Translate this page into:
Inhibition of nasopharyngeal cells mediated via G2/M cell cycle arrest, blocking PI3K/AKT/mTOR signalling pathway and suppression of cell migration and invasion
⁎Corresponding author at: Department of Otorhinolaryngology Head and Neck Surgery, the First People’s Hospital of Chenzhou, Chenzhou, Hunan, China.
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Nasopharyngeal carcinoma (NPC) is a less common but destructive cancer. Because of distant metastasis and deleterious side effects, there is persistent need to identify effective treatment options for nasopharyngeal cancer. In the current research work, the anticancer effects of Abietic acid were evaluated against the cisplatin resistant nasopharyngeal cancer cells. MTT (3-(4,5-Dimethylthiazol-2-Yl)-2,5-Diphenyltetrazolium Bromide) assay was executed for monitoring cell proliferation rate. Annexin V/propidium iodide and acridine orange/ethidium bromide assays were executed to investigate effects on cell apoptosis. Flow cytometry was employed to observe the effects on different phases of cell cycle. Cancer Cell migration and cell invasion was checked by transwell assays. Western blotting was done to evaluate impact on protein expression. It was found that Abietic acid exerts potent antiproliferative against the nasopharyngeal cancerous cell line and exhibited an IC50 of 20 μM. However the toxic effects of abietic acid in comparison to cancerous cells were seen to be insignificant against the normal cells. The antitumor impact of abietic acid was exerted through apoptosis induction and alteration of apoptosis related proteins (Bax, caspase 3 and 9). Abietic acid also caused (ROS) reactive oxygen species arbitrated changes in the MMP (mitochondrial transmembrane potential) and triggered cell cycle arrest at G2/M phase. Finally, Abietic acid could also inhibit the invasiveness of cancer cells and migration of the NPC cells. Abietic acid could also block the PI3K/AKT/mTOR biochemical signalling cascade in the HNE1 cells which remains a significant therapeutic objective of anticancer drugs. In conclusion, the results indicate that abietic acid might be a promising drug candidate and promising treatment option for nasopharyngeal carcinoma.
Keywords
Nasopharyngeal cells
Cell cycle arrest
Suppression of cell migration
Anticancer drugs
1 Introduction
Plants which get exposed to harsh environmental conditions synthesize secondary metabolites to save themselves. These metabolites have diverse structures and have shown the tendency to act as drugs in human beings (Antonisamy et al., 2015; Balamurugan, 2015; Kannan and Agastian, 2015; Rathi et al., 2015; Newman and Cragg,2016; Valsalam et al., 2019a,b). Diterpenoids constitute a large group of plant secondary metabolites and have shown tremendous pharmacological potential (Zhang et al., 2004). Recent studies about Abietane diterpenoids have reported that they inhibit the progress of carcinoma cells, for example, abietane diterpenoids isolated from salvia have revealed the potential of apoptosis induction via several mechanisms (Akaberi et al., 2015; Rajkumari et al., 2019; Gurusamy et al., 2019; Arokiyaraj et al., 2015). Similarly, abietane diterpenoids from Picea glehni have shown promising anticancer activity (Kinouchi et al., 2000). Abietic acid is an abietane diterpenoid with immense pharmacological potential. Although the anti-inflammatory as well as anti-microbial effects of Abietic acid are well known (Savluchinske-Feio et al., 2006; Abdulla, 2008), the anticancer effects of Abietic acid against nasopharyngeal carcinoma have not been investigated. This current research work was designed to demonstrate the antitumor impact of abietic acid against the cisplatin resistant human NPC HNE1 cells. In Southeast Asia and Southern China NPC is one of the foremost ubiquitous malignant tumors. The primary stage metastasis of nasopharyngeal carcinoma makes it one of lethal cancers (Xiao et al., 2018). Radiotherapy and cisplatin chemotherapy show synergic effects on improving the overall five year survival rate to 50–60% (8). Major burden in currently available treatment strategies are constant relapses and distant metastasis (Adham et al., 2012). Generally, surgical removal, systemic chemotherapy or radiotherapy is employed for nasopharyngeal carcinoma. However owing to the severe adverse effects of these treatment strategies, the patient’s quality of life drastically impaired (Chang and Adami, 2006; Haleshappa et al., 2017). In the current study, the anticancer activity of abietic acid was examined for the first time against the cisplatin resistant nasopharyngeal HNE1 cancer cells and the NP69 normal cells. It was established that abietic acid declined the cell viability of HNE1 cancer cells with minimal effects on the normal NP69 cells indicative of the selective cancer cells activity of test molecule (abietic acid). Investigation of the mechanism of the action of abietic acid revealed that abietic acid triggered apoptotic cell death of the HNE1 cancerous cells which was also allied with declination of Bax, caspase-3 and −9 and enhancement of Bcl-2 expressions. However, apoptosis alone could not be responsible for such a potent anticancer property of abietic acid. Therefore cell cycle examination of the abietic acid treated HNE1 cells was performed and results showed that abietic acid triggers G2/M arrest. Cell migration and cell invasion is the initial step for cancer metastasis (Hazan et al., 2000). Herein we observed that abietic acid could inhibit both migration as well as invasion of the cancer cells indicative of potent anticancer effects of abietic acid.
