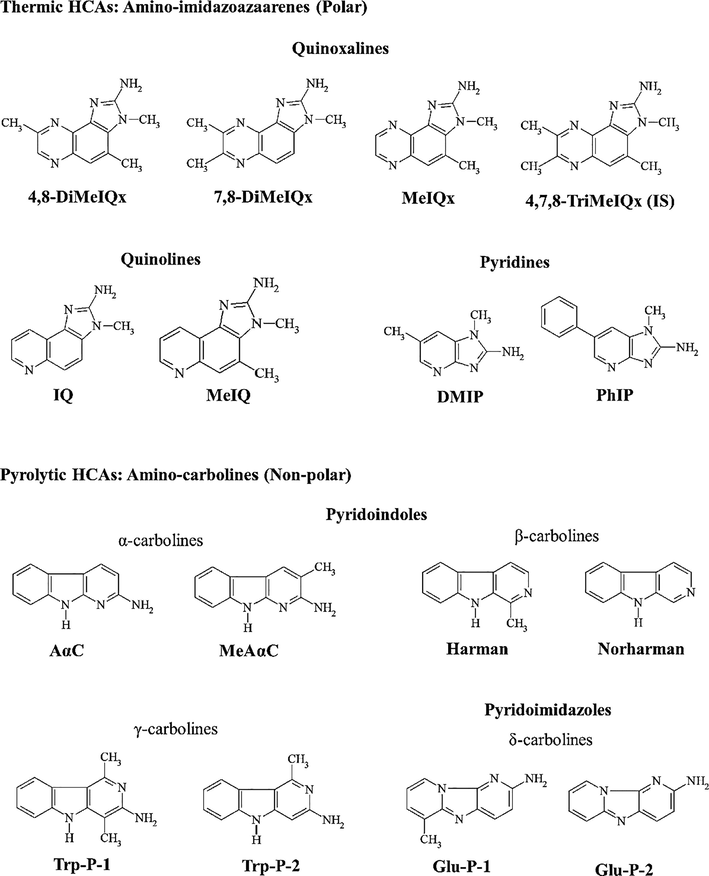
Structure and abbreviated names of the studied HCAs in beef patties, 4,7,8-TriMeIQx used as internal standard.
Stock solution of HCAs was prepared at concentration of 200 µg/g in methanol. The stock solution was used for dilution purposes. For HCAs calibration curves, standard mixtures of HCAs at levels 0.003, 0.03, 0.1, 0.5, 1.0 µg/g containing 4,7,8-TriMeIQx as internal standard (0.5 µg/g) were prepared weight by weight (w/w). Prior to the ultra-performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) analysis, standard and samples were filtered through Macherey-Nagel PTFE syringe filter (0.22 μm) (Düren, Germany).
The cooking temperature of the samples was monitored by wire probes with Normadics TC6 software (Cole-Parmer, Vernon Hills, USA). The samples were cooked using gas cooker (Gibson, Cairo, Egypt). The cooked samples were blended by means of Microtron MB 800 laboratory blender (Kinematica AG, Luzern, Switzerland). Then, the samples were freeze dried using Bechtop Freeze Dryer (SP Scientific, New York, USA). The Visiprep and Visidry manifolds (Supelco, Gland, Switzerland) were used for SPE and solvent evaporation purposes, correspondingly.
2.2 Sample preparation
Minced beef (1000 g) and Za'atar (blend of thyme (30.18 g), sesame seeds (30.13 g) and sumac (30.08 g) were obtained from the hypermarket, Riyadh, Saud Arabia. Two tablespoons (∼30 g) Za'atar were added to the minced beef (500 g) in a large bowl and mix thoroughly until beef is well coated. Then, the samples were transferred to a plastic food storage bag and stored in a refrigerator for different marination periods (1, 2, 4, 6, 12 and 24 h). The samples were prepared in patties and pan-fried using frying pan (Tefal, Paris, France). The pan surface was greased with a low quantity of olive oil so as to avoid any stickiness of the patties. The cooking temperature (200 °C) was monitored by the wire probe, the probe was also calibrated prior to the cooking process. The sample cooking period was eight minutes (4 min/side). The cooking temperature variation vs time has been demonstrated in Fig. 2. After cooking process, the samples weight loss was measured as the variance between raw and cooked sample weight. Then, the samples were ground and homogenized and lyophilized prior to SPE method. The sample description and weight loss have been presented in Table 1. CS, control sample (no Za'atar added); Za'atar (blend of thyme, sesame seeds and sumac); *4.10 min/side
Variation of cooking temperature vs. time.
