Translate this page into:
Influence of light wavelengths, light intensity, temperature, and pH on biosynthesis of extracellular and intracellular pigment and biomass of Pseudomonasaeruginosa NR1
⁎Corresponding author. prashantdixit74@gmail.com (Prashant P. Dixit)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The purpose of the present study was to check whether the light is an inducing agent or not for the intracellular and extracellular pigment production by Pseudomonas aeruginosa NR1? Is the produced pigment a photoprotective in nature? The aim of the present research study was to evaluate the influence of different light wavelengths, light intensity, temperature, and pH on biomass and intracellular and extracellular pigment production. A strain of Pseudomonas that was thermoalkalophilic and produces red non-diffusible intracellular and green-diffusible extracellular pigment was isolated, until now no one has reported this type of strain. For determining the effect of different light wavelengths, we introduced and designed a novel ‘selective light wavelength method’, which gave us better results than previously used glass paper wrapping method. Extracellular pigment production was found maximum under darkness, red and blue light wavelengths. Same results were obtained in glass paper wrapping method, but the concentration of pigment produced by bacteria was greater in our method which suggests us that the ‘selective light wavelength method’ is better (for pigment yield) between these methods.
The outcome of this study refutes the photo protective role of extracellular pigment, claimed by many researchers. Red and light wavelength was found to suppress the intracellular pigment production. Extracellular pigment production by Pseudomonas aeruginosa NR1 was maximum in darkness with 4.042 ± 0.03 OD/mL and minimum in green light (Glass paper plates (GPP), 2.151 ± 0.24 OD/mL; Selective lamp plates (SLP), 2.232 ± 0.28 OD/mL). Maximum intracellular pigment (SLP, 4.672 ± 0.15 OD/mL; GPP, 4.395 ± 0.03 OD/mL) and biomass concentrations (Selective lamp method-830 ± 5.7 µg/0.01 L; Glass paper method, 810 ± 2.88 µg/0.01 L) were achieved under yellow light wavelength. Minimum intracellular pigment yield (GPP, 2.124 ± 0.01 OD/mL; SLP, 2.252 ± 0.0 OD/mL and biomass yield (Glass paper method, 363.3 ± 3.33 µg/0.01 L; selective lamp method-385 ± 2.88 µg/0.01 L) was recorded under red light wavelength. Direct relationship was observed between biomass production and intracellular pigment production. Maximum extracellular (3.548 ± 2.2 OD/mL) and intracellular pigment (3.856 ± 0.02 OD/mL) and biomass concentration (800 ± 1.44 µg/100 mL) were observed when pH was 7. The intracellular pigment concentration (3.698 ± 0.01 OD/ml) and biomass production (656 ± 3.05 µg/ml) were maximal at a temperature of 30 °C. The maximum concentration of extracellular pigment was recorded at 35 °C (3.924 ± 0.04 OD/ml).
Keywords
Pseudomonas aeruginosa NR1
Light wavelength
Light intensity
Biomass
Intracellular
Extracellular pigment concentration
1 Introduction
Environmental factors such as temperature, light etc., are important for regulating the developmental and physiological processes for humans, animals and microbes. We are well familiar with the fact that humans and animals sense and behave accordingly to temperature, light and other many environmental factors. Microbes also can sense, respond and behave accordingly in different environmental conditions and therefore the ability to sense and respond to light in microbes is considered evolutionary conserved mechanism (Arden et al., 1966). For microbial growth and secondary metabolite production, environmental factors are very important. There is a high influence of medium composition and the operating conditions e.g., temperature, pH, availability and type of light wavelength, degree of aeration etc., on microbial cell growth, development, reproduction and metabolite production. This influence has always been a big hindrance in optimizing the biotechnological process (Chen et al., 2015). Optimization of bacterial and fungal pigment production has become an important aspect now days because many microbial pigments have been reported to have medicinal properties such as anticancer (Ferreira et al., 2004; Kodach et al., 2006), anti protozoan (Matz et al., 2004), antiviral (Sánchez et al., 2006), antibacterial (Nakamura et al., 2003), antioxidant (Konzen et al., 2006), anti-hepatotoxic (Gallardo-Casas et al., 2010), antidiabetic (Soni et al., 2009), or anti-inflammatory (Gitte et al., 2015) activity.
