Inhibition of multi-drug resistant microbial pathogens using an eco-friendly root extract of Furcraea foetida mediated silver nanoparticles
⁎Corresponding authors. razia.bt@motherteresawomenuniv.ac.in (M. Razia), chandrubdubio@gmail.com (Murugesan Chandrasekaran), ovideb21@gmail.com (Debnath Ovi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
Advancement in the biological process of nanoparticles synthesis is an important area of nanotechnology. The aim of this research is to utilize the root extract of Furcraea foetida as reduction and stabilization agents to biosynthesize. Silver nanoparticles (AgNPs) from AgNO3 via a simple green method.
Methods
By utilizing UV–VIS spectroscopy, FTIR, XRD, Zeta potential, and HR-TEM techniques, the nanoparticles have been characterized. The anti-Candida properties of AgNPs were tested using the well-diffusion method in agar plates against five Candida species.
Results
With an average diameter of 15 nm, the produced AgNPs are spherical. They were found to be stable and non-aggregated. The synthesized nanoparticles were active against all the tested organisms and showed better efficacy compared to Amphotericin B in most cases. The highest level of inhibition was found against C. albicans at 100 µg concentration.
Conclusion
The results prove that F. foetida is a very good bioreductant and can be used for one-pot synthesis of stable AgNPs with good anti-Candida activity.
Keywords
Biosynthesis
Furcraea foetida
AgNPs
Anti-Candida activity
Bioreductant
- AgNPs
-
Silver nanoparticles
- FFR AgNPs
-
Silver nanoparticles synthesized using Furcraea foetida root extact
Abbreviations
1 Introduction
Candida species especially Candida albicans, is a commonly found infectious yeast in medical devices which provides adhesion sites for other fungal and bacterial pathogens and leads to mixed biofilm formation which presents a major threat to public health (Boucherit-Otmani et al., 2019). Immuno-compromised hospital patients get opportunistic bloodstream infections caused by different Candida spp. which leads to a high level of morbidity and mortality rate (Wiwing and Cucunawangsih, 2020). Silver is known worldwide as an excellent antimicrobial agent for many years. It shows low toxicity in humans whereas has numerous uses in vitro and in vivo (Farooqui et al., 2010). Silver nanoparticles (AgNPs) and silver are the most extensively utilized and recognized uses in the pharmaceutical sector (Becker, 1999; Arokiyaraj et al., 2015). Silver-based topical dressings are commonly utilized for the treatment of infections in burns, chronic ulcers as well as open wounds. The recent advances in research on metallic nanoparticles led to special attention on AgNPs as a possible antimicrobial agent (Baker et al., 2005; Firdhouse and Lalitha, 2013; Al-Dhabi et al., 2018). Silver has an inhibitory effect on a large number of bacterial strains and other microorganisms that are common to industrial and medical procedures (Jiang et al., 2004). The specific surface area of nanoparticles is directly related to their biological efficacy when surface energy is increased (Willems, 2005). Compared to other types of nanomaterials, AgNPs have proved to show great promise in terms of biomedical applications and are the most effective antimicrobial agents due to their high-volume surface area ratio (Bhattacharya and Mukherjee, 2008; Hirst et al., 2009).
Green nanoparticles are advantageous in comparison to other biological processes through the use of plants because it avoids the tedious cell culture maintenance procedure (Shankar et al., 2004; Judy et al., 2021). Biologically synthesized nanoparticles show impressive antibacterial, antifungal properties (Surendra et al., 2016; Arasu et al., 2019a; Al-Dhabi et al., 2019; Valsalam et al., 2019a; Roopan et al., 2019; Gurusamy et al., 2019), antioxidant (Arasu et al., 2019b) and anticancer activities (Magdalane et al., 2018; Valsalam et al., 2019b) To the best of our knowledge, there is no information on the AgNPs synthesis using Furcraea foetida (L.) Haw., which is a monocot plant of the Asparagaceae family. This plant is commonly known as the giant cabuaya, Mauritius hemp, and green-aloe. It is native to Northern South America and the Caribbean. Many cultivars are used as ornamental plants in different parts of the world. It is also cultivated in many countries for fibre extraction. To understand the biological synthesis process and to discover novel eco-friendly methods of prefabricating nanomaterials, it is essential to investigate the formation of nanoparticles utilizing F. foetida root extract. This work centers on one-pot green synthesis of AgNPs utilizing the root extract of the plant F. foetida and analyzing its anti-fungal activity.