Marination
Raw minced beef (g)
Za'atar (thyme, sesame seeds and sumac, g)
Total weight (raw minced beef and Za'atar, g)
Raw beef patties thickness (cm)
Marination temperature (°C)
Cooking temperature (°C)
Cooking time (min)*
Cooked beef patties (g)
Weight loss (%)
CS
300.13
30.15
330.28
0.8
4
200
8.20
171.14
48.18
1 h
300.10
30.11
330.21
0.8
4
200
8.20
175.12
46.97
2 h
300.14
30.14
330.28
0.8
4
200
8.20
179.32
45.71
4 h
300.13
30.14
330.27
0.8
4
200
8.20
183.35
44.48
6 h
300.10
30.16
330.26
0.8
4
200
8.20
188.11
43.04
12 h
300.12
30.13
330.25
0.8
4
200
8.20
195.32
40.86
24 h
300.14
30.10
330.24
0.8
4
200
8.20
198.68
39.84
2.3 HCAs extraction
HCAs extraction was performed using previously developed method (Toribio et al., 2007) with a little change in SPE procedure. 1 g of freeze dried meat sample was added to a falcon tube (60 mL) containing 12 mL of 1 M sodium hydroxide. The meat solution was homogenized by a dispersing Ultra-Turrax T25 digital device (IKA, Staufen, Germany). After that, the sample was mixed with 13 g of refill material (hydromatrix bulk, diatomaceous earth). The mixed sample was transferred to an empty extraction cartridge (Extrelut NT20) which was connected to PRS extraction cartridge (500 mg). Previously, the PRS cartridge was preconditioned by 5 mL hydrochloric acid (0.1 M), 10 mL Milli-Q water and 5 mL methanol. The HCAs was extracted from refill material using 75 mL dichloromethane as an extracting solvent to PRS cartridge. After complete elution of dichloromethane, the PRS cartridge was vacuum dried followed by washing with a water and methanol mixture (15 mL, 4:6, v/v) and 5 mL Milli-Q water in order to remove unwanted matrices. Afterward, the PRS cartridge was online to C18 (100 mg) cartridge which was preconditioned using methanol and Milli-Q water (5 mL each). From PRS, the HCAs were eluted to C18 using 0.5 M ammonium acetate solution (20 mL, pH 8.5). After elution, C18 cartridge was rinsed by Milli-Q water (5 mL) followed by vacuum drying. From C18 cartridge, the HCAs were eluted to 1.5 mL Eppendorf tube using 0.8 mL mixture of methanol and ammonia (9:1, v/v). The extracted sample was evaporated to dryness using nitrogen gas and then mixed with 0.1 mL methanol containing 4,7,8-TriMeIQx (internal standard, 0.5 µg/g). Prior to the UPLC-MS/MS analysis, the sample was filtered through 0.22 µm PTFE filter.
2.4 HCAs quantification
In order to reduce the matrix influences that obstruct with compound measurement signals, the quantification of HCAs in samples was carried out by standard addition method. The studied samples were comprising unmarinated sample (control sample) and marinated samples spiked with known amounts of polar and non-polar HCAs. The reduction rate of the HCAs formation was calculated based on the difference amounts of the HCAs found in the unmarinated and marinated samples. The HCAs percent recovery was calculated from the slope of the linear regression achieved between the amounts of HCAs added and found. Statistical analysis was performed by the analysis of variance (ANOVA) program. The sample analysis was carried out in triplicates (n = 3).
2.5 Instrumentation (UPLC-MS/MS)
Sample analysis was performed by UPLC (Waters Acquity system, Milford, USA). The system was linked to binary pump, vacuum degasser, auto-sampler and column with temperature control units. The system was coupled to a triple quadrupole mass analyzer interfaced with an ion source (electrospray ionization, ESI, Micromass, Milford, USA). An analytical column based on reversed phase BEH C18 with particle size 1.7 μm and dimension 50 mm × 2.1 mm id was applied for the polar and non-polar HCAs separation (Waters, Milford, USA). To maintain the column efficiency and its safety, a BEH C18 guard column (VanGuard™) with particle size 1.7 µm was used while performing the analysis. The separation of polar and non-polar HCAs was attained by the optimal binary mobile phase: solvent A (acetonitrile, 100%) and solvent B 30 mM buffer HCOOH/NH₄HCO₂ (pH 4.7). the mobile phase was used in gradient elution mode at 1 mL/min flow rate. The elution of mobile phase was: A, 5% (0–0.1 min); A 5–30% (0.1–1.5 min); A, 30–60% (1.5–1.8 min); A, 60% (1.8–2.3 min); restore to its original settings (2.3–2.6 min); column equilibration (2.6–3 min). The sample was automatically injected with a volume of 10 µL. In order to avoid any matrix influence in the column, it was cleaned with a solution mixture of methanol and Milli-Q after every five injections (Barcelo-Barrachina et al., 2006).
Mass spectrometer (MS) system was used in positive ionization mode and data acquisition was acquired in multiple reaction monitoring (MRM) method. The optimal ESI source parameters were applied as, source temperature (120 °C), desolvation temperature (350 °C), desolvation gas (600 L/h), cone gas (60 L/h), cone voltage (35 V), capillary voltage (3.5 kV). Nitrogen generator, model NM30LA (Peak Scientific, Inchinnan, United Kingdom) was used to generate nitrogen gas of high purity and used as cone gas. Argon was used as collision gas. An oerlikon rotary pump, model SOGEVACSV40 BI (Cedex, France) was used to supply the primary vacuum to the MS system. The MRM parameters applied with the triple quadrupole mass analyzer were showed in Table 2. MassLynx V4.1 software was used to acquire the data (Waters, Milford, USA) (Barcelo-Barrachina et al., 2006). aMultiple Reaction Monitoring (MRM) mode was used for acquisition purpose; bDwell time (25 ms, time in which each m/z ion signal was acquired); cInternal standard.