There is a very strong influence of light and temperature on bacterial and fungal pigment production. In this context, Serratia marcescens can produce prodigiosin only at low temperature (28 °C), but not at 37 °C (Xu et al., 2014; Nora et al., 2017). Light-dependent accumulation of carotenoid pigments has been studied in Neurospora (Rau and Mitzka-Schnabel, 1985). In fungi, many types of photo receptors have been identified (Corrochano, 2007; Herrera-Estrella and Horwitz, 2007). Light has been an inducing agent for pigment (carotenoid) production for several species of non photosynthetic representatives of the genera Mycobacterium, Flavobacterium dehydrogenans, Myxococcus, Micrococcus roseus (Yasumasa et al., 1975). All these reports suggest us that there is a huge influence of these factors on pigment production, and they also regulate the pigment production mechanism. As bacteria also sense and respond to light, there is a possibility that bacteria could also possess photo receptors like fungi, which should be detected. Considering all these facts and possibilities, in present research study, the effect of presence or absence of light (total darkness), type of light wavelength, light intensity, temperature and pH on Pseudomonas aeruginosa NR1growth, and on intracellular and extracellular pigment and biomass production was investigated.
2 Materials and methods
2.1 Materials
All the glassware and Petri dishes used in experimentation were purchased from Scott Duran manufacturer (DURAN®, Germany). The glassware consists of good quality glass with clear, colourless properties, having negligibly low absorption spectra in the range about 310 to 2200 nm. Petri dishes used in experiments could transmit light of all wavelengths by more than 90%. Philips 20Watts lamps (Philips India Ltd.) were used as a light source for experimentation. All other chemicals used in experimentation were of analytical grade, and were procured from Merck, India. Light intensity was controlled in both experimental setups by using the same 20 Watt lamps, and by keeping all plates and flasks equidistant (15 cm) from the light source.
2.2 Isolation of pigment producing bacterium
The pigment producing bacterium was isolated from dead Rhinoceros beetle. The insect was collected aseptically and was brought into the laboratory. The feathers of the insect were cut and were washed with sterile distilled water. This water was serially diluted up to a factor of 10-6 with sterile distilled water. Diluted samples were used for the isolation of pigmented bacteria. A loopful of diluted sample from 10-6 tube was withdrawn with Nichrome wire loop, and was streaked on sterile nutrient agar (Hi-Media, Mumbai) plates. Plates were incubated at 37 °C in incubator for 48 h. A colony with intense and bright pigment producing ability was selected for the further research studies. The selected bacterium was able to produce dark red intracellular (Fig. 3) and green extracellular pigments (Fig. 4).
. 16S rRNA of Pseudomonas aeruginosa NR1.
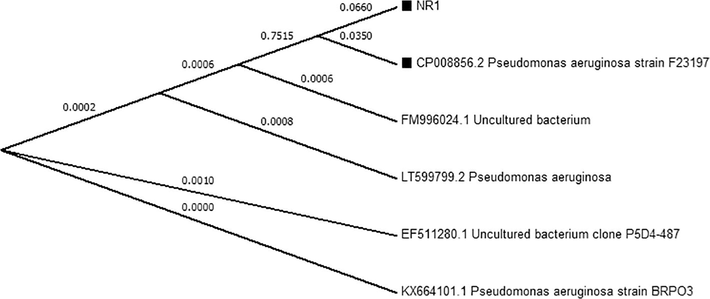
. Phylogenetic tree of P. aeruginosa NR1.
. Intracellular pigment produced by P. aeruginosa NR1.

. Extracellular pigment produced by P. aeruginosa NR1.
2.3 Identification of isolated pigmented bacterium
The isolated bacterium was identified by morphological, biochemical characteristics, and by the 16S rRNA gene sequencing method. The 16S rRNA gene was amplified by using following 16S universal primers.
-
16S Forward primer: 5′ AGA GTT TGA TCC TGG CTC AG 3′
-
16S Reverse primer: 5′ AAG GAG GTG ATC CAG CCG CA 3′
2.4 Effect of different light wavelengths on pigment and biomass production
Effect of different light wavelengths on pigment and biomass production was evaluated by using two methods. One was glass paper wrapping method, which has been used by researchers (Soumya et al., 2014; Babitha et al., 2008) to deduce effect of light wavelengths on microbial pigment and biomass production; the second was by using the selective lamp light wavelength method, which is a novel technique and for the first time introduced by our research group. First method was based on the principle that the color glass paper allows only selective wavelength of light to pass through it. If red colored glass paper is used, then only red color light wavelength will pass through the paper. In the second method, only selective colored light wavelength was provided to flasks and plates, which were kept in controlled confined environment. Colored lamps (Philips, 20 W) were used for providing selective wavelengths.
