Translate this page into:
Influence of prolonged ambient storage condition on the physicochemical properties of uncooked and cooked salted duck egg yolk
⁎Corresponding author. paramee.n@psu.ac.th (Paramee Noonim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Salted duck egg yolk (SDEY) is rich in numerous beneficial nutrients, particularly proteins and fatty acids, and can be used in various edible condiments. However, uncooked SDEY can readily deteriorate when stored under ambient condition. Thus, the present study examined the changes in the shelf life of SDEY in uncooked and cooked forms stored under ambient condition (28 ± 5 °C) for 15 days. The SDEY was tested every 3 days for various physicochemical properties. The color attributes of SDEY, such as lightness (L*), redness (a*), and yellowness (b*), experienced a notable increase when cooked. This enhancement in color parameters was further amplified with ongoing storage. Additionally, the total color change (ΔE) exhibited a similar upward trend, with cooked SDEY displaying significantly higher values compared to the uncooked samples. The moisture content of SDEY was higher in uncooked samples than the cooked SDEY. On the other hand, water activity was not significantly different between the two samples. Additionally, the uncooked SDEY displayed a significantly higher pH level than the cooked SDEY. Notably, the pH values remained unaffected by the extended storage period. The total salt content was similar in both uncooked and cooked SDEY samples, yet both demonstrated a consistent decrease over extended storage periods. Similarly, all samples experienced a continuous decline in textural characteristics during the storage period. Notably, the cooked samples exhibited increased hardness, cohesiveness, springiness, and chewiness, in contrast to the uncooked SDEY, which showed a higher prevalence of adhesiveness. The total plate count and yeast and mold growth of SDEY continuously increased with storage, but cooked SDEY demonstrated better control against microbial growth. Overall, this study found that cooked SDEY is a better and more economical option for storage under ambient condition compared to the uncooked samples.
Keywords
SDEY
Cooking
Ambient temperature
Physicochemical
Qualities
1 Introduction
Approximately 30 % of total egg consumption, either in fresh or preserved form in Southeast Asia, is attributed to duck eggs, whether fresh or preserved. Fresh eggs have a short shelf life, and among the other forms of eggs, salted duck eggs are the most cost-effective option (Ganesan et al., 2014). Salted duck eggs are prepared by submerging them in a supersaturated salt solution or encasing them in a blend of soil paste and salt. This process spans roughly 10 to 30 days, with the salting duration being adjusted based on the desired characteristics of the resulting egg yolk (Venkatachalam et al., 2019). The salting or fermentation process makes the salted duck egg white becomes watery, and the yolk becomes hardened during the extended process, and during the salting process, dehydration causes the yolk to transform into a gel-like state (Cheng et al., 2018). Salted duck egg yolk (SDEY) contains more protein and lipids as compared to other macro and micronutrients (Benjakul and Kaewmanee, 2017). The SDEY is distinguished by attributes such as its solidified state, oil-rich texture, and aromatic qualities. Owing to its profound taste profile and significant nutritional constituents, the SDEY is extensively incorporated into a diverse array of culinary applications. Its inclusion in food items such as moon cakes and pasta is largely driven by its capacity to elevate the culinary characteristics and sensory appeal of these preparations (Ai et al., 2018). The reactions in the egg in the process of salting would influence the changes in the physicochemical and texture properties of the egg yolk. In comparison with the uncooked SDEY, the cooked SDEY has better rheological behavior and characterization.
The cooked SDEY exhibits a superior granular texture, establishing it as an especially desirable form of salted duck egg products compared to others (Harlina et al., 2012). Major factors contributing to the degradation of SDEY include texture loss due to water migration, the Maillard reaction, off-flavor development from lipid oxidation, and microbial growth during storage (Wang et al., 2020). Various methods are employed to mitigate the quality deterioration of uncooked SDEY, including vacuum packaging and an extended salting period to infuse more salt. However, these methods are time-consuming and not necessarily cost-effective (Dong et al., 2022). Boiling, a basic and commonly used cooking method, renders the SDEY palatable while also inhibiting unwanted enzymes and microbial growth (Wang et al., 2023). Furthermore, the boiling process enhances the bioavailability of xanthophyll, lutein, and zeaxanthin in the egg yolk (Nimalaratne et al., 2012). Although many studies have investigated changes in SDEY characteristics during the salting process, research on the changes in SDEY during the extension of its shelf life post-salting is relatively limited. This study aimed to explore the changes in physicochemical properties and microbial stability of both uncooked and cooked SDEY during prolonged ambient storage.
2 Material and methods
2.1 Raw materials and reagents
The study utilized fresh duck eggs (Anas platyrhynchos) [NO. 1 (75–80 g) and NO. 2 (70–75 g)], sourced from a local agricultural establishment in the Chaiya district, Surat Thani province, Thailand. Additionally, elements necessary for the fermentation process, including salt, mud, and ash, were acquired from regional producers in Surat Thani. All employed chemicals and reagents were of analytical grade, secured from Sigma Aldrich, Thailand. In addition, the agar mediums for microbial assessment were procured from HiMedia, based in Mumbai, India.