2 Materials and methods
2.1 Chemicals
All analytical reactive utilized in the analysis were analytic and were acquired from Sigma-Aldrich, India. PDA (Potato Dextrose Agar) & Amphotericin B have been bought from Hi-Media, India.
2.2 Microorganisms
The assessment of anti-Candida activity was carried out using five different strains. The following organisms have been acquired from MTCC (“Microbial Type Culture Collection and Gene Bank”): Candida albicans (183), C. tropicalis (184), C. glabrata (3019), C. parapsilosis (7043), and C. krusei (9215).
2.3 Collection and processing of plant samples
The plant F. foetida was collected from Kodaikanal, Dindigul district, Tamil Nadu. It was washed three times with tap water and then cleaned using distilled water to remove external debris. The roots of the plant were separated, chopped into small pieces, and dried in a hot air oven at 40 °C. They were pulverized using an electric grinder and stored in airtight containers for further use.
2.4 Extract preparation
Aqueous extract of the roots of F. foetida was made with 1 g of powdered plant sample and 50 ml double-distilled water are mixed in a conical flask. The mixture was heated at 80 °C for 30 min to denature the enzymes in the extract. Filter the solution using Whatman No. 1 for eliminating residues.
2.5 Silver nanoparticles Biosynthesis
In the synthesis of nanoparticles, 15 ml of the aqueous extract has been combined in a 300 ml Erlenmeyer beaker of 1 mM AgNO3 solution and left 24 h to react at room temperature. The nanoparticles were separated by centrifugation at 10,000 rpm for 10 min. The pellets were then redispersed three times in deionized water for removing water-soluble biomolecules including secondary metabolites and proteins (Raut et al., 2010). The pellets have been freeze-dried using a lyophilizer and stored at room temperature until further analysis. The freeze-dried AgNPs were re-suspended in distilled water for all characterization assays as well as procedures.
2.6 AgNPs characterization
Different methods characterized the synthesized AgNPs. UV–VIS spectroscopy is an essential technique and is one of the simplest ways to confirm the formation of nanoparticles compared to other methods. Reaction medium readings were obtained at distinct periods to check the reduction process. UV–Vis spectroscopic studies were carried out using a Shimadzu –UV 260 spectrophotometer in a range between 200 nm and 800 nm. FT-IR analysis was carried out with a scan range between 400 and 4000 cm−1 using an FTIR spectroscope (Shimadzu, IR Affinity 1, Japan). This method was used to identify the possible bio-molecules and metal ions interaction responsible for AgNPs’ formation and stabilization. The distinct vibration modes have been discovered and allocated to identify the different functional groups in the AgNPs.
An XRD method was used to investigate the development of a single-phase compound. The XRD data have been obtained by X-ray diffractometer (XPERT-PRO, PW3050/60) at room temperature, with CuKα radiation (λ = 1.5406 Å) between 20° & 90°.The XY data from this experiment (2θ vs intensity are generated with the Origin Pro program and the angular sites of the peaks are also acquired with this. Using Henry’s equation, Zeta potential is to be determined. A titrator MPT-2a and Zetasizer Nano ZS (Malvern) were used to conduct the analysis. It is an essential characterization of the stability of the synthesized AgNPs.
HR-TEM is one of the most commonly used techniques for the characterization of nanomaterials. It is used to obtain quantitative measures of particle size, morphology, and size distribution. HR-TEM analysis was performed on a JEOL JEM-2100 (HT) electron microscope, with an accelerating voltage of 200 kV. The freeze-dried pellet was redispersed in deionized water and a drop of it was put on Cu grids pre-coated with carbon films.