HCAs
HCAs precursor ion (m/z) tentative attribution
HCAs quantification
HCAs confirmation
HCAs product ion (m/z) tentative attribution
Collision energy (eV)
HCAs product ion (m/z) tentative attribution
Collision energy (eV)
Polar
4,8-DiMeIQx
228 [M + H]+
213 [M + H − CH3]+•
32
187 [M + H − C2NH3]+
26
7,8-DiMeIQx
228 [M + H]+
172 [M + H − CH3 − C2NH3]+•
36
213 [M + H − NH3]+•
26
MeIQx
214 [M + H]+
199 [M + H − CH3]+•
32
172 [M + H − CH3 − HCN]+•
32
4,7,8-TriMeIQxc
242 [M + H]+
227 [M + H − CH3]+•
26
201 [M + H − C2NH3]+
32
IQ
199 [M + H]+
184 [M + H − CH3]+•
32
157 [M + H − CH3 − HCN]+•
36
MeIQ
213 [M + H]+
198 [M + H − CH3]+•
26
197 [M + H − CH3 − H]+
32
DMIP
163 [M + H]+
148 [M + H − CH3]+•
26
147 [M + H − CH3 − H]+
32
PhIP
225 [M + H]+
210 [M + H − CH3]+•
26
183 [M + H − CH3 − HCN]+•
32
Non-polar
AαC
184 [M + H]+
167 [M + H − NH3]+
26
140 [M + H − NH3 − HCN]+
32
MeAαC
198 [M + H]+
181 [M + H − NH3]+
26
154 [M + H − NH3 − HCN]+
32
Harman
183 [M + H]+
115 [M + H − CH3CN − HCN]+
32
168 [M + H − CH3]+•
32
Norharman
169 [M + H]+
115 [M + H − 2HCN]+
32
142 [M + H − HCN]+
26
Trp-P-1
212 [M + H]+
195 [M + H − NH3]+
26
168 [M + H − NH3 − HCN]+
32
Trp-P-2
198 [M + H]+
154 [M + H − NH3 − HCN]+
32
181 [M + H − NH3]+
26
Glu-P-1
199 [M + H]+
172 [M + H − HCN]+
26
184 [M + H − CH3]+•
26
Glu-P-2
185 [M + H]+
158 [M + H − HCN]+
26
131 [M + H − HCN − HCN]+
32
3 Results and discussion
Natural food ingredients including herbs are the great source of numerous biologically active compounds. They have been described to inhibit the formation of HCAs because of their antioxidant properties. These biologically active compounds behave as a radical scavenger to trap free radicals produced in diverse routes of the formation of HCAs (Kikugawa, 1999; Vitaglione and Fogliano, 2004). In the present study, culinary herbs Za'atar was used to evaluate the inhibitory effect on the formation of polar and non-polar HCAs in fried beef patties. The weight loss of the fried beef patties at different marination time has been presented in Table 1, demonstrates that there was no big difference in weight loss between the control sample (48.18%) and the fried beef patties (39.84–46.97%). The achieved values were found in good agreement with those obtained in earlier work (Khan et al., 2019). The HCAs formation steadily increased with higher percentage of sample weight loss (Fig. 3). This occurs because of high weight loss involve larger HCAs precursors transference to the meat surface during cooking process (Persson et al., 2003). Concerning heat transfer, the cooking pan temperature was set aside within the identical temperature range in each samples. Nonetheless, the existence of Za'atar in sample marination from 1 to 24 h led to a quantity of fluid in frying pan, which could have lower the temperature of confined portions of the sample, therefore decrease the weight loss. The HCAs amounts achieved in studied samples are presented in Table 3. The outcomes have revealed that the Za'atar marinades applied in this investigation effectively reduced the formation of HCAs in fried beef patties. The amounts of polar HCAs 4,8-DiMeIQx (2.04 ng/g), 7,8-DiMeIQx (0.74 ng/g), MeIQx (2.88 ng/g), IQ (0.54 ng/g), MeIQ (0.75 ng/g), DMIP (1.66 ng/g) and PhIP (6.32 ng) and non-polar AαC (<LOQ), MeAαC (<LOQ), harman (3.62 ng/g), norharman (7.89 ng/g), Trp-P-1 (<LOD), Trp-P-2 (<LOD), Glu-P-1 (not detected) and Glu-P-2 (not detected) was quantified in control sample. The LOD values of polar and non-polar amines in control sample were ranged between 0.01 ng/g and 0.02 ng/g, whereas in marinated samples the HCAs range were between 0.01 ng/g and 0.06 ng/g. In comparison to control sample, the LOD values were found at higher levels in marinated samples. These variations might be happened because of samples containing Za'atar have greater matrix influences on the analysis and mass accuracy may differ because of the matrix influences applying triple quadrupole MS system. The recovery values of polar and non-polar amines were obtained in control sample between 38% and 69%, however, the marinated samples contained lower recovery between 28% and 67%, this appears due to the similar cause produced in LOD. The LOD and recovery values (Table 4) were found to be identical to those reported in earlier work (Toribio et al., 2007). *Unmarinated; sd, standard deviation (n = 3); h, hour; nd, not detected; LOD, below detection limit (signal-to-noise, 3:1); <LOQ: below quantification limit (signal-to-noise, 10:1). *Unmarinated; sd, standard deviation; R, recovery; h, hour; LOD, detection limit (signal-to-noise, 3:1).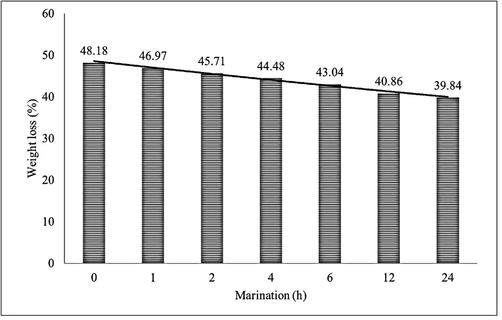
Influence of marination on sample weight loss.