2 Materials and methods
2.1 Cell culture conditions
Current study involves HNE1 (nasopharyngeal cancer cell line) and NP69 (normal nasopharyngeal cell line) which were obtained from Cancer Research Institute of Beijing (Beijing, China). Dulbecco’s modified Eagle’s medium bought from Invitrogen Life Technologies, Massachusetts, USA was used to maintain and culture procured cell lines. After supplementation of FBS (fetal bovine serum) (10%) penicillin G (100 U/ml) and streptomycin (100 μg/ml) (Himedia, Pennsylvania, USA), media was put in a CO2 (5%) incubator at 37 °C of temperature.
2.2 Cell viability assay
Briefly, 96-well plates were used for seeding of the HNE1 and the NP69 cells at around 70% confluence and then treated with 0–640 µM of Abietic acid. Afterwards these cells were incubated for one complete day followed by further incubation with MTT (2.5 mg/ml) for 4 h. All the media was completely drawn-off after 4 h of incubation and the formazan crystals remaining were solubilized in DMSO (dimethyl sulfoxide) (100 µl). Finally, through absorbance measurement at 570 nm with spectrophotometer (BD Biosciences, San Jose, CA, USA) cellular viability of indicated HNE1 and the NP69 cells was estimated.
2.3 Acridine orange/ethidium bromide (AO/EB) and annexin V/PI staining assay
For this assay, the cells (with a cell density of 0.6 × 106 cells per well) were cultured in 6-well plates and then subjected to 12 h of incubation. Subsequently, the nasopharyngeal cancerous cells were subjected to abietic acid treatment of increasing doses viz., 0, 10, 20 and 40 µM for 24 h. Afterwards, glass slides were prepared, loaded with cell culture and then stained with DAPI. The glass slides laden with cell cultures were then covered with cover slips and then using a fluorescence microscope (BioTek Instruments, Inc., Winooski, VT, USA,) the cells were analysed. Quantification of apoptosis was done with annexin V/PI staining assay which was performed as reported already (Hua et al., 2018).
2.4 Measurement of ROS (reactive oxygen species) and MMP (mitochondrial membrane potential)
The HNE1 cells were cultured for 24 h at 37 °C and then treated with increasing concentrations of abietic acid viz., 0, 10, 20 and 40 µM and then left as such for 12 h. Following drug treatment, for estimation of ROS generation the abietic acid treated cells were exposed with DCH-DA (5 μM). MMP estimation were performed through rhodamine 123 (5 µM) treatment of cells and then the cells were analysed under flow cytometer (FACSCalibur flow cytometer, BD Biosciences, San Jose, CA, USA).
2.5 Cell cycle analysis by flow cytometry
The HNE1 nasopharyngeal cells were initially incubated with increasing doses of abietic acid viz., 0, 10, 20, and 40 µM for 24 h. Following treatment, the nasopharyngeal cells were stained with PI (propidium iodide) for 20 min. Staining was followed by cell cycle phase distribution analysis via FACS flow cytometer (BD Biosciences, San Jose, CA, USA).
2.6 Cell migration and cell invasion assays
The impact of abietic acid on HNE1 nasopharyngeal cells was examined by using transwell chamber assay, the cells at a density of 1x104 cells per ml of cell culture were placed in upper chamber and grown in it with a 8 μm pore size polycarbonate filters. These chambers were then placed into 24-well plates and then incubated at 37 °C for 24 h. For invasion assay, a little modification was done and the chambers were covered with extracellular matrix gel (30 μl) (Sigma Aldrich, USA). After this, swabbing was performed to separate non-migrated cells as well as non-invaded cells from other cells. Following this the cells which migrated and invaded to other parts were fixed with methanol for 30 min followed by crystal violet staining (0.5%) for about 20 min. The cells were then washed with PBS and finally using light microscope, the cells were counted using 5 random fields.