2.2 Salting procedure, cooking, and storage
The salting process involved a mix of mud, salt, and water in a ratio of 3:1:1 w/w, a ratio that is commonly used by local salted egg producers. This mixture was thoroughly blended to form a salted mud coating with a thin paste-like consistency. Fresh duck eggs were subsequently submerged in this prepared mud coating and given an additional layer of rice ash. A total of 1,000 coated eggs were then transferred into a plastic container and left to undergo salt infusion, also known as fermentation, at room temperature (28 ± 5 °C) for a duration of 12 days. After fermentation, the salted duck eggs were thoroughly cleaned to remove the mud coating. They were drained and allowed to air-dry on a rack for additional analysis. The fermented eggs were then separated into two batches of 500 each. The eggs in the first batch underwent a rigorous washing process, while those in the second batch were not only washed but also boiled in water for 20 min and subsequently cooled down to room temperature. Both batches were then stored at room temperature (28 ± 5 °C) for a period of 15 days. Every three days, thirty salted duck egg samples were taken from each batch for quality analysis. The uncooked salted duck egg samples were cracked to collect the yolks. Similarly, the shells of the cooked salted duck eggs were peeled off to retrieve the yolks. Subsequently, these yolks underwent various quality analyses, as described in section 2.3.
2.3 Quality analysis
2.3.1 Color
Color coordinators, including lightness (L*), redness (a*) and yellowness (b*) of egg yolks, were measured with a colorimeter (T-BOTA, HP-200, Japan) by using the method of AOAC (2000). Total Color of the egg yolks was measured using the following formula:
2.3.2 Moisture content and water activity
Each yolk sample was gently ground into a smooth paste. Subsequently, 2 g of yolk paste was used to determine the total moisture content. Before initiating the analysis, the samples were placed into the sample tray. Moisture content was then measured employing an infrared moisture analyzer (Sartorius, Model MA37, Germany). For water activity, in a similar manner, 2 g of this yolk paste was transferred into the sampling cup. Water activity was then assessed using a water activity meter (Aqualab, Model CX3TE, USA).
2.3.3 pH
Salted duck egg yolk was blended with distilled water up to 20 percent of the weight of salted duck egg yolk. pH was measured with pH meter (Mettler-Toledo, Germany) in accordance with the method of AOAC (2000).
2.3.4 Salt content
The salt content in salted duck egg yolks was analyzed in accordance with the AOAC method (AOAC, 2000). Ten grams of a ground salted duck yolk sample were mixed with 50 ml of distilled water. The volume was then adjusted to 100 ml, from which a 10 ml aliquot was extracted. To this aliquot, 1 ml of potassium chromate was added, and the solution was titrated with 0.1 M silver nitrate up to the endpoint (brick red color). The salt content was calculated using the following formula:
Here, 'a' represents the volume of 0.1 M AgNO3 utilized for titration (in ml), 'b' is the volume of water used for adjustment, 'c' refers to the concentration of AgNO3 (M), 'd' denotes the weight of the sample (in g), and 'e' signifies the volume of the sample used for titration (in ml).
2.3.5 Textural characteristic analysis
The texture profile (hardness, adhesiveness, cohesiveness, springiness, and chewiness) analysis (TPA) was undertaken using a texture analyzer (Brookfield Texture Analyzer, Model CT3, Germany) fitted with a 50 mm plastic cylinder probe. Each test run involved a batch of five samples, which were subjected to a 50 % strain press. The test parameters were defined with a trigger load setting at 5 g, an operational measuring speed of 2 mm/s, and a subsequent post-test speed of 10 mm/s.
2.3.6 Microbiological qualities
2.3.6.1 Total plate count
Salted duck egg samples were aseptically bisected using a sterile knife. The egg yolk from ten salted duck eggs was collected for total plate count analysis as per the guidelines of BAM (2001a). Twenty-five grams of the ground samples were combined with 225 ml of phosphate buffer. Decimal dilutions of the food homogenate were prepared, and 1 ml of each dilution was dispensed onto duplicate Petri dishes. Plate count agar was then added and mixed thoroughly and allowed to solidify. The colony-forming units (CFU) were counted, calculated, and the results were recorded as CFU/g.
2.3.6.2 Yeast and mold
Salted duck egg samples were aseptically cut in half using a sterile knife. The egg yolk from ten salted duck eggs was sampled for total plate count analysis, following the BAM (2001b) methodology. Twenty-five grams of ground samples were combined with 225 ml of 0.1 % peptone water, and the mixture was homogenized in a stomacher for two minutes. Decimal dilutions were carried out to 10^-6. Subsequently, 0.1 ml of each dilution was dispensed onto pre-poured solidified potato dextrose agar plates. The results were reported as CFU/g.