HCAs
Control sample*(ng/g) ± sd
Beef marination with Za'atar
1 h(ng/g) ± sd
2 h(ng/g) ± sd
4 h(ng/g) ± sd
6 h(ng/g) ± sd
12 hng/g) ± sd
24 hng/g) ± sd
Polar
4,8-DiMeIQx
2.04 ± 0.13
1.98 ± 0.11
1.96 ± 0.11
1.52 ± 0.08
1.40 ± 0.06
1.23 ± 0.04
0.72 ± 0.06
7,8-DiMeIQx
0.74 ± 0.06
0.66 ± 0.05
0.63 ± 0.05
0.44 ± 0.03
0.35 ± 0.02
0.26 ± 0.01
0.28 ± 0.03
MeIQx
2.88 ± 0.15
2.73 ± 0.12
2.69 ± 0.11
2.21 ± 0.08
1.77 ± 0.07
1.43 ± 0.04
1.17 ± 0.08
IQ
0.54 ± 0.04
0.35 ± 0.04
<LOQ
<LOD
nd
nd
nd
MeIQ
0.75 ± 0.06
0.42 ± 0.04
<LOQ
<LOD
nd
nd
nd
DMIP
1.66 ± 0.10
1.22 ± 0.08
1.17 ± 0.07
1.04 ± 0.06
0.88 ± 0.07
0.62 ± 0.05
0.46 ± 0.03
PhIP
6.32 ± 0.55
5.08 ± 0.42
4.96 ± 0.40
4.76 ± 0.36
4.20 ± 0.30
3.69 ± 0.22
2.14 ± 0.12
Non-polar
AαC
<LOQ
<LOD
nd
nd
nd
nd
nd
MeAαC
<LOQ
<LOD
nd
nd
nd
nd
nd
Harman
3.62 ± 0.21
4.10 ± 0.33
4.22 ± 0.32
4.25 ± 0.37
4.78 ± 0.36
4.90 ± 0.28
5.62 ± 0.43
Norharman
7.89 ± 0.65
9.21 ± 0.82
9.29 ± 0.79
9.36 ± 0.80
9.93 ± 0.91
10.22 ± 1.0
12.65 ± 1.33
Trp-P-1
<LOD
nd
nd
nd
nd
nd
nd
Trp-P-2
<LOD
nd
nd
nd
nd
nd
nd
Glu-P-1
nd
nd
nd
nd
nd
nd
nd
Glu-P-2
nd
nd
nd
nd
nd
nd
nd
HCAs
Control sample*
Beef marination with Za'atar
1 h
2 h
4 h
6 h
12 h
24 h
LOD(ng/g ± sd)
R(%)
LOD(ng/g ± sd)
R(%)
LOD(ng/g ± sd)
R(%)
LOD(ng/g ± sd)
R(%)
LOD(ng/g ± sd)
R(%)
LOD(ng/g ± sd)
R(%)
LOD(ng/g ± sd)
R(%)
Polar
4,8-DiMeIQx
0.02 ± 0.01
65
0.02 ± 0.01
64
0.03 ± 0.01
63
0.03 ± 0.01
62
0.04 ± 0.01
59
0.04 ± 0.01
58
0.05 ± 0.02
55
7,8-DiMeIQx
0.02 ± 0.01
60
0.02 ± 0.01
60
0.03 ± 0.01
58
0.03 ± 0.01
57
0.04 ± 0.01
55
0.05 ± 0.02
53
0.06 ± 0.02
49
MeIQx
0.02 ± 0.01
51
0.02 ± 0.01
50
0.03 ± 0.01
46
0.03 ± 0.01
44
0.04 ± 0.01
42
0.05 ± 0.02
40
0.05 ± 0.02
37
IQ
0.01 ± 0.006
42
0.02 ± 0.01
41
0.02 ± 0.01
38
0.03 ± 0.01
37
0.04 ± 0.01
35
0.04 ± 0.01
34
0.05 ± 0.02
32
MeIQ
0.01 ± 0.005
39
0.02 ± 0.01
39
0.02 ± 0.01
36
0.02 ± 0.01
36
0.04 ± 0.01
35
0.05 ± 0.02
33
0.05 ± 0.02
29
DMIP
0.01 ± 0.006
38
0.01 ± 0.005
38
0.02 ± 0.01
34
0.03 ± 0.01
34
0.03 ± 0.01
33
0.05 ± 0.02
32
0.05 ± 0.02
28
PhIP
0.01 ± 0.006
45
0.01 ± 0.006
44
0.02 ± 0.01
40
0.02 ± 0.01
39
0.03 ± 0.01
37
0.04 ± 0.01
35
0.04 ± 0.01
32
Non-polar
AαC
0.01 ± 0.006
67
0.02 ± 0.01
67
0.02 ± 0.01
64
0.03 ± 0.01
62
0.04 ± 0.01
59
0.05 ± 0.02
58
0.06 ± 0.02
54
MeAαC
0.01 ± 0.005
69
0.02 ± 0.01
67
0.02 ± 0.01
62
0.03 ± 0.01
59
0.04 ± 0.01
57
0.05 ± 0.02
55
0.06 ± 0.02
53
Harman
0.01 ± 0.005
64
0.01 ± 0.005
63
0.02 ± 0.01
58
0.02 ± 0.01
58
0.03 ± 0.01
55
0.04 ± 0.01
54
0.05 ± 0.02
51
Norharman
0.01 ± 0.005
62
0.01 ± 0.005
61
0.02 ± 0.01
57
0.02 ± 0.01
56
0.03 ± 0.01
55
0.04 ± 0.01
54
0.05 ± 0.02
50
Trp-P-1
0.02 ± 0.01
45
0.02 ± 0.01
45
0.03 ± 0.01
42
0.03 ± 0.01
40
0.04 ± 0.01
38
0.05 ± 0.02
37
0.05 ± 0.02
35
Trp-P-2
0.02 ± 0.01
48
0.02 ± 0.01
46
0.03 ± 0.01
44
0.03 ± 0.01
43
0.04 ± 0.01
40
0.05 ± 0.02
39
0.05 ± 0.02
35
Glu-P-1
0.01 ± 0.006
58
0.01 ± 0.005
56
0.02 ± 0.01
53
0.03 ± 0.01
53
0.04 ± 0.01
51
0.04 ± 0.01
50
0.06 ± 0.02
48
Glu-P-2
0.01 ± 0.006
64
0.01 ± 0.005
62
0.02 ± 0.01
58
0.03 ± 0.01
58
0.04 ± 0.01
56
0.04 ± 0.01
55
0.06 ± 0.02
53
HCAs values in control sample (except β-carbolines) were found to be higher than those identified in marinated fried beef patties. Relatively, the amounts of β-carbolines gradually increased with marination time, 44.76% at 12 h of marination. Tryptophan (α-amino acid) is the main precursor of β-carbolines which contribute their formation during cooking process. Tryptophan could have been discharge from the culinary herbs/spices applied for marination purposes (Rönner et al., 2000) and could enhance their formation, this might be the clarification of this occurrence. In general, the use of food condiments during meat cooking can be the advantageous and prevent the HCAs formation. Nevertheless, the increase in β-carbolines levels, which may have human neurotoxicity and play an important role in chronic diseases (Pfau and Skog, 2004). Earlier studies have reported that β-carbolines can be increased with the recipes comprising various natural food contaminants (Santacana, 2012; Tengilimoglu-Metin et al., 2017). Therefore, it is significant that future investigation emphases on β-carbolines formation and toxicity mechanisms, from which limited evidence is identified at present. The amounts of 7,8-DiMeIQx, IQ, MeIQ, AαC, MeAαC, Trp-P-1, Trp-P-2, Glu-P-1 and Glu-P-2 were found either low concentrations or not identified in control sample. Nevertheless, these amines are not repeatedly detected in thermally processed meat samples. The precursors for the formation of these amines existing in meat might be inadequate, either produced some other products or degradation of some HCAs may occurred. In the case of α- and γ-carbolines (AαC, MeAαC, Trp-P-1 and Trp-P-2) formation, they needed high cooking temperatures (Jägerstad et al., 1998). The amines formation could also be influenced by turning the meat samples frequently during cooking process.