2.5 Experimental set up for method one (glass wrapping method)
For this method, five different color glass papers were used. The papers used in this research study were green, red, yellow, brown, blue in color (Fig. 5). Each glass paper was wrapped around the one flask and a plate and these wrapped plates and flasks were kept around the illumination source, 15 cm away from the light source (Fig. 6). One flask and one plate were directly kept under illumination without wrapping to check effect of direct light on pigment and biomass production. To detect effect of darkness on pigment and biomass production, one flask and one plate were kept in total darkness. For intracellular pigment production, the organism was grown on sterile nutrient agar plates; for extracellular pigment production, as it was diffusible only in liquid medium, the organism was grown in sterile nutrient broth flasks. Flasks and plates were inoculated with the organism with equal volumes (2 mL) of 24 h old bacterial culture and were incubated in a box that had a single illumination source provided centrally.
. Glass papers wrapped to plates and flasks.

Wrapped flasks and plated under light source.
2.6 Experimental set up for method two (selective wavelength method)
We designed ‘a selective light wavelength’ experiment for evaluating the effect of light on pigment and biomass production. For this experiment, a wooden box was constructed. This box was equally subdivided in nine chambers by partitioning the box with wooden blocks (Fig. 7). The partition was done in such a manner that no light rays from one chamber could pass to another chamber. An electric holder plate was designed with 10 lamp holders (Fig. 8). This plate was designed in such a way that in each chamber a holder socket was present in a central position. At every holder socket, one colored selective wavelength lamp was fixed. The box was closed, so that no external light was able to enter the box. Red, orange, blue, green, yellow, white, pink lamps and one more lamp with multiple light wavelengths that exerts red, blue, green, yellow, white light wavelength one by one repeatedly were used for the study. In one chamber no lamp was fixed, so that total darkness is achieved for incubation in absence of light.
. Equal chambers made in box.

. Electric plate constructed for the experiment.
2.7 Effect of temperature on pigment and biomass production.
To evaluate effect of temperature on pigment and biomass production, sterile nutrient broth flasks and nutrient agar plates were prepared. For each temperature, one flask and one plate were investigated. All plates and flasks were inoculated with equal amounts of bacterial culture and kept at different temperatures such as 25 °C, 35 °C, 45 °C, 55 °C, 65 °C. After 2 days of incubation, the amount of pigment and biomass was estimated.
2.8 Effect of pH on pigment and biomass production.
To check the effect of pH, one nutrient broth flask and one nutrient agar plate with pH 3, 4, 5, 6, 7, 8, 9, 10 were prepared and inoculated with equal amounts of bacterial culture and were kept for incubation for 2 days at 30 °C. Amount of pigment and biomass was estimated by using spectrophotometric analysis (Elico UV–Visible double beam spectrophotometer).
2.9 Effect of light intensity on pigment production
For detecting the effect of increased light intensity on extracellular and intracellular pigment production, and to get information regarding role of extracellular pigment in photoprotection, this study was undertaken. To study the light intensity effect on pigment production, the plates and flasks producing intracellular and extracellular pigment, respectively, were kept and incubated under increasing light intensity environment by using 15 W, 20 W, 25 W, 30 W, 40 W, 50 W white light lamps.
2.10 Inoculation
2 mL of inoculum medium was used for all flasks. Twenty-four hours old broth of P. aeruginosa NR1 was used for inoculation. Plates and flasks were inoculated with desired bacterial culture and all were kept for incubation at their places for 72 h at 35 °C. The room temperature was maintained at 35 °C with the help of room heater.