2.4 Statistical analysis
All experiments in this study were conducted in triplicate. The significance of differences was assessed through a one-way analysis of variance (ANOVA). To compare differences between means, Duncan's New Multiple Range Test (DMRT) was utilized. The above statistical approach was performed using the SPSS software (V6) for Windows.
3 Results and discussion
3.1 Physicochemical attributes
3.1.1 Color
Egg color can be measured subjectively or objectively. Regarding yolk color, the subjective (human) assessment implies using the Yolk Color Fan Scale- Roche, which is widely accepted as the standard for assessing yolk color (Milovanovic et al., 2021). The changes in color attributes of the uncooked and cooked salted duck egg yolks that underwent prolonged storage under ambient storage are shown in Fig. 1. In general, significant changes in color characteristics of the egg yolk were observed either by the treatment variations and as well as the study period. The significant changes in egg yolk color characteristics observed in this study can be attributed to various factors, including chemical reactions during storage, treatment variations, storage conditions, microbial activity, and natural egg variation (Li et al., 2022). In general, yolk coloration (yellow red) depends on a various number of variables such as animal health and physiology, feed and dietary factors and product features with the capability to storage carotenoids and xanthophylls (Baker and Gunther, 2004; An et al., 2010). Among the color parameters, the lightness of the yolk retained more values and followed by yellowness and others. The L* values were continuously increased in the samples during the prolonged storage periods. In comparison with the cooked samples, the uncooked yolk had the lowest L* values as compared with the cooked samples. On the other hand, the changes of L* values between intervals were somewhat very high in the cooked samples as compared with the uncooked ones. Kaewmanee et al. (2011) found that cooking has induced protein aggregation and followed by the formation of cluster and/or coagulation occurred in the salted duck egg white and yolk, and thus, consequently increased lightness values as compared with the uncooked samples. Similarly, the redness of the yolk samples also tends to increase along with the increased storage period. At the initial period of storage, the uncooked yolk redness was slightly high; however, as storage was prolonged, the cooked yolk color tended to increase, and at the end, it was again weakened against the uncooked yolks. Lutein and zeaxanthin are the major contributors to the darker salted duck egg yolks. Uncooked egg yolk might induce the free oil from the yolk to mix with carotenoids and exude from the egg yolk, thus increasing the redness. However, the thermal application from cooking reduced the oil and yolk interaction and thus slightly decreased the redness. Furthermore, the curing period and NaCl content in the yolk might impact the textural properties of the yolk and thus adversely influence the scattering of light and color change in the cooked yolks (Lian et al., 2014). On the other hand, yellowness was significantly high in the cooked samples as compared with the uncooked ones. Furthermore, the changes in the yellowness of cooked samples were non-significantly different between the 6th and 15th days of storage. In contrast, slightly non-significant changes were found in the yellowness of the uncooked yolk during the first three intervals, and afterwards, it gradually increased. Wang et al. (2021) reported that changes in uncooked yolk during prolonged storage were influenced by the air in the environment; when the eggs were stored in the absence of air, they turned slightly brown, and when yolks received enough air, they turned back to yellow. Furthermore, the total color of the yolk samples was shown that the increased storage period had significantly influenced their values. In comparison with the uncooked samples, the cooked yolk samples were significantly high in retaining the total color values. The changes in the appearance of the SDEY, both uncooked and cooked samples under prolonged storage in ambient condition, are shown in Fig. 2.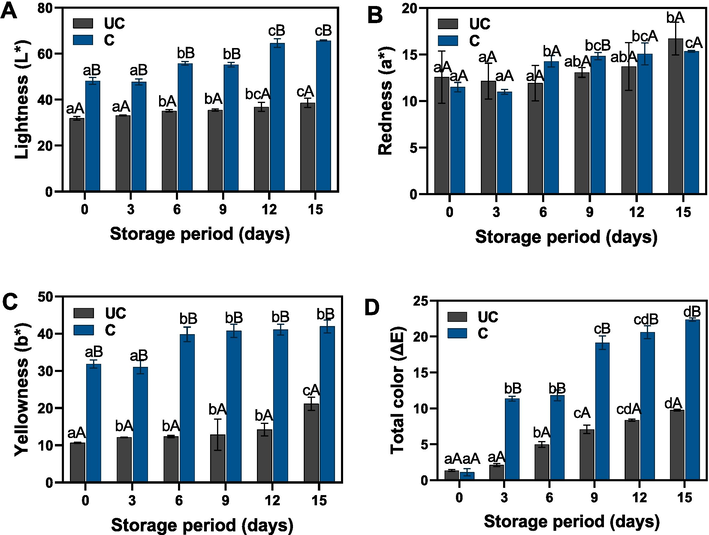
Changes in color characteristics of uncooked and cooked SDEY during prolonged ambient storage. Note: UC represents uncooked yolk and C represents cooked yolk.