2.7 Western blotting assay
The HNE1 nasopharyngeal cells were cultured and then harvested and then washed twice with ice-cold PBS. Cells were treated with changing concentrations of abietic acid viz 0, 10, 20, and 40 µM followed by preparation of lysates. Protein content was measured using BCA assay and separation of 30 μM of proteins was performed through electrophoresis via SDS-PAGE gels. Electrophoresis was followed by transference to Hybond-C super membrane (Amersham Pharmacia Biotech, Piscataway, NJ). Strictly sticking to manufacturer’s protocol and under enhanced chemiluminescence protein bands were observed.
2.8 Statistical analysis
Whole of the experimental data is presented as mean ± SD. One-way analysis of variance ANOVA was utilized for data analysis and for multiple comparisons Student-Newman-Keuls test was used. P < 0.05 was considered for revealing statistically significant differences.
3 Results
3.1 Abietic acid inhibits the proliferation of HNE1 NPC cells
The antiproliferative effects of Abietic acid (Fig. 1A) were inspected on the normal NP69 and HNE1 NPC cells by MTT assay at concentration stretched from 0 to 640 µM. Abietic acid was found to hamper the growth and development of the HNE1 cells concentration dependently (Fig. 1B). The IC50 of Abietic acid against the HNE1 cells was observed to be 20 µM. However, proliferation rate of NP69 normal cells didn’t alter much after drug exposure. The IC50 of Abietic acid against the normal NP69 cells was >100 µM (Fig. 1B).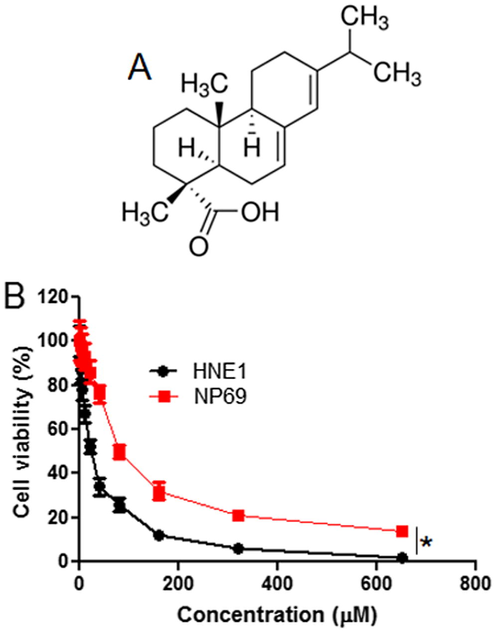
(A) Chemical structure of Abietic acid (B) MTT assay showing the effect of abietic acid on the viability of the nasopharyngeal carcinoma HNE1 and normal NP69 cells. The figure depicts that Abietic acid decrease the viability of HNE1 NPC cells with comparatively lower effects on the normal NP69 cells. Triplicate repetitions were given to individual experiments and data are expressed as mean ± SD (*p < 0.05).
4 Abietic acid induces apoptotic cell death of HNE1 cells via ROS production
AO/EB staining was performed to detect the apoptotic effect by Abietic acid treated HNE1 cells. It was quite evident that the number of apoptotic cells amplified on increasing the dose of Abietic acid as shown by AO/EB (Fig. 2) as well as the annexin V/PI staining (Fig. 3). Apoptosis allied protein expressions further validated the apoptosis inducing impact of Abietic acid on HNE1 carcinoma cells. It was established through results of western blotting analysis that Abietic acid augmented the manifestation of Bax, caspase-3 and -9 in dose-reliant fashion. Further the manifestation of Bcl-2 diminished upon Abietic acid treatment (Fig. 4). The ROS and the MMP levels were measured in the HNE1NPC cells and it was concluded that abietic acid resulted in considerable production of ROS and also caused declination in percentage of MMP in HNE1 carcinoma cells (Fig. 5).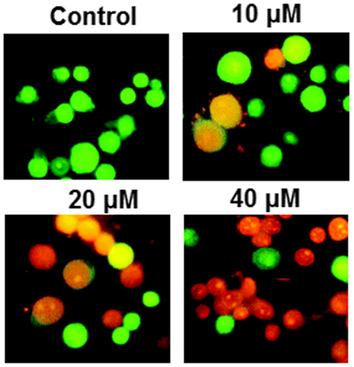
Induction of apoptosis in the HNE1 cells by Abietic acid treatment as evident from the AO/EB staining. The figure depicts that Abietic acid induces concentration dependent apoptosis in the HEN1 cells. Triplicate repetitions were given to individual experiments (orange color cells depict early apoptosis and Red color cells depict late apoptosis).