2.11 Intracellular and extracellular pigment estimation
Extracellular pigment concentration was estimated by measuring absorption of pigmented solution at its λmax, i.e., 382 nm. Broth was centrifuged with relative centrifugal force of 3600 × g for 20 min by using REMI research centrifuge (REMI, India). The supernatant was filtered and cleared, colored filtrate was obtained. Equal amount of Chloroform was used for the complete extraction of extracellular pigment from the aqueous broth. The colored chloroform was used for spectrophotometric analysis (Elico UV–Visible double beam spectrophotometer). For estimating intracellular pigment concentration, the intracellular pigment was extracted by using acetone. 10 mL of acetone was added in a Petri plate with well grown pigmented colonies. The pigment slowly started to dissolve in solvent. When colonies turned colorless, the acetone was removed and collected in a test tube. 5 mL of acetone was again added to extract the remaining traces of pigment completely from colonies. The red colored acetone was filtered with Whatmann filter paper GF/C disc (47 mm). The absorption maxima were determined for the red pigment, and the concentration of pigment in each plate was estimated by measuring the absorption in spectrophotometer (Elico UV–Visible double beam spectrophotometer). Acetone was used as blank in the analysis.
2.12 Biomass estimation
The bacterial biomass was estimated as dry weight by the conventional method described by Li and Mira (2010) with some minor modification. Broth was centrifuged with relative centrifugal force of 3600 × g for 20 min by using REMI research centrifuge (REMI, Centrifuge, India) for 20 min. The pellet was washed with distilled water and was mixed in 5 mL distilled water. The empty weight (in gram) of the evaporating dish was measured and recorded (initial weight, IW). The bacterial mass was kept in evaporating dish. The dish was kept in oven at 100 °C until all water was evaporated, and only bacterial mass remained in dish. After complete drying, the final weight (in gram) of the dish with the dried bacterial mass was recorded (Final weight, FW). The actual weight of bacterial cells was determined by subtracting the initial weight from the final weight. where,
-
FW – final dish weight (with dried bacterial mass)
-
IW – initial dish (empty dish weight)
2.13 Statistical analysis
Each experiment was repeated at least three times. Numerical data are presented as mean ± SD. All the results were analyzed by means of one-way ANOVA. All statistical analyses were performed by using SPSS 11.5 software. P < 0.05, P < 0.01 was considered statistically significant.
3 Result and discussion
3.1 Isolation and identification of pigmented bacterium-
The red colored bacterium was identified as P. aeruginosa NR1 by 16srRNA gene sequencing and considering it’s morphological and biochemical characteristics. The bacterium was able to catabolize many sugars including glucose, galactose, sucrose, maltose, fructose, mannose, dextrose, xylose, but was found unable to catabolize rhamnose, lactose, arabinose, and mannitol (Table 1). Moreover, the bacterium was oxidase and catalase positive. The 16S rRNA gene sequence and phylogenic tree is shown in Figs. 1 and 2 respectively.