To our dietary source, the PhIP is the principal contributor, and its amounts in thermally processed meat samples ranged at higher concentrations up to 56.4 ng/g (Damašius et al., 2011). As far as our information, this is the highest value of PhIP found in cooked beef. Usually, PhIP is detected lower than 10 ng/g in such types of cooked meat (Khan and Naushad, 2014; Khan et al., 2015; Kizil et al., 2011; Santacana, 2012). Nonetheless, the highest levels of PhIP have been determined in cooked chicken (70 ng/g) (Sinha et al., 1995). Lately, Khan et al. had identified the highest amount of PhIP (121 ng/g) in thermally processed seafood (swordfish) (Khan et al., 2013). However, the lowest amount of PhIP (0.12 ng/g) obtained in cooked offal products (Khan et al., 2019). Earlier reported that PhIP could arise from phenylacetaldehyde condensation (essential α-amino acid phenylalanine degradation product) with creatine amino acid (Murkovic, 2004). In this work, the overall HCAs reduction (with the exception of β-carbolines) was obtained for the samples marinade with Za'atar. The outcomes have revealed that the HCAs formation can be inhibited by means of culinary herbs/spices. While their influences were largely reliant on marination time for instance the lowest HCAs (with the exception of β-carbolines) concentration (nd-2.14 ng/g) were obtained in the sample marinated for 24 h. The HCAs levels produced by Za'atar marinades were at lower amounts, and found in good agreement with those obtained in earlier studies (Damašius et al., 2011; Lee et al., 2020; Puangsombat and Smith, 2010; Tengilimoglu-Metin et al., 2017; Tsen et al., 2006; Paola Vitaglione et al., 2002). Za'atar is chemically characterized by the presence of great quantities of polyphenolic compounds and demonstrates high antioxidants activities (El-Guendouz et al., 2019; Kosar et al., 2007; Shahidi et al., 2006). The antioxidants from such blend may have inhibited radical reactions leading to the HCAs formation, even though the mechanism of theses chemical reaction are very complex and is not so far strong capability to predict the influence of culinary herbs/spices on the HCAs amounts produced (Murkovic et al., 1998). Numerous studies have described the efficiency of applying the marinades of various herbs/spices (turmeric, curry leaf, ginger, lemon grass, basil, oregano, marjoram, rosemary, sweet grass, savory, thyme, coriander, chili pepper, capsaicin and hawthorn) on the inhibition of HCAs formation in cooked meats (Alsohaimi et al., 2019; Damašius et al., 2011; Sepahpour et al., 2018; Tengilimoglu-Metin et al., 2017; Zeng et al., 2017), nevertheless in some study, herb marinades promote the HCAs formation for instance the amount of PhIP reduced in cooked beef using extracts of thyme, oregano and savory (Damašius et al., 2011). In contrast, the amount of PhIP increased by applying the extract of basil, sweet grass, coriander and rosemary (Damašius et al., 2011). For the clarification of this existence, it was assumed that some herbs/spices extracts contribute additional constituents to the path of HCAs formation which can probable increase or obstruct the formation of HCAs in meat products. The HCAs formation occurs through Maillard reaction, and dominance of the Maillard reaction is of great significance to attain cooked meat quality and reduce the formation of such type of carcinogens. In addition, to inhibit or diminish HCAs formation, investigations have frequently concentrated on phenolic compounds due to their illustrious antioxidant characteristics. Outcomes from the present study elucidates that Za'atar is a favorable inhibitor of HCAs amounts cooked beef patties, and having inhibitory influences ranged between 59.38% and 72.29% at 24 h of marination while IQ and MeIQ reduced to 100% at 2 h of marination. In contrast, harman and nonharman levels were gradually increased with time, 44.76% at 24 h of marination. The variation of HCAs formation occurred due to the presence of natural antioxidants in Za'atar which usually stimulate the oxidative effect, and thus leading to the reduction and/or formation of HCAs in cooked meat. The achieved HCAs values from this work could be applied to estimate the HCAs human intake globally and add to Za'atar in such types of food products that diminish the risk of HCAs exposure, and thus to get healthier food quality and security. Even though inhibition by means of culinary herbs/spices is because of diverse mechanisms. Nevertheless, there is a relation between phenolic antioxidants and HCAs inhibition (Vitaglione and Fogliano, 2004).
4 Conclusions
At the present time, the innovative approaches for inhibiting the formation of HCA carcinogens have great concern. Cooking methods may have a substantial role in the formation of HCAs, and in specific, the use of antioxidants prior to food preparation may be an influential approach to reduce the amounts of foodborne carcinogens. Za'atar is chemically characterized by the presence of great quantities of polyphenolic compounds and demonstrate high antioxidants activities (El-Guendouz et al., 2019; Kosar et al., 2007; Shahidi et al., 2006). Its inhibitory potential on HCAs formation has been persuasively revealed in the current investigation. The highest HCAs inhibition rate was identified in the samples marinade for 24 h. The outcomes were also specifying that the influence of Za'atar marinade depends on the types of HCAs present in the cooked meat products. The levels of HCAs quinoxalines, pyridines, α-carbolines, γ-carbolines and δ-carbolines reduced to 59.38%-72.29% at 24 h of marination while quinolones reduced to 100% at 2 h marination. On contrary, β-carbolines levels were gradually increased with time, 44.76% at 24 h of marination. Our findings demonstrate that the HCAs exposure can be reduced at higher levels by natural food condiments especially of herbs source present in the cooked meat.
Acknowledgments
The authors would like to thank the Researchers Supporting Project No. (RSP-2021/138) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Intestinal microbiota and its association with colon cancer and red/processed meat consumption. J. Gastroenterol. Hepatol. 2020
- [Google Scholar]
- Factors that affect the content of heterocyclic aromatic amines in foods. Compr. Rev. Food Sci. Food Saf.. 2011;10(2):52-108.
- [Google Scholar]
- Exposure to heterocyclic aromatic amines from the consumption of cooked red meat and its effect on human cancer risk: a review. Food Addit. Contam.. 2008;25(1):2-24.
- [Google Scholar]
- Emergence of mutagenic/carcinogenic heterocyclic amines in traditional Saudi chicken dishes prepared from local restaurants. Food Chem. Toxicol.. 2019;132:110677.
- [CrossRef] [Google Scholar]
- Ultra-performance liquid chromatography–tandem mass spectrometry for the analysis of heterocyclic amines in food. J. Chromatogr. A. 2006;1125(2):195-203.
- [Google Scholar]
- Doneness preferences, meat and meat-derived heterocyclic amines intake, and N-acetyltransferase 2 polymorphisms: association with colorectal adenoma in Japanese Brazilians. Eur. J. Cancer Prev.. 2020;29(1):7-14.
- [Google Scholar]
- Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am. J. Epidemiol.. 2003;157(5):434-445.
- [Google Scholar]
- Assessment of the influence of some spice extracts on the formation of heterocyclic amines in meat. Food Chem.. 2011;126(1):149-156.
- [Google Scholar]
- Antioxidant activity of thyme waste extract in O/W emulsions. Antioxidants. 2019;8(8):243.
- [CrossRef] [Google Scholar]
- Tobacco smoke carcinogens and lung cancer. J. Natl. Cancer Inst.. 1999;91(14):1194-1210.
- [Google Scholar]
- Humans, I. W. G. o. t. E. o. C. R. t., Cancer, I. A. f. R. o., & Organization, W. H. (1993). Some naturally occurring substances: food items and constituents, heterocyclic aromatic amines and mycotoxins (Vol. 56): World Health Organization.
- Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) in the rat. Mutat. Res./Fundam. Mol. Mech. Mutagen.. 1997;376(1-2):107-114.
- [Google Scholar]
- Chemistry, formation and occurrence of genotoxic heterocyclic amines identified in model systems and cooked foods. Zeitschrift Lebensmitteluntersuchung und-Forschung A. 1998;207(6):419-427.
- [Google Scholar]
- Mutagenic heterocyclic amine content in thermally processed offal products. Food Chem.. 2009;112(4):838-843.
- [Google Scholar]
- Influence of food condiments on the formation of carcinogenic heterocyclic amines in cooked chicken and determination by LC-MS/MS. Food Addit. Contamin.: Part A. 2015;32(3):307-314.
- [Google Scholar]
- Shrimp as a substantial source of carcinogenic heterocyclic amines. Food Res. Int.. 2020;109977
- [Google Scholar]
- Blueberry, raspberry, and strawberry extracts reduce the formation of carcinogenic heterocyclic amines in fried camel, beef and chicken meats. Food Control. 2021;123:107852.
- [CrossRef] [Google Scholar]
- Cooking with elaborate recipes can reduce the formation of mutagenic heterocyclic amines and promote co-mutagenic amines. Food Addit. Contamin.: Part A. 2019;36(3):385-395.
- [Google Scholar]
- Identification of seafood as an important dietary source of heterocyclic amines by chemometry and chromatography–mass spectrometry. Chem. Res. Toxicol.. 2013;26(6):1014-1022.
- [Google Scholar]
- Khan, M.R., Naushad, M., 2014. UPLC–MS/MS Analysis of Heterocyclic Amines in Cooked Food. Ultra Performance Liquid Chromatography Mass Spectrometry: Evaluation and Applications in Food Analysis, 129.
- Presence of heterocyclic amine carcinogens in home-cooked and fast-food camel meat burgers commonly consumed in Saudi Arabia. Sci. Rep.. 2017;7(1):1-7.
- [Google Scholar]
- Effect of natural food condiments on carcinogenic/mutagenic heterocyclic amines formation in thermally processed camel meat. J. Food Process. Preserv.. 2017;41(1):e12819.
- [CrossRef] [Google Scholar]
- Solid phase extraction and ultra performance liquid chromatography-tandem mass spectrometric identification of carcinogenic/mutagenic heterocyclic amines in cooked camel meat. RSC Adv.. 2015;5(4):2479-2485.
- [Google Scholar]
- Involvement of free radicals in the formation of heterocyclic amines and prevention by antioxidants. Cancer Lett.. 1999;143(2):123-126.
- [Google Scholar]
- A review on the formation of carcinogenic/mutagenic heterocyclic aromatic amines. J. Food Process Technol.. 2011;2(5):1-5.
- [Google Scholar]
- Antioxidant activity and phenolic composition of sumac (Rhus coriaria L.) extracts. Food Chem.. 2007;103(3):952-959.
- [Google Scholar]
- Overview of the effect of natural products on reduction of potential carcinogenic substances in meat products. Trends Food Sci. Technol.. 2020;99:568-579.
- [Google Scholar]
- Amino acids effects on heterocyclic amines formation and physicochemical properties in pan-fried beef patties. J. Food Sci.. 2020;85(4):1361-1370.
- [Google Scholar]
- Association between meat consumption and risk of breast cancer: Findings from the Sister Study. Int. J. Cancer. 2020;146(8):2156-2165.
- [Google Scholar]
- Marmot, M., Atinmo, T., Byers, T., Chen, J., Hirohata, T., Jackson, A., . . . Leitzmann, C., 2007. Food, nutrition, physical activity, and the prevention of cancer: a global perspective.
- Formation of heterocyclic aromatic amines in model systems. J. Chromatogr. B. 2004;802(1):3-10.
- [Google Scholar]
- Antioxidant spices reduce the formation of heterocyclic amines in fried meat. Zeitschrift Lebensmitteluntersuchung und-Forschung A. 1998;207(6):477-480.
- [Google Scholar]
- Effects of cooking methods on the formation of heterocyclic aromatic amines of two different species trout. Food Chem.. 2007;104(1):67-72.
- [Google Scholar]
- The inhibitory effect of black pepper on formation of heterocyclic aromatic amines in high-fat meatball. Food Control. 2011;22(3-4):596-600.
- [Google Scholar]
- Formation of mutagenic/carcinogenic heterocyclic amines in dry-heated model systems, meats, and meat drippings. J. Agric. Food. Chem.. 1999;47(3):1098-1108.
- [Google Scholar]
- Effect of high water-holding capacity on the formation of heterocyclic amines in fried beefburgers. J. Agric. Food. Chem.. 2003;51(15):4472-4477.
- [Google Scholar]
- Exposure to β-carbolines norharman and harman. J. Chromatogr. B. 2004;802(1):115-126.
- [Google Scholar]
- Program, N.T., 2019. 14th report on carcinogens. 2016. Research Triangle Park: National Toxicology Program, US DUSEPArtment of Health and Human Services. June. Available at URL: http://ntp. niehs. nih. gov/ntp/roc/twelfth/roc12. pdf.
- Inhibition of heterocyclic amine formation in beef patties by ethanolic extracts of rosemary. J. Food Sci.. 2010;75(2):T40-T47.
- [Google Scholar]
- Formation of tetrahydro-β-carbolines and β-carbolines during the reaction of L-tryptophan with D-glucose. J. Agric. Food. Chem.. 2000;48(6):2111-2116.
- [Google Scholar]
- Consumption of meat, traditional and modern processed meat and colorectal cancer risk among the Moroccan population: A large-scale case–control study. Int. J. Cancer. 2020;146(5):1333-1345.
- [Google Scholar]
- Santacana, R.B., 2012. Food Borne Carcinogens: A Dead End? Edited by Margarita Pesheva, Martin Dimitrov and Teodora Stefkova Stoycheva, 163.
- Inhibitory effect of mixture herbs/spices on formation of heterocyclic amines and mutagenic activity of grilled beef. Food Addit. Contamin.: Part A. 2018;35(10):1911-1927.
- [Google Scholar]
- Antioxidant activity of white and black sesame seeds and their hull fractions. Food Chem.. 2006;99(3):478-483.
- [Google Scholar]
- High concentrations of the carcinogen 2-amino-1-methyl-6-phenylimidazo-[4, 5-b] pyridine (PhIP) occur in chicken but are dependent on the cooking method. Cancer Res.. 1995;55(20):4516-4519.
- [Google Scholar]
- Factors affecting the formation and yield of heterocyclic amines. In: Paper presented at the Princess Takamatsu symposia. 1995.
- [Google Scholar]
- Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci.. 2004;95(4):290-299.
- [Google Scholar]
- Inhibitory effect of hawthorn extract on heterocyclic aromatic amine formation in beef and chicken breast meat. Food Res. Int.. 2017;99:586-595.
- [Google Scholar]
- Heterocyclic amines in griddled beef steak analysed using a single extract clean-up procedure. Food Chem. Toxicol.. 2007;45(4):667-675.
- [Google Scholar]
- Effects of rosemary extracts on the reduction of heterocyclic amines in beef patties. J. Food Sci.. 2006;71(8):C469-C473.
- [Google Scholar]
- Formation and biochemistry of carcinogenic heterocyclic aromatic amines in cooked meats. Toxicol. Lett.. 2007;168(3):219-227.
- [Google Scholar]
- Use of antioxidants to minimize the human health risk associated to mutagenic/carcinogenic heterocyclic amines in food. J. Chromatogr. B. 2004;802(1):189-199.
- [Google Scholar]
- Carotenoids from tomatoes inhibit heterocyclic amine formation. Eur. Food Res. Technol.. 2002;215(2):108-113.
- [Google Scholar]
- Inhibitory effect of selected hydrocolloids on 2-amino-1-methyl-6-phenylimidazo [4, 5-b] pyridine (PhIP) formation in chemical models and beef patties. J. Hazard. Mater.. 2020;402:123486
- [Google Scholar]
- Inhibitory profiles of chilli pepper and capsaicin on heterocyclic amine formation in roast beef patties. Food Chem.. 2017;221:404-411.
- [Google Scholar]
- Determination of 14 heterocyclic aromatic amines in meat products using solid-phase extraction and supercritical fluid chromatography coupled to triple quadrupole mass spectrometry. J. Sep. Sci.. 2020;43(7):1372-1381.
- [Google Scholar]