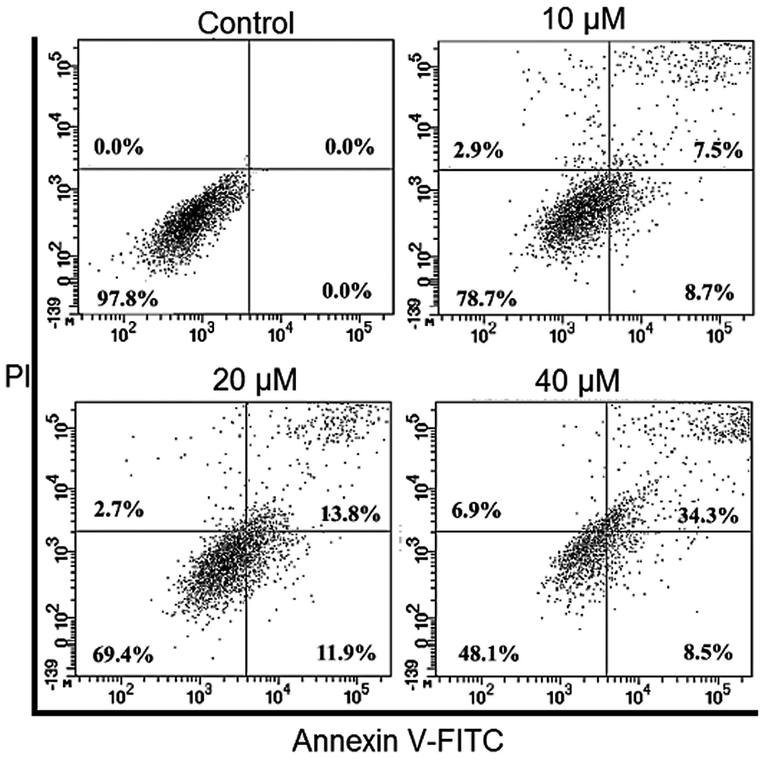
At each phase of cell cycle Annexin V/PI staining representation of percentage of apoptotic cells. The figure depicts that the apoptotic cell percentage increases with increase in the concentration of Abietic acid. Triplicate repetitions were given to individual experiments.
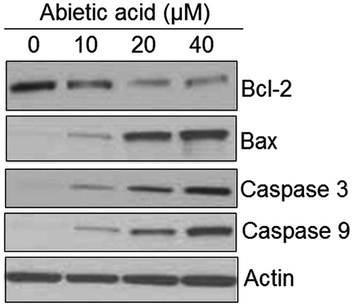
Representation of abietic acid exposure on the expression apoptosis concomitant proteins as illustrated by the western blotting analysis. The figure depicts that the expression of Bax, caspase-3 and 9 is augmented while as that of Bcl-2 is dwindled by abietic acid treatment. Triplicate repetitions were given to individual experiments.
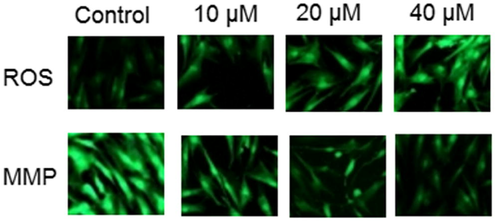
Fluorescence microscopy of abietic acid treated HNE1 cells showing increase in ROS and reduction in MMP levels after abietic acid treatment to HNE1 cells. The figure depicts that Abietic acid increases ROS and decreases MMP concentration dependently in HNE1 cells. The experiments were performed in triplicates.
4.1 Abietic acid arrests the HNE1 cells at G2/M phase
To further decipher the mechanism of action of anti-proliferative effects of Abietic acid, flow cytometry was set up to check the cycle phase distribution of cancerous HNE1 cells at 0, 10, 20 and 40 µM concentrations of Abietic acid. Results unveiled that with increasing abietic acid drug concentration the percentage number of cells at G2/M-phase augmented considerably with 3.7% (approx.) in control to 41.2% at 40 µM concentration of Abietic acid (Fig. 6).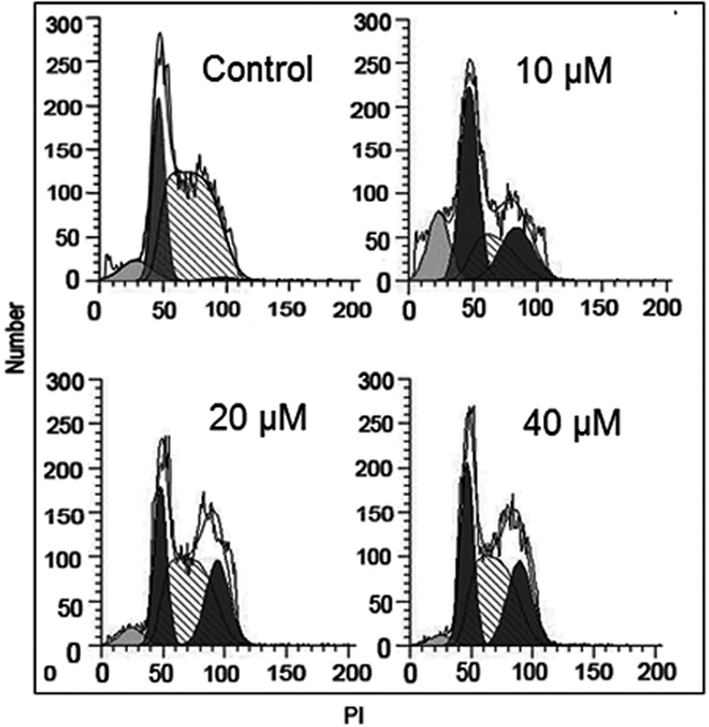
Impact of Abietic acid on distribution of different phases of the cell cycle of HNE1 cells as portrayed by flow cytometery. The figure depicts that Abietic acid induces G2/M arrest of the HNE1 cells. The experiments were performed in triplicate.
5 Abietic acid inhibits cell migration and cell invasion of HNE1 cancer cells
Next, the effect of Abietic acid on the cell migration and cell invasion of the HNE1 cancer cells was investigated by wound healing and transwell assays respectively. The results showed that at IC50, Abietic acid could inhibit the migration of the HNE1 cancer cells (Fig. 7). Similar trend was perceived in case cell invasion (Fig. 8).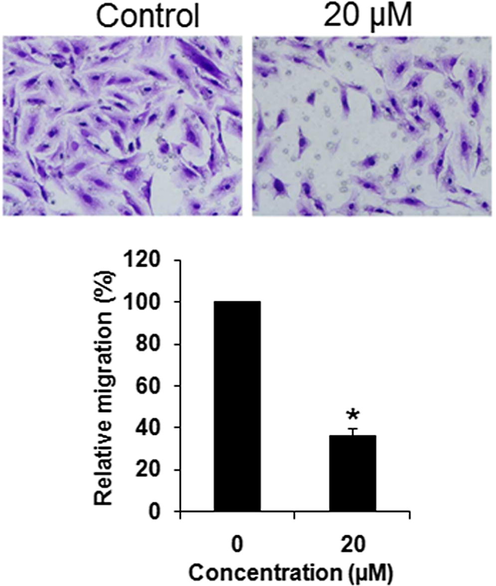
Effect of abietic acid on the migration of the HNE1 cells as depicted by transwell assays. The figure depicts that abietic acid inhibits the migration of the HNE1 cells. Triplicate repetitions were given to individual experiments and data are expressed as mean ± SD (*p < 0.05).
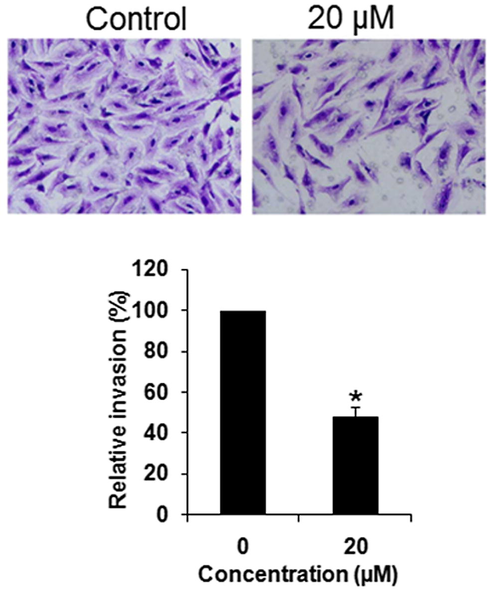
Effect of abietic acid on the invasion of the HNE1 cells as depicted by transwell assays. The figure depicts that abietic acid inhibits the invasion of the HNE1 cells. Triplicate repetitions were given to individual experiments and data are expressed as mean ± SD (*p < 0.05).
5.1 Abietic acid inhibits the PI3K/AKT/mTORsignalling pathway of HNE1 cancer cells
PI3K/AKT/mTOR signalling pathway is among important therupetic targets for the management of cancer. Hence, the consequences of Abietic acid treatment was also inspected on this signalling pathway. The results showed that Abietic acid caused a significant suppression in the expression of p-PI3K and p-AKT and p-mTOR dose dependently while no effect was found on total PI3K, AKT and mTOR protein expression (Fig. 9).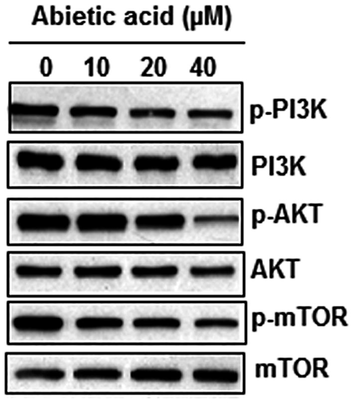
Representation of Western blotting analysis depicting the effect of abietic acid over PI3K/AKT/mTOR signalling pathway of HNE1 cells. The figure depicts that abietic acid blocks the PI3K/AKT/mTOR signalling pathway in HNE1 cells. Triplicate repetitions were given to individual experiments.
6 Discussion
Nasopharyngeal carcinoma (NPC) is one of the prevalent types of neck and head malignancy (Seow, 2017). The treatment of nasopharyngeal carcinoma is obstructed by early metastasis and the adverse effects of the accessible existing chemotherapeutic agents (Chen et al., 2018). Moreover with the progress of drug resistance in NPC cells it is even difficult to overcome NPC (Wang et al., 2015; Zhang et al., 2014). Plants serve as a pool of chemical entities that can assist in curbing various diseases including cancer (Adham et al., 2012). Herein, Abietic acid- naturally available plant extracted diterpenoid, was testified for its anticancer potency against the HNE1NPC and normal nasopharyngeal NP69 cells. It was found that Abietic acidshowsdose-dependent growth inhibitory effects on the HNE1 cells. However, Abietic acid showed relatively less toxic effects over normal NP69 cells. These observations were in agreement with investigations wherein abietane diterpenoids have been revealed to inhibit the growth cancer cells (Burmistrova et al., 2013). Previous studies regarding Abietic acid and its derivatives have also depicted its suppressive effects on growth and progression of different types of cancer cells (Lin et al., 2006). Many plant derived molecules have been reported to interfere with the ROS levels in the cancer cells (Hua et al., 2018). In the current study, it was found that Abietic acid triggered the formation ROS in the HNE1 cells and this was also accompanied by the disruption of MMP. Since, apoptosis is a vital process to eliminate the cancer cells to maintain the tissue homeostasis and (Lopez and Tait, 2015), we carried out AO/EB double staining as well as the annexin V/PI staining of the Abietic acid treated-HNE1 cells and it was found that Abietic acid triggered apoptosis in the HNE1 cells. The Abietic acid-induced apoptosis was also accompanied by upsurge of caspases, 3 and 9 and Bax and decline in Bcl-2. Abietic acid treatment could also halt the HNE1 cells at cell cycle G2/M-phase and dose dependently. These observations are also well supported wherein abietane diterpenoids have been reported to trigger cell cycle arrest (Chen et al., 2010). The cancer cell migration and invasion is considered to be imperative for the metastasis of cancer cells. Herein we observed that abietic acid could inhibit both migration capacity and invasion ability of the HNE1 cells. Finally, abietic acid could also block the PI3K/AKT/mTOR signalling pathway in the HNE1 cells which has been reported to be an essential therapeutic biochemical target of anticancer drugs (Porta et al., 2014).
7 Conclusion
The results indicate that abietic acid targets nasopharyngeal cancer cells by initiating apoptosis and cell cycle arrest along with inhibiting cellular invasion and migration and mTOR/PI3K/AKT signalling pathway.
Acknowledgement
This study was supported by Hunan Provincial Health and Health Committee's Scientific Research Project in 2019 (No: C2019001)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Anti-inflammatory activity of heterocyclic systems using abietic acid as starting material. MonatsheftefürChemie-Chem. Monthly. 2008;139(6):697.
- [Google Scholar]
- Nasopharyngeal carcinoma in Indonesia: epidemiology, incidence, signs, and symptoms at presentation. Chin. J. Cancer. 2012;31(4):185.
- [Google Scholar]
- Multiple pro-apoptotic targets of abietane diterpenoids from Salvia species. Fitoterapia. 2015;100:118-132.
- [Google Scholar]
- Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in Wistar rats.South Ind. J. Biol. Sci.. 2015;1:34-37.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci.. 2015;1:47-51.
- [Google Scholar]
- Antiproliferative activity of abietane diterpenoids against human tumor cells. J. Nat. Prod.. 2013;76(8):1413-1423.
- [Google Scholar]
- The enigmatic epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol. Prevent. Biomarkers. 2006;15(10):1765-1777.
- [Google Scholar]
- Terpenoids induce cell cycle arrest and apoptosis from the stems of Celastruskusanoi associated with reactive oxygen species. J. Agric. Food Chem.. 2010;58(6):3808-3812.
- [Google Scholar]
- A retrospective study to compare five induction chemotherapy regimens prior to radiotherapy in the reduction of regional lymph node size in patients with nasopharyngeal carcinoma. Med. Sci. Monitor.. 2018;24:2562.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol. B. 2019;193:118-130.
- [Google Scholar]
- Epidemiology and outcomes of nasopharyngeal carcinoma: Experience from a regional cancer center in Southern India. South Asian J. Cancer. 2017;6:122.
- [Google Scholar]
- Exogenous expression of N-cadherin in breast cancer cells induces cell migration, invasion, and metastasis. J. Cell Biol.. 2000;148(4):779-790.
- [Google Scholar]
- Daidzein exerts anticancer activity towards SKOV3 human ovarian cancer cells by inducing apoptosis and cell cycle arrest, and inhibiting the Raf/MEK/ERK cascade. Int. J. Mol. Med.. 2018;41(6):3485-3492.
- [Google Scholar]
- In vitro regeneration of a rare antidiabetic plant Epaltes divaricata L. South Ind. J. Biol. Sci.. 2015;1:52-59.
- [Google Scholar]
- Potential Antitumor-Promoting Diterpenoids from the Stem Bark of Picea g lehni. J. Nat. Prod.. 2000;63(6):817-820.
- [Google Scholar]
- Lin CH, Chuang HS, inventors; Xiamen Ever-Health Bio-Tech Co Ltd, assignee. Use of abietic acid and derivatives thereof for inhibiting cancer. United States patent US 7,015,248. 2006.
- Mitochondrial apoptosis: killing cancer using the enemy within. Br. J. Cancer. 2015;112(6):957.
- [Google Scholar]
- Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod.. 2016;79:629-661.
- [Google Scholar]
- Kaviyarasu K. Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill andevaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol., B 2019
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci.. 2015;1:60-65.
- [Google Scholar]
- Antimicrobial activity of resin acid derivatives. Appl. Microbiol. Biotechnol.. 2006;72(3):430-436.
- [Google Scholar]
- Synergistic combinations of small molecule kinase inhibitors: implications for reducing toxicities in nasopharyngeal carcinoma treatment. J. NasoPharyngeal Carcinoma (4):4.
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol., B. 2019;191:65-74.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B: Biol. 2019
- [CrossRef] [Google Scholar]
- Triptolide induces apoptosis and synergizes with cisplatin in cisplatin-resistant HNE1/DDP NPC cells. Folia Biol.. 2015;61(5):195.
- [Google Scholar]
- Medical history, medication use, and risk of nasopharyngeal carcinoma. Am. J. Epidemiol.. 2018;26:1-7.
- [Google Scholar]
- Epithelial-mesenchymal transition is necessary for acquired resistance to cisplatin and increases the metastatic potential of nasopharyngeal carcinoma cells. Int. J. Mol. Med.. 2014;33(1):151-159.
- [Google Scholar]
- Anticancer effect of two diterpenoid compounds isolated from Annonaglabra Linn. Acta Pharmacol. Sin.. 2004;25:937-942.
- [Google Scholar]