Glucose Fermentation
Sucrose fermentation
Fructose fermentation
Lactose fermentation
Arabinose Fermentation
Galactose fermentation
Dextrose fermentation
Rhamnose fermentation
Mannitol Fermentation
Mannose fermentation
Positive
Positive
Positive
Negative
Negative
Positive
Positive
Negative
Negative
Positive
Xylose fermentation
Maltose fermentation
Indole test
Methyl red test
VP Test
Citrate Test
Amylase enzyme
Chitinase enzyme
Gelatinase Enzyme
Lipase enzyme
Positive
Positive
Negative
Negative
Positive
Positive
Positive
Positive
Negative
Negative
3.2 Effect of light wavelengths, light intensity, temperature and pH on pigment and biomass production.
Propst and Lubin (1979) have also reported the light mediated changes in P. aeruginosa cultures. In their research study, they found that cultures of Pseudomonas aeruginosa PA0 grown under uninterrupted broad-spectrum light showed pigmentation different from dark-grown cultures; dark-grown bacteria produced pigments, which resulted in blue-purple colored agar, while light-grown organisms produced red colored plates. We didn’t observe complete change in pigment color, but a slight change in color shade was observed when our bacterium was kept under light and total darkness. In our experiment, higher concentration of pigment was obtained for the flasks and plates of ‘selective wavelengths lamp light method’ than for the ‘glass paper wrapped’ flasks and plates (Table 2). This higher concentration of pigment in selective wavelengths method shows its superiority and efficacy over the glass paper wrapping method. For intracellular pigment production, yellow light wavelength was found most suitable in our experiment. Under yellow light wavelength, maximum intracellular pigment production was recorded (SLP- 4.672 ± 0.1 OD/ml; GPP- 4.395 ± 0.03 OD/ml), followed by green (SLP – 3.816 ± 0.02 OD/ml; GPP –3.611 ± 0.07 OD/ml), orange (SLP – 3.500 ± 0.03 OD/ml), and pink (SLP – 2.913 ± 0.04 OD/ml). Minimum intracellular pigment production was observed under red (GPP – 2.124 ± 0.01 OD/ml; SLP- 2.252 ± 0.02 OD/ml), blue (GPP- 2.308 ± 0.01 OD/ml; SLP – 2.412 ± 0.02 OD/ml) and white light wavelengths (SLP – 2.890 ± 0.03 OD/ml). Our light experiments revealed that under blue and white light, intracellular pigment production decreases, which is in accordance with the findings by Nobutaka et al. (2004) and Chen et al. (2016). As a difference between our result and those by Nobutaka et al. (2004) results, red light negatively influenced the pigment formation in our experiments, while in Nobutaka et al. (2004) red light didn’t affect the pigment and biomass concentration. Our result that under blue light the intracellular pigment production decreases, was similar with the result by De Lucca et al., (2012), as they reported that the blue light effectively inhibits bacterial and fungal growth, whereas Miyake et al. (2005) in a research study reported opposite observation that under blue light pigment production was maximum by Monascus ruber N. Darkness was not that much effective for the organism for intracellular pigment production. On the other hand, extracellular pigment was produced maximum under darkness (4.042 ± 0.13 OD/mL). This result is in accordance with Babitha et al., (2008), Soumya et al., (2014), and Velmurugan et al., 2010, who also reported that darkness was beneficial for the pigment production by their organism. Our this result is significant as this observation refutes the photo protective role of our pigment as proposed by Dieringer et al. (1977), Yong and Lee (1991), Salih et al., (2000), and Seagle et al., (2005). Using red light wavelength (Selective lamp flask 3.714 ± 0.2 OD/mL; Glass paper flask, 3.317 ± 0.07 OD/mL) and blue wavelength (Selective lamp flask 3.638 ± 0.19; Glass paper flask, 3.590 ± 0.02), extracellular pigment production was also pronounced. The lowest extracellular pigment concentration was obtained under green light wavelength (GPP-2.151 OD/mL; SLP- 2.232 OD/mL). The biomass was produced maximum under yellow (Selective lamp flask 830 ± 5.7 µg/0.01 L; Glass paper flask, 810 ± 2.88 µg/0.01 L) and green light (Selective lamp flask 731 ± 4.4 µg/0.01 L; Glass paper flask, 708 ± 4.4 µg/0.01 L) wavelengths. This means that the biomass and intracellular pigment was produced at the maximum concentration under yellow and green light. This result was expected since the concentration of intracellular pigment should be directly proportional to the concentration of biomass; however, in a research published by Alejandro et al. (2011), the opposite observation was reported namely no direct relationship between biomass concentration and pigment production. Pigment production by the organism was not completely inhibited under any particular light wavelength.
Concentration
Type of light
Red Paper
(620–750)Blue Paper
(450–495)Green Paper
(495–570)Yellow Paper
(570–590)Brown paper
Red Lamp
Bulb (620–750)Blue Lamp
Bulb (450–495)Green Lamp
Bulb (495–570)Yellow Lamp
(570–590)Orange Lamp
(590–620)Pink Lamp
bulb(455–390)White Lamp
Bulb (400–700)Multiple color LED
Dark
Intracellular pigment 540 nm
2.124 ± 0.01
2.308 ± 0.0
3.611 ± 0.07
4.395 ± 0.03
2.721 ± 0.02
2.2352±
2.412 ±0.02
3.816 ± 0.02