Appearance of uncooked and cooked SDEY under prolonged storage in ambient condition. Note: UC represents uncooked yolk and C represents cooked yolk.
3.1.2 Moisture content and water activity
Moisture content and water activity are the major physicochemical factors in identifying the quality of the salted duck egg yolk. Changes in moisture content and water activity of uncooked and cooked salted duck egg yolks under extended ambient storage condition are illustrated in Fig. 3A-B. The results demonstrate that moisture and water activity levels in these samples were maintained within a stable range, exhibiting no significant deviations throughout the storage duration. A marked difference in moisture content was observed between cooked and uncooked samples, indicating that the cooking process may have a notable influence on moisture levels within salted duck egg yolks. However, the data revealed that storage duration did not exert a significant impact on yolk moisture content, regardless of whether the sample was cooked or uncooked. This suggests that moisture content in salted duck egg yolks remains consistent during storage, independent of the storage period. The salting process in duck eggs may facilitate the movement of water-soluble components, such as proteins and carbohydrates, towards the yolk's surface. These hydrophilic components have an affinity for water and can lead to increased moisture retention within the yolk. However, in the tested egg yolks, the salting process was terminated, and the availability of hydrophilic components in the yolk was limited. This could be the reason why no significant differences in moisture content were observed during storage. Quan and Benjakul (2017) found that uncooked egg yolks exposed to an extended salting period retained higher moisture content. This is in accordance with our study, where the uncooked egg yolk retains more moisture content than the cooked ones. The moisture content in the cooked egg yolk is dependent on several factors, including boiling time, temperature and the initial moisture content of the yolk. Kaewmanee et al. (2008) reported that the solidification of salted duck egg yolk plays a crucial role in the moisture content, and the increase of the solidification adversely decreases the moisture content. Paula et al. (2005) investigated the effect of boiling temperature on the moisture content of egg yolk. Their findings suggested that increased temperature did not significantly affect the moisture content, indicating that once the egg yolk was completely solidified (70 °C, 6 min), the temperature had no further impact on the solidification process. Regarding water activity, no significant differences were observed in the samples, regardless of sample type (cooked or uncooked) or storage duration. This finding suggests that water activity in salted duck egg yolks is not significantly influenced by the cooking process or the length of storage, remaining relatively stable throughout the storage period. The consistency of water activity levels may have important implications for maintaining quality and safety in salted duck egg yolks during extended storage. This study demonstrates that the water activity of the yolks remains within a critical range where microbial growth can occur. However, the salting process and cooking method could reduce the impact of microbial growth on the salted duck egg yolks (see Table 1). Note: CFU represent the colony forming unit. EAPC (Estimated Aerobic Plate Count). Thailand Community Product Standard specify microbial standard for raw salted duck egg as Yeast and Mold not more than 500 CFU/g while for cooked salted duck egg as TPC not more than 1 × 106 CFU/g and Yeast and Mold not more than 100 CFU/g (TCBS, 2007).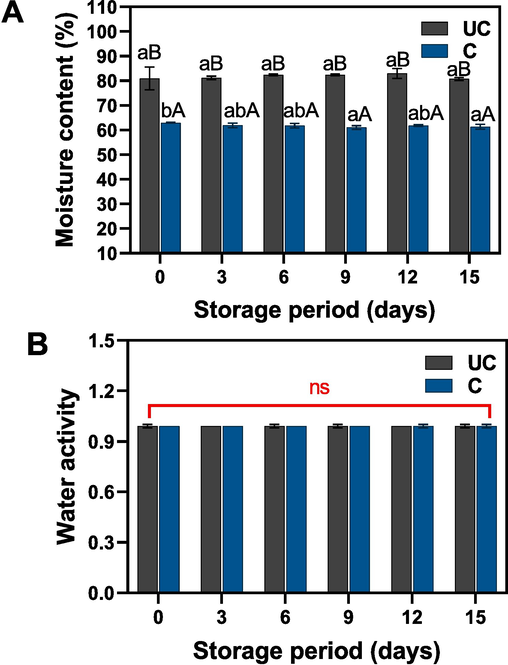
Changes in moisture content and water activity of uncooked and cooked SDEY during prolonged ambient storage. Note: UC represents uncooked yolk and C represents cooked yolk.
Storage period (days)
TPC (CFU/g)
Yeast and Mold (CFU/g)
Uncooked
Cooked
Uncooked
Cooked
0
<250
<250
<100
<100
3
<250
<250
<100
<100
6
1.2 × 103
4.6 × 102
<100
<100
9
1.9 × 103
5.0 × 102
5.5 × 102 EAPC
1.7 × 102 EAPC
12
2.5 × 106
2.7 × 106
2.5 × 106
1.4 × 103
15
2.6 × 106
3.3 × 106
2.6 × 107
1.5 × 105
3.1.3 pH and salt content
Fig. 4A depicts the changes in pH levels of uncooked and cooked salted duck egg yolks under extended ambient storage condition. Overall, significant differences in pH were observed between the sample types; however, there were minimal differences in pH within each group's samples during storage. For up to 9 days of storage, only small differences in pH were observed among samples, irrespective of the storage period. After this point, a slight drop in pH was noted. The overall pH range for the samples fell between 6.4 and 8.2. The uncooked samples maintained higher pH values (between 8.0 and 8.2), with a 2.5 % change from the initial values by the end of storage, compared to the cooked samples (between 6.4 and 7.4), which exhibited a 13.51 % change at the end of storage. Fig. 4B displays the changes in salt content for the different salted duck egg yolks during storage. The results showed significant changes in the salt content of the yolk samples. Compared to uncooked samples, the cooked yolk samples retained more salt content. Furthermore, storage condition significantly impacted the total salt content of the samples. A rapid decrease in salt content was observed as the storage period increased, with the rate of decline becoming more pronounced after six days of storage. Changes in the salt content of the yolk, whether cooked or uncooked, could be influenced by various factors, including temperature and humidity during storage (Cao et al., 2021). A decrease in salt content might be attributable to the total moisture content of the egg yolk (Kaewmanee et al., 2009). This is in accordance with our study, in which the yolk with higher moisture content also exhibited higher salt content. Xu et al. (2018) reported that heat treatment could reduce the size of protein granules and make lipid spheres in the yolk finer, resulting in a structurally weaker yolk. This process would release moisture content from the yolk, subsequently leading to a decrease in salt content. Furthermore, an increase in microbial activity in the yolk could adversely decrease the salt content, which may be mainly due to the metabolization by the microorganisms.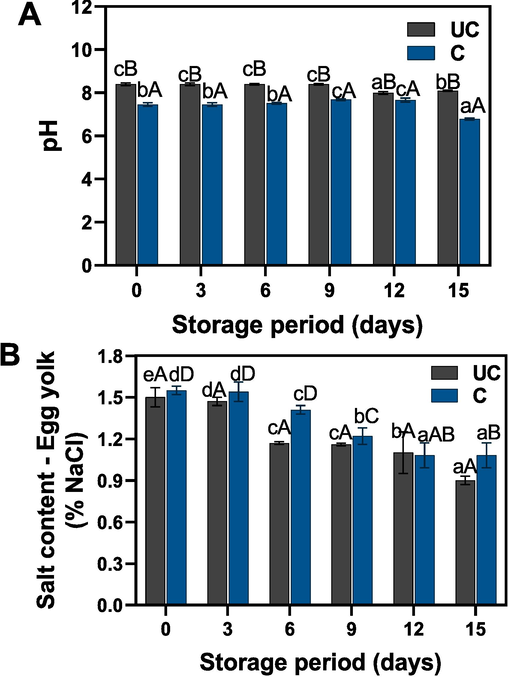
Changes in pH and salt content of uncooked and cooked SDEY during prolonged ambient storage. Note: UC represents uncooked yolk and C represents cooked yolk.
3.2 Textural characteristics
The textural characteristics (hardness, adhesiveness, cohesiveness, springiness, and chewiness) of uncooked and cooked salted duck egg yolks stored for extended periods at room temperature are depicted in Fig. 5A-E. Overall, a significant pattern in these characteristics was observed across the samples, irrespective of the type and storage duration. Hardness measurements revealed a significant improvement in this attribute for cooked yolk samples when compared to their uncooked counterparts. However, the storage period negatively impacted the overall hardness of the samples. An increase in storage duration led to a substantial decrease in hardness values for both sample types when compared to their initial values. The cooked yolk sample had a slight increase in hardness during the initial storage period. Liu et al. (2022) reported that in the initial phase of salting, hardness tends to increase due to the diffusion of salt content into the egg yolk. This doesn't significantly impact protein aggregation but rather causes the egg yolk globules to break apart, leading to the discharge of yolk granules. This process fosters gelation and amplifies the yolk's hardness. Li et al. (2023) suggested that the moisture content of the yolk is significantly interconnected with its hardness, as the moisture level in the yolk decrease or increase could significantly affect the hardness level. The development of a gel network is due to protein denaturation, which intensifies interactions between molecules and transforms the egg yolk into a structure with increased hardness and springiness (Paraskevopoulou et al., 1997). Similarly, the springiness of the yolk samples was influenced by both the type and the storage period. Cooked yolk samples maintained higher springiness values compared to uncooked ones. Between the 3rd and 12th day of storage, there was little change in the springiness of the uncooked yolk samples. Xu et al. (2017) also reported that in comparison with the uncooked yolk, the cooked yolk samples retain more springiness and hardness values. Adhesiveness is primarily a feature associated with surface properties, resulting from the collective influence of both adhesive and cohesive forces. The adhesiveness of the yolk samples is presented in Fig. 5B, with the results showing a significant decrease in adhesiveness with prolonged storage. Although this decline was more pronounced in cooked samples when compared to their initial values, the downward trend was more noticeable in uncooked samples over time. Meanwhile, cooked samples maintained stable adhesiveness values with minimal changes between the 3rd and 12th day of storage, followed by a sharp decrease towards the end of the storage period. A higher level of adhesiveness in uncooked samples could be attributed to the presence of fat content, whereas the cooked samples might have lost their fat content during the cooking process, as cooking conditions can significantly influence the degree of fat loss. Xue et al. (2023) reported that prolonged storage conditions could reduce yolk granules, which are particularly vulnerable due to the salting process. This reduction may cause an outflow of lipids and proteins, leading to a loss of adhesiveness. The cohesiveness values of the yolk samples varied significantly between cooked and uncooked types. Moreover, prolonged storage gradually decreased these values. Regardless of the type of yolk, there was a consistent decline in chewiness in correlation with extended storage. Compared to all other textural characteristics, the chewiness of uncooked samples was markedly lower than that of the cooked ones. Both samples showed a continuous decrease in chewiness, but this reduction was more evident over the storage period in cooked samples compared to the uncooked ones. Cohesiveness and chewiness of egg yolk samples, whether cooked or uncooked, can be impacted by cooking and storage duration. The difference in cohesiveness between cooked and uncooked samples may arise from heat-induced changes in the yolk's protein structure during cooking (Ahlborn et al., 2022). Meanwhile, prolonged storage may reduce these values due to protein and moisture changes over time. Furthermore, chewiness decreases consistently with extended storage in both types, indicating ageing-related texture alterations. The lower chewiness in uncooked samples could stem from the absence of heat-induced protein changes that create a firmer texture in cooked samples. The more pronounced chewiness reduction in cooked samples over time may be due to their increased susceptibility to protein degradation and moisture loss during storage following initial heat treatment (Binsi et al., 2014).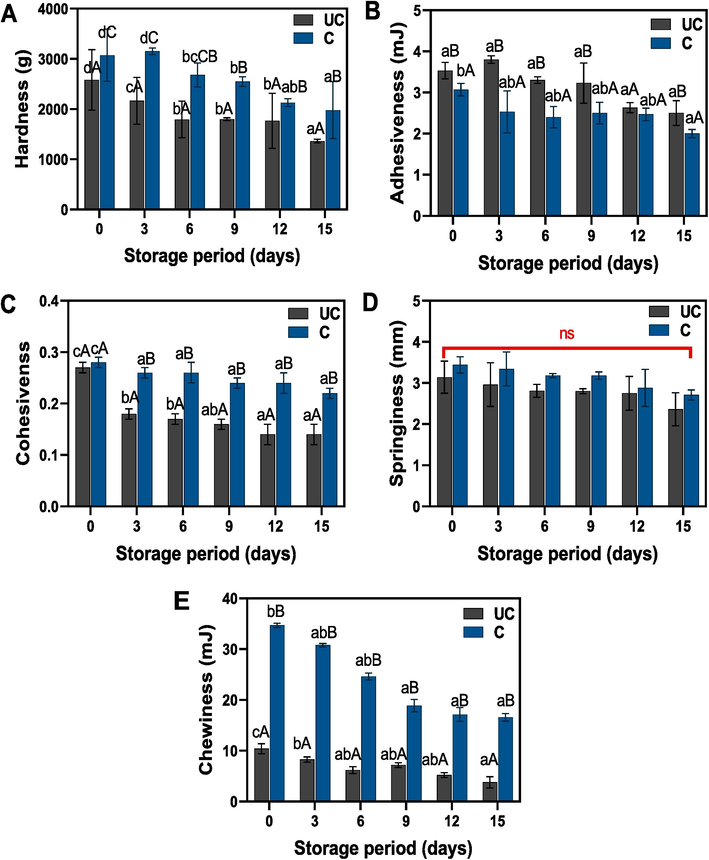
Changes in textural characteristics of uncooked and cooked SDEY during prolonged ambient storage. Note: UC represents uncooked yolk and C represents cooked yolk.
3.3 Microbial analysis
Table 1 presents the microbial growth in both uncooked and cooked salted duck egg yolks, which were stored at room temperature for an extended period. The Total Plate Count (TPC) indicates the overall microbial growth in the yolk samples tested. Notably, the TPC count was significantly higher in these samples. Egg yolks provide an excellent medium for microbial growth due to their nutrient content. Regardless of yolk type, TPC growth consistently increased in the samples. During the first three days of storage, yolk samples displayed undetectable TPC. However, as the storage period extended, microbial colonies began to form, reaching high counts by the end of the storage period. Though TPC levels consistently increased, cooked yolk samples had significantly lower TPC levels compared to uncooked ones. Badr (2006) reported that among pathogenic bacteria, Salmonella is the predominant species that grows in egg yolk. Yang et al. (2021) found that salted duck egg is a potential source for numerous pathogenic bacteria, with a total of 77 bacteria from 14 genera identified; among these, Bacillus and Exiguobacterium mexicanum was the dominant genus and species, respectively. Similar to TPC, yeast and mold growth in the yolk samples also consistently increased with extended storage. During the first six days of storage, Yeast and mold growth was minimal in both cooked and uncooked yolk samples but saw a rapid increase thereafter. Compared to uncooked samples, cooked yolks were more effective in controlling Yeast and mold growth. Overall, the collected microbial growth data indicates that Yeast and mold were the most predominant forms of microbial growth found in the yolk samples. Bailey and Fletcher (1987) reported that hard-boiled egg yolk is safer compared to fresh ones. However, they noted that heat treatment alone is not sufficient to control microbial growth and that the addition of citric acid and sodium benzoate to the boiling water could effectively control microbial growth. Wahba et al. (2014) observed microbial growth in stored egg yolk and found that yeast and mold growth was predominant in salt-treated egg yolk. According to TCPS (2007) of salted duck eggs, both cooked and uncooked SDEY stored under ambient condition had shelf life for 6 days.
4 Conclusion
The current study investigated the impact of ambient storage condition on the physicochemical properties of uncooked and cooked salted duck egg yolks. Significant color changes were observed, primarily influenced by whether the egg yolk was cooked or uncooked and by extended storage condition. Cooked yolk samples showed higher lightness and yellowness, while uncooked samples initially exhibited more redness, which gradually decreased. Despite a reduction in moisture due to cooking, the moisture content and water activity remained relatively stable during storage. This stability is essential to maintaining yolk quality and safety, as these parameters fall within a range supportive of microbial growth. Extended ambient storage noticeably altered the pH and salt content. Salt content declined with increased storage duration, while pH changes within each group were minimal, with a slight decrease observed after nine days. Textural properties also changed significantly during storage, with cooked samples showing higher initial hardness and springiness. Microbial growth, especially Total Plate Count (TPC), Yeast, and mold, steadily increased over time. Overall, the study found that cooking is the best preservation method for salted duck egg yolk. It significantly reduces microbial growth and enhances overall quality. However, despite the benefits of cooking, the extension of storage life at ambient temperature is limited and overall shelf lift of uncooked and cooked salted duck egg yolks were six days, suggesting the need for refrigerated storage for extended periods.
Acknowledgement
The authors are very grateful to Prince of Songkla University, Hatyai campus for the financial support through the research grant (Grant number SIT600127S). Furthermore, space and equipment support for the Food Innovation and Product Development Laboratory and this research were financially supported by Prince of Songkla University, Surat Thani campus (2016).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A comparison of egg white and egg yolk in gluten free bread. Food Chem. Adv.. 2022;1:100142
- [CrossRef] [Google Scholar]
- The investigation of the changes in physicochemical, texture and rheological characteristics of salted duck egg yolk during salting. LWT – Food Sci. Technol.. 2018;88:119-125.
- [CrossRef] [Google Scholar]
- Effect of different oil sources and vitamin E in breeder diet on egg quality, hatchability and development of the Neonatal offspring. Asian-Australas. J. Anim. Sci.. 2010;23(2):234-239.
- [Google Scholar]
- Official Methods of Analysis of the Association of Official Analytical Chemists (seventeenth ed.). Gaithersburg, MD: AOAC International; 2000.
- Effect of gamma radiation and cold storage on chemical and organoleptic properties and microbiological status of liquid egg white and yolk. Food Chem.. 2006;97:285-293.
- [Google Scholar]
- The influence of added egg yolk on the microbiological quality of hard-cooked eggs stored in a citric acid/sodium benzoate solution. Poult. Sci.. 1987;66:861-865.
- [Google Scholar]
- The role of carotenoids in consumer choice and the likely benefits from their inclusion into products for human consumption. Trends Food Sci. Technol.. 2004;15(10):484-488.
- [CrossRef] [Google Scholar]
- BAM, 2001a. Bacteriological Analytical Manual Chapter 3: Aerobic Plate Count. http://www.cfsan.fda.gov/∼ebam/bam-3.html (assessed 19 June 2022).
- BAM, 2001b. Bacteriological Analytical Manual Chapter 18: Yeasts, Molds and Mycotoxins. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-18-yeasts-molds-and-mycotoxins (assessed 19 June 2022).
- Sodium chloride preservation in duck eggs. In: Hester P., ed. Egg Innovation and Strategies for Improvement. Oxford: Academic press; 2017. p. :415-426.
- [Google Scholar]
- Compositional and chill storage characteristics of microwave-blanched sutchi catfish (Pangasianodon hypophthalmus) fillet. Int. J. Food Sci. Technol.. 2014;49:364-372.
- [Google Scholar]
- Changes in lipid properties of duck egg yolks under extreme processing conditions. Poult. Sci.. 2021;100:101140
- [CrossRef] [Google Scholar]
- S. Cheng T. Zhang X. Wang Y. Song H. Wang H. Wang P. Yang M. Tan Influence of salting processes on water and lipid dynamics, physicochemical and microstructure of duck egg LWT-Food Sci. Technol. 95 2018 413 149 10.1016/j.lwt.2018.04.074.
- Water and lipid migration in salted duck eggs during storage with different packaging conditions as studied using LF-NMR and MRI techniques. J. Food Sci.. 2022;87:2009-2017.
- [CrossRef] [Google Scholar]
- Comparative study on the nutritional value of pidan and salted duck egg. Korean Journal of Food Science and Animal Resources. 2014;34(1):1-6.
- [CrossRef] [Google Scholar]
- The effect of supplementation garlic oil as an antibacterial activity and salting time on the characteristics of salted egg. J. Appl. Food Technol.. 2012;1(4):121-128.
- [Google Scholar]
- Effect of salting processes on chemical composition, textural properties and microstructure of duck egg. J. Sci. Food Agric.. 2008;89:625-633.
- [CrossRef] [Google Scholar]
- Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chem.. 2009;112:560-569.
- [Google Scholar]
- Effects of salting processes and time on the chemical composition, textural properties, and microstructure of cooked duck egg. J. Food Sci.. 2011;76(2):S139-S147.
- [CrossRef] [Google Scholar]
- The Quality Characteristics Formation and Control of Salted Eggs: A Review. Foods. 2022;11:2949.
- [CrossRef] [Google Scholar]
- Effect of salt penetration and water migration on cooked salted egg yolk gel during storage: Physicochemical properties, structural characteristics and flavor changes. Food Chem.. 2023;404:134510
- [CrossRef] [Google Scholar]
- Use of sodium dodecyl sulfate pretreatment and 2-stage curing for improved quality of slated duck eggs. J. Food Sci.. 2014;79(3):E354-E361.
- [Google Scholar]
- Change in rapid salting kinetics and characteristics of hen egg yolks. J. Food Eng.. 2022;329:111090
- [CrossRef] [Google Scholar]
- J. Food Meas. Charact.. 2021;15:5097-5112.
- [CrossRef]
- Effect of domestic cooking methods on egg yolk xanthophylls. J. Agric. Food Chem.. 2012;60:12547-12552.
- [CrossRef] [Google Scholar]
- Texture profile analysis of heat-formed gels and cakes prepared with low cholesterol egg yolk concentrates. J. Food Sci.. 1997;62(1):208-211.
- [Google Scholar]
- Thermal inactivation of Salmonella Enteritidis by boiling and frying egg methods. J. Food Saf.. 2005;25:43-57.
- [Google Scholar]
- Quality, protease inhibitor and gelling property of duck egg albumen as affected by storage conditions. J. Food Sci. Technol.. 2017;55(2):513-522.
- [Google Scholar]
- TCPS (Thailand Community Product Standard) No.27/2007: Salted duck egg (in Thai) 2007.
- Influences of different coating materials on the quality changes of hard-boiled salted duck eggs under ambient storage. Braz. Arch. Biol. Technol.. 2019;62:e19180471.
- [Google Scholar]
- The effect of different preservation methods on EGG quality and validity. Assiut Vet. Med. J.. 2014;60(143):42-48.
- [Google Scholar]
- Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted duck egg yolks. Foods. 2020;9:449.
- [CrossRef] [Google Scholar]
- Quantitative metabolome analysis of boiled chicken egg yolk. Curr. Res. Food Sci.. 2023;6:100409
- [CrossRef] [Google Scholar]
- Effects of packaging methods on the quality of heavy metals-free preserved duck eggs during storage. Poult. Sci.. 2021;100:101051
- [CrossRef] [Google Scholar]
- Effects of salting treatment on the physicochemical properties, textural properties and microstructures of duck eggs. PLoS One. 2017;12(8):e0182912.
- [Google Scholar]
- Changes in aggregation behavior of raw and cooked salted egg yolks during pickling. Food Hydrocoll.. 2018;80:68-77.
- [CrossRef] [Google Scholar]
- Formation mechanism of salted egg yolk mudding during storage: protein oxidation, gel structure, and conformation. Food Chem.. 2023;413:135632
- [CrossRef] [Google Scholar]
- Salted duck eggs: the source for pathogens and antibiotic resistant bacteria. J. Food Sci. Technol.. 2021;58(12):4722-4729.
- [CrossRef] [Google Scholar]