2.7 Anti-Candida activity
Anti-Candida activity of the synthesized AgNPs has been tested using the well-diffusion method. 20 ml of molten as well as cooled PDA media has been added into sterilized Petri-plates. Wells are loaded with 25, 50, 75, and 100 µg of AgNPs suspended in 100 µl of sterile double distilled water. For 24 h the plates have been incubated at a temperature of 37 °C and then examined for the presence of zones of inhibition. A meter ruler was used to measure the diameter of zones of inhibition. The mean values of each organism were noted and presented in millimeters (mm). The antibiotic Amphotericin B (20 mcg) was employed as a positive control as well as wells loaded with sterile water were used as a negative control. All procedures were done in triplicates.
3 Results and discussion
3.1 Synthesis of Silver nanoparticles
The usage of plant extracts for the synthesis of nanoparticles is suited for therapeutic applications as it is free of toxic contaminants (Mittal et al., 2013). The resultant color change from transparent liquid to reddish-brown colloid indicates that AgNPs synthesis has been effective.
3.2 UV–Vis spectroscopy
This analysis showed sharp absorbance at around 440 nm for AgNPs synthesized from the root extract (Fig. 1). The greatest absorption is seen at 440 nm and the intensity gradually rises as a function of the reaction time. At 60 mins the absorption intensity was 1.5a.u. A well-defined absorption peak of 420–450 nm shows the existence of SPR (“Surface Plasmon Resonance”) of metallic AgNPs (Henglein, 1993). The biosynthesized FFR AgNPs showed a sharp peak within this range. The concentrations of AgNPs could be monitored quantitatively as there was no shift in the peak wavelength of the reaction (Song and Kim, 2009).
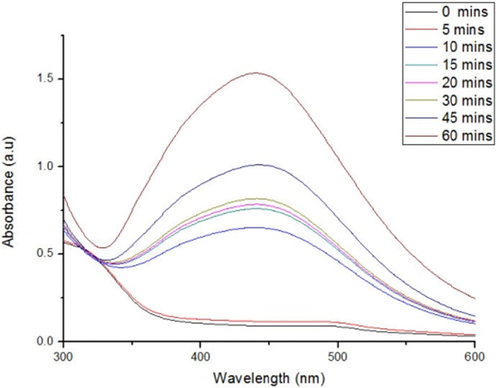
- UV–vis spectra for AgNPs synthesized from root extract of F. foetida.
3.3 Fourier transforms infrared spectroscopy (FT-IR)
Biosynthesized AgNPs were measured by FTIR to determine the interaction between the bio-organics of the nanoparticle sand root extract. The absorption peaks of FFR AgNPs in the FTIR spectrum at 444.57, 618.09, 1109.07, 1415.91, 1556.99, 1634.38, and 2092.30 cm−1 (Fig. 2). This shows the presence of alkynes, aliphatic amines, primary amines, nitro compounds, and aromatic groups of compounds.
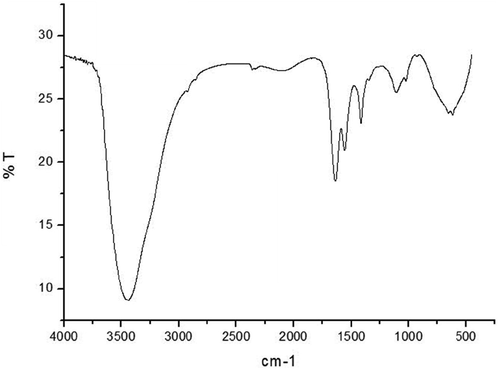
- FTIR spectrum of AgNPs synthesized using F. foetida root extract.
3.4 X-ray powder diffraction investigations
XRD (“X-Ray Diffraction”) patterns of the AgNPs show that the AgNPs structure is FCC (face-centered cubic). Furthermore, at 2 Å the diffraction peaks values of 38, 44, 64, and 78 of FFR AgNPs could be attributed to (1 1 1), (2 0 0), (2 2 0), (3 1 1) planes of centrosymmetric cubic Ag (Fig. 3). According to the JCPDS (Joint committee on powder diffraction standards), the resulted value is acquired with 01–071-3762 file no.
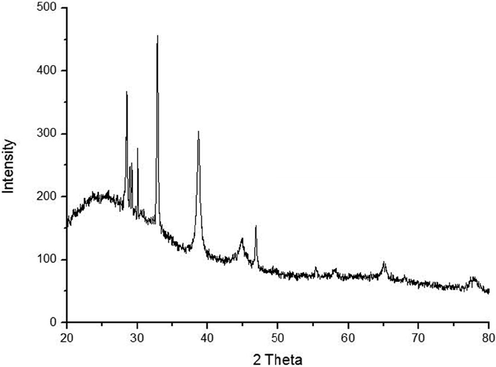
- XRD spectra of AgNPs synthesized from F. foetida root extract.
3.5 Zeta potential analysis
The zeta potential of the biosynthesized AgNPs root extract was found as a sharp peak at −10.6 mV (Fig. 4). It indicates that the nanoparticles’ surface is negatively charged as well as dispersed into the media. The negative value of zeta potential proves that they are very stable and confirms the repulsion among the particles preventing agglomeration. The AgNPs were spherical as seen in TEM micrographs.
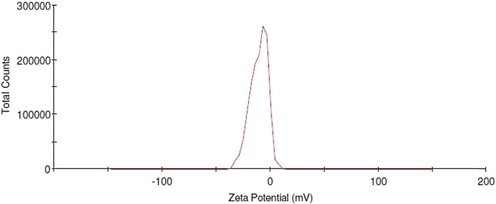
- Zeta potential analysis of AgNPs synthesized from F. foetida root extract.
3.6 HR-TEM analysis
HR-TEM (“High Resolution-Transmission Electron Microscopy”) analysis has been performed to understand the morphology as well as the size of the AgNPs. FFR AgNPs demonstrated the synthesis of poly-dispersed high-density spherical AgNPs, with moderate variation in size from 12 to 19 nm. Most of the nanoparticles were scattered and aggregation was rare as observed from the micrographs (Fig. 5). SAED (Selected Area Electron Diffraction) patterns for the sample have been produced to validate the nature of the nanoparticles. The ring-like diffraction pattern in SAED shows that FFR AgNPs are entirely crystalline and may be indexed based on the silver FCC structure (Fig. 6). This supports the results obtained by XRD analysis.
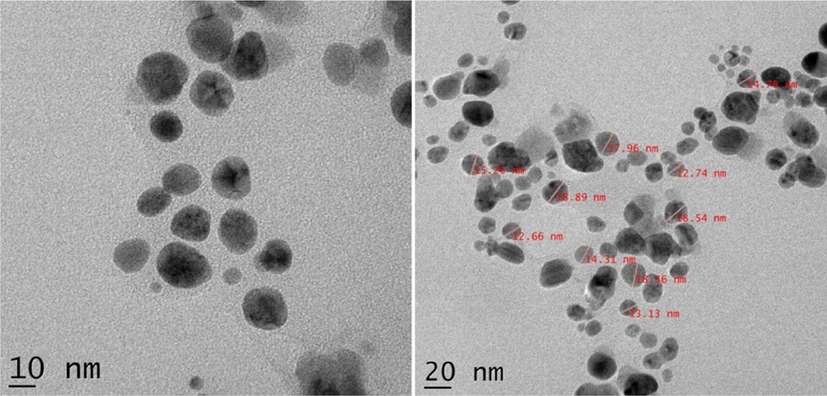
- HR-TEM micrographs of FFR AgNPs.
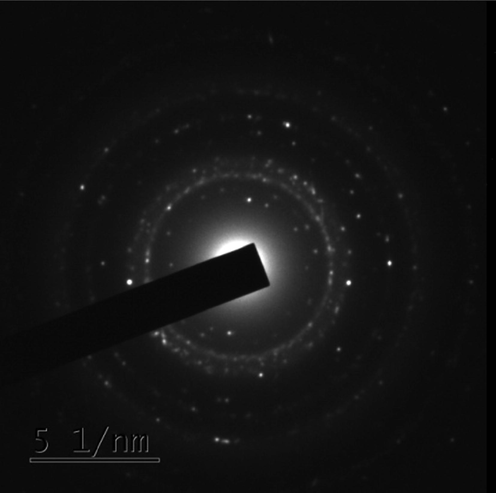
- SAED pattern of FFR AgNPs.
Previously spherical AgNPs have been successfully synthesized using plant extracts of Acalypha indica (20–30 nm) (Krishnaraj et al., 2010), Allium sativum (4–22 nm) (Ahamed et al., 2011), Citrus sinensis peel (35 nm) (Kaviyaet al., 2011), Rhododendron dauricam (25–40 nm) (Mittal et al., 2012), Sesuvium portulacastrum (5–20 nm) (Nabikhan et al., 2010) and Syzygium cumini (29–92 nm) (Kumar et al, 2010; Banerjee and Narendhirakannan, 2011).
3.7 Anti-Candida activity
This study has measured the antifungal impact of FFR AgNPs at 25, 50, 75, and 100 µg concentrations were based on the inhibitory zone. All the tests were done in triplicates. The AgNPs showed potential against five test organisms. The AgNPs effectively inhibited the growth of all five Candida species. At 100 µg concentration, they showed a higher level of inhibition compared to that of Amphotericin B against all organisms except C. glabrata (Fig. 7). The greatest impact of nanoparticles on C. albicans was the 16 ± 2 nm inhibitory zone (Table 1).
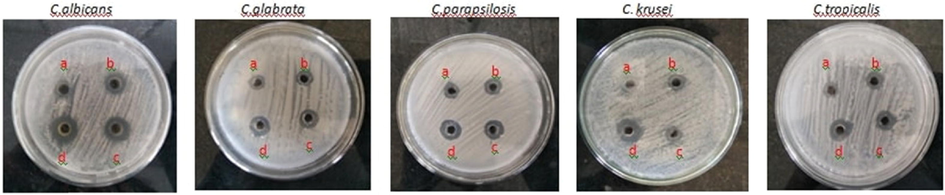
- Anti-Candida activity of FFR AgNPs.
| Organism | Zone of inhibition (mm) | |||||
|---|---|---|---|---|---|---|
| Negative Control | Positive Control | FFR AgNPs | ||||
| 25 µg | 50 µg | 75 µg | 100 µg | |||
| Candida albicans | Nil | 10 ± 2 | 6 ± 2 | 9 ± 3 | 14 ± 3 | 16 ± 2 |
| C. tropicalis | Nil | 8 ± 3 | 5 ± 2 | 8 ± 3 | 10 ± 4 | 12 ± 2 |
| C. glabrata | Nil | 7 ± 2 | 3 ± 1 | 4 ± 2 | 4.5 ± 2 | 5 ± 1 |
| C. parapsilosis | Nil | 10 ± 3 | 4 ± 2 | 7 ± 2 | 9 ± 1 | 11 ± 2 |
| C. krusei | Nil | 9 ± 2 | 6 ± 3 | 10 ± 3 | 12 ± 2 | 13 ± 4 |
*Values are mean of triplicate measurements ± standard deviation.
Only a few studies on the antifungal effect of AgNPs have been published as the topic has received only marginal attention (Zeng et al., 2007; Falletta et al., 2008; Roe et al., 2008). In 2008 a study dealing more specifically with the activity of AgNPs against dermatophytes was published (Kim et al., 2008). AgNPs were again reported to be able to suppress the pathogenic yeasts Candida krusei & Candida albicans in 2018 (Ahmed et al., 2018). The anti-Candida activity of stable AgNPs of size 12.5 nm against C. tropicalis & C. albicans have also been assessed and AgNPs were concluded to be an option for the treatment of fungal infection (Mallmann et al., 2015).
The most commonly studied organism is Candida albicans compared to other Candida species. AgNP anti-Candida activity was measured against pathogenic Candida species using an MFC (minimum fungicidal concentration) as well as MIC (minimum inhibitory concentration) determination and it was found that the MICs of stabilized AgNPs were comparable or better than the MICs of conventional antifungal agents (Panáček et al, 2009). The FFR AgNPs exhibited greater efficacy against most Candida species in comparison to Amphotericin B which is in support of the study.
4 Conclusion
F. foetida is an effective reducing agent to successfully synthesize and stabilize AgNPs. The average size of the synthesized AgNPs was less than 15 nm. The characterization techniques show that the nanoparticles are stable and crystalline in nature. Pathogenic fungi such as Candida continue to develop resistance to many antibiotics thus alternative methods are needed. The synthesized AgNPs show good activity against all the fungal species used in the study. The present study showed a simple, rapid, and eco-friendly route to synthesize stable AgNPs.
Acknowledgement
This work was carried out with the support of ‘Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01529302)’ Rural Development Administration, Republic of Korea. The authors extend their appreciation to the Researchers supporting project number (RSP-2021/193), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Green synthesis, characterization and evaluation of biocompatibility of silver nanoparticles. Physica E Low Dimens. Syst.. 2011;43(6):1266-1271.
- [CrossRef] [Google Scholar]
- Analyzing formation of silver nanoparticles from the filamentous fungus Fusarium oxysporum and their antimicrobial activity. Turk. J. Biol.. 2018;42(1):54-62.
- [CrossRef] [Google Scholar]
- Green biosynthesis of silver nanoparticles produced from marine Streptomyces sp. Al-Dhabi-89 and their potential applications against wound infection and drug resistant clinical pathogens. J. Photochem. Photobiol. B: Biol.. 2018;189:176-184.
- [Google Scholar]
- Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J. Photochem. Photobiol. B: Biol.. 2019;197:111529
- [Google Scholar]
- One step green synthesis of larvicidal, and azo dye degrading antibacterial nanoparticles by response surface methodology. J. Photochem. Photobiol. B: Biol.. 2019;190:154-162.
- [Google Scholar]
- Synthesis and characterization of ZnO nanoflakes anchored carbon nanoplates for antioxidant and anticancer activity in MCF7 cell lines. Mater. Sci. Eng.: C.. 2019;102:536-540.
- [Google Scholar]
- Biosynthesized silver nanoparticles using floral extract of Chrysanthemum indicum L.—potential for malaria vector control. Environ. Sci. Pollut. Res.. 2015;22(13):9759-9765.
- [Google Scholar]
- Synthesis and antibacterial properties of silver nanoparticles. J. Nanosci. Nanotechnol.. 2005;5(2):244-249.
- [CrossRef] [Google Scholar]
- Biosynthesis of silver nanoparticles from Syzygiumcumini (L.) seed extract and evaluation of their in vitro antioxidant activities. Dig. J. Nanomater. Biostruct.. 2011;6(3):961-968.
- [Google Scholar]
- Silver ions in the treatment of local infections. Metal-based Drugs. 1999;6(4-5):311-314.
- [Google Scholar]
- Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev.. 2008;60(11):1289-1306.
- [CrossRef] [Google Scholar]
- Mixed species biofilms of Candida albicans isolated from vascular catheters at the university hospital center of Tlemcen-Algeria. J. Infect. Public Health. 2019;12(1):123.
- [CrossRef] [Google Scholar]
- Clusters of poly (acrylates) and silver nanoparticles: structure and applications for antimicrobial fabrics. J. Phys. Chem. C.. 2008;112(31):11758-11766.
- [CrossRef] [Google Scholar]
- Extraction of silver nanoparticles from the leaf extracts of Clerodendruminerme. Dig. J. Nanomater. Biostruct.. 2010;5(1):43-49.
- [Google Scholar]
- Fabrication of antimicrobial perspiration pads and cotton cloth using Amaranthusdubius mediated silver nanoparticles. J. Chem.. 2013;2013:1-5.
- [CrossRef] [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol. B: Biol.. 2019;193:118-130.
- [Google Scholar]
- Physicochemical properties of small metal particles in solution: “microelectrode” reactions, chemisorption, composite metal particles, and the atom-to-metal transition. J. Phys. Chem.. 1993;97(21):5457-5471.
- [CrossRef] [Google Scholar]
- Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5(24):2848-2856.
- [CrossRef] [Google Scholar]
- Plasma-enhanced deposition of silver nanoparticles onto polymer and metal surfaces for the generation of antimicrobial characteristics. J. Appl. Polym. Sci.. 2004;93(3):1411-1422.
- [CrossRef] [Google Scholar]
- Environmental photochemistry in Solanum trilobatum mediated plasmonic nanoparticles as a probe for the detection of Cd2+ ions in water. Environ. Res.. 2021;202:111918
- [Google Scholar]
- Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc.. 2011;79(3):594-598.
- [CrossRef] [Google Scholar]
- Antifungal effect of silver nanoparticles on dermatophytes. J. Microbiol. Biotechnol.. 2008;18(8):1482-1484.
- [Google Scholar]
- Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloid Surf. B.. 2010;76(1):50-56.
- [CrossRef] [Google Scholar]
- Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol.. 2010;85(10):1301-1309.
- [CrossRef] [Google Scholar]
- Photocatalytic decomposition effect of erbium doped cerium oxide nanostructures driven by visible light irradiation: Investigation of cytotoxicity, antibacterial growth inhibition using catalyst. J. Photochem. Photobiol. B: Biol.. 2018;185:275-282.
- [CrossRef] [Google Scholar]
- Antifungal activity of silver nanoparticles obtained by green synthesis. Rev. Inst. Med. Trop. Sao Paulo. 2015;57(2):165-167.
- [CrossRef] [Google Scholar]
- Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv.. 2013;31(2):346-356.
- [CrossRef] [Google Scholar]
- Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed. Eng.. 2012;4(3)
- [CrossRef] [Google Scholar]
- Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant Sesuvium portulacastrum L. Colloids Surf. B. 2010;79(2):488-493.
- [CrossRef] [Google Scholar]
- Antifungal activity of silver nanoparticles against Candida spp. Biomaterials. 2009;30(31):6333-6340.
- [CrossRef] [Google Scholar]
- Extracellular synthesis of silver nanoparticles using dried leaves of Pongamiapinnata(L) pierre. Nano-Micro Lett.. 2010;2(2):106-113.
- [CrossRef] [Google Scholar]
- Antimicrobial surface functionalization of plastic catheters by silver nanoparticles. J. Antimicrob. Chemother.. 2008;61(4):869-876.
- [CrossRef] [Google Scholar]
- CuO/C nanocomposite: synthesis and optimization using sucrose as carbon source and its antifungal activity. Mater. Sci. Eng.. 2019;101:404-414.
- [Google Scholar]
- Rapid synthesis of Au, Ag, and bimetallic Au core–Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J. Colloid Interface Sci.. 2004;275(2):496-502.
- [CrossRef] [Google Scholar]
- Rapid biological synthesis of silver nanoparticles using plant leaf extracts. Bioprocess Biosyst. Eng.. 2009;32(1):79-84.
- [CrossRef] [Google Scholar]
- Vegetable peel waste for the production of ZnO nanoparticles and its toxicological efficiency, antifungal, hemolytic, and antibacterial activities. Nanoscale Res. Lett.. 2016;11(546)
- [Google Scholar]
- Rapid biosynthesis and characterization of silver nanoparticles from the leaf extract of Tropaeolum majus L. and its enhanced in-vitro antibacterial, antifungal, antioxidant and anticancer properties. J. Photochem. Photobiol. B: Biol.. 2019;191:65-74.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol. B: Biol.. 2019;201:111670
- [Google Scholar]
- Roadmap Report on Nanoparticles. Barcelona, Spain: W&W Espanasl; 2005. p. :157.
- Candidemia in an Indonesian Teaching Hospital: a 9-year observational study. J. Infect. Public Health.. 2020;13(2):345.
- [CrossRef] [Google Scholar]
- Silver nanoparticles directly formed on natural macroporous matrix and their anti-microbial activities. Nanotechnology. 2007;18(5):055605.
- [CrossRef] [Google Scholar]