4.672 ± 0.15
3.500 ± 0.03
2.913 ± 0.04
2.890 ± 0.03
2.601 ± 0.04
3.206 ± 0.02
Extracellular pigment 382 nm
3.317 ± 0.007
3.590 ± 0.02
2.151 ± 0.24
3.206 ± 0.32
3.296 ± 0.25
3.714 ± 0.21
3.638 ± 0.19
2.232 ± 0.28
3.154 ± 0.55
2.269 ± 0.18
2.486 ± 0.24
2.432 ± 0.31
2.583 ± 0.19
4.042 ± 0.13
Biomass(µg/10 mL)
363.3 ± 3.33
413 ± 3.33
708 ± 4.40
810 ± 2.88
650 ± 2.88
385 ± 2.88
470 ± 5.77
731.6 ± 4.4
830 ± 5.7
690 ± 5.7
651.6 ± 4.4
571.3 ± 2.4
530 ± 2.40
676.6 ± 3.5
Temperature (0C) →
Factor ↓25
30
35
40
45
50
55
60
65
Intracellular pigment/color 540 nm
4.539 ± 0.04/orange
3.698 ± 0.01/dark Red
4.433 ± 0.02/dark red
3.414 ± 0.2/light pink
NO
No
No
No
No
Extracellular 382 nm pigment/absorbance/color
2.448 ± 0.02/yellow green
3.257 ± 0.07/green
3.924 ± 0.04/green
3.230 ± 0.01/Green
No
No
No
No
No
Growth
Yes/++
Yes/+++
Yes/+++
Yes/++
Yes/++
Yes/+
Yes/+
NO
No
Biomass(microgram/ml)
618.3 ± 4.4
656 ± 3.05
592.3 ± 1.452
551.6 ± 6.0
530 ± 5.7
480 ± 2.8
358.3 ± 4.4
–
–
pH → at 33 °C
Factor ↓pH 3
pH 4
pH 5
pH 6
pH 7
pH 8
pH 9
pH 10
pH 11
Intracellular pigment/color 540 nm
No
No
2.548 ± 0.35/light pink
3.753 ± 2.14/dark red
3.856 ± 0.02/dark red
3.524 ± 3.5/dark red
3.342 ± 2.6/dark pink
2.015 ± 4.3/light pink
No
Extracellular pigment
No
No
2.861 ± 1.86 light Yellowish green
3.048 ± 0.75
green3.548 ± 2.2
Dark Green3.564 ± 1.5
Dark Green2.965 ± 3.6
Dark Green1.946 ± 2.6
Light greenNo Light green
Growth
No
No
Yes/+
Yes/++
Yes/+++
+++/Green
++/green
+++/green
No
Biomass(microgram/100 mL)
–
–
500 ± 0.50
650 ± 1.12
800 ± 1.44
800 ± 0.85
750 ± 0.65
600 ± 2.1
–
In our research study, we used two methods to detect the effect of light wavelengths on pigment production. Both methods, i.e., glass paper wrapping method and selective wavelength lamp method, gave good results, but more satisfactory and more efficient results were obtained in method two, i.e., selective lamp light wavelength method; as the pigment concentrations were higher under selective light lamps than the glass paper wrapped flasks and plates. In different light wavelengths, we observed some differences in color shade of extracellular pigment. This might be due to the variation in composition of pigment as suggested by Velmurugan et al. (2010). Our result and conclusion is also in agreement with Miyake et al. (2005), who stated that pigment concentration changes with the variation in light wavelength. When bacterial plates and flasks were incubated under increasing light intensity, no considerable changes in pigment concentration were observed (Table 5). These results also refute the role of our pigment in photoprotection. The effect of temperature on extracellular and intracellular pigment and biomass production is shown in Table 3. The maximum extracellular (3.924 ± 0.04 OD/mL) and intracellular pigment concentration (3.698 ± 0.01 OD/mL) was recorded when temperature was 35 °C and 30 °C, respectively. Maximum biomass concentration (656 ± 3.05 µg/0.01 L) was obtained when temperature was 30 °C. Above 45 °C, neither intra- nor extracellular pigment production was observed. Growth was observed up to 55 °C, but not at higher temperature. This shows that this bacterium is a moderate thermopile. Maximum intracellular pigment (3.856 ± 0.02 OD/mL), extracellular pigment (3.548 ± 2.2 OD/ml) and biomass concentration (800 µg/0.01 L) was recorded at pH 7. Above pH 10 and below pH 5, no pigment production was recorded. The bacterial growth was observed up to pH 10, which means the isolated bacterium is an alkalophilic strain. Table 4 shows the effect of pH on extracellular, intracellular pigment and biomass production by the bacterium. It is apparent from this research study that the pigment of isolated bacterium doesn’t have any role in photoprotection. This fact raises many questions in mind like why does the bacterium produce the pigment? Many bacterial and fungal pigments have been well recognized for their virulent activities (Liu and Nizet, 2009; Hall et al., 2016; Ortiz-Castro et al., 2014; Carbonell et al., 2000). As we had isolated the pigment producing bacterium from dead insect, we may speculate that the pigment of the bacterium might have acted as virulent factor for the insect and it might have served a reason for insect’s death. Another role of the pigment (considering its source of isolation) might be in degradation of cellular components (like chitin) of insect for making energy for bacterial cellular processes. This presumption seems more convincing after considering the ability of the bacterium to produce chitinase enzyme (the isolated bacterium was able to produce chitin in colossal amount). The pigment might be having roles other than these two hypothesized roles. The pigment may help the bacterium in competition with other microbes for getting needed nutrients for its growth and survival or it could have a role in bacterial resistance to naturally produced antimicrobial compounds by other microbes or plants. The pigment might be helpful for the bacterium to cope up with harsh and extreme conditions like high temperature, high pressure, high salinity and cold. Many researchers have reported the role of pigments in salt tolerance by haloarchae (Rebecca et al., 2016; Oren, 2010).
Light intensity
15 Watt
20 Watt
30 Watt
40 Watt
50 Watt
Darkness
Extracellular pigment OD at 382 nm
2.431 ± 0.2
2.315 ± 0.33
2.442 ± 0.03
2.492 ± 0.2
2.496 ± 1.1
4.154 ± 0.15
Intracellular pigment OD at 540 nm
2.875 ± 1.3
2.877 ± 0.33
2.814 ± 0.1
2.770 ± 0.05
2.814 ± 4.1
3.296 ± 0.35
4 Conclusion
From this study, we conclude that we have isolated a thermoalkalophilic strain of P. aeruginosa that produces red non-diffusible intracellular and green diffusible extracellular pigments. We also infer that there is an influence of light wavelength, pH and temperature on pigment production and biomass production of P. aeruginosa NR1. However, temperature displays the major influence factor, because slight difference in temperature completely inhibited the pigment production. Light intensity doesn’t affect considerably on pigment production by the bacterium. From this study, we infer that there is a strong effect of different light wavelengths on pigment production by P. aeruginosa NR1 as pigment production was doubled under suitable light wavelength. Biomass production is also influenced by pH and temperature. For pH and temperature, this was expected as it is well known fact that bacterial growth is influenced by pH and temperature. However, our study demonstrates that bacterial growth and therefore biomass production is also influenced by light wavelength. We conclude that darkness, red and blue light are suitable conditions for maximum extracellular pigment production, while yellow and green light is suitable for maximum biomass and intracellular pigment production by P. aeruginosa NR1. We also conclude that our designed selective wavelength lamp model for light experiments is a more suitable technique than the paper wrapping method. Our model is also advantageous over paper wrapping method as, once designed, it is easy to handle and also gives maximum pigment yield.
Conflict of interest
The authors declare that they have no conflict of interest.
Statement of human rights/animal welfare
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
“Informed consent was obtained from all individual participants included in the study.”
Acknowledgment
The authors are thankful to DST INSPIRE, New Delhi, India for providing fellowship to first author (IF150419) of this manuscript.
References
- Red pigment production by Penicillium purpurogenum GH2 is influenced by pH and temperature. J. Zhejiang Univ. Sci. B. 2011;12(12):961-968.
- [CrossRef] [Google Scholar]
- New components of the mammalian receptor potential and their relation to visual photochemistry. Vision Res.. 1966;6:373.
- [CrossRef] [Google Scholar]
- Effect of light on growth, pigment production and culture morphology of Monascus purpureus in solid-state fermentation. World J. Microbiol. Biotechnol.. 2008;24:2671-2675.
- [Google Scholar]
- Clinical relevance and virulence factors of pigmented Serratia marcescens. FEMS Immun. Med. Microbiol.. 2000;28(2):143-149.
- [CrossRef] [Google Scholar]
- Applications of a lipopeptide biosurfactant, surfactin, produced by microorganisms. Biochem. Eng. J.. 2015;103:158-169.
- [CrossRef] [Google Scholar]
- Effects of blue light on pigment biosynthesis of Monascus. J. Microbiol.. 2016;54:305-310.
- [Google Scholar]
- Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochemical & photobiological sciences. Euro. Photochem. Assoc. Euro. Soc. Photobiol.. 2007;6:725-736.
- [Google Scholar]
- Blue light (470 nm) effectively inhibits bacterial and fungal growth. Lett. Appl. Microbiol.. 2012;55:460-466.
- [Google Scholar]
- Molecular mechanism of violacein-mediated human leukemia cell death. Blood. 2004;104:1459-1467.
- [Google Scholar]
- Phycobiliproteins from Spirulina maxima and Pseudanabaena tenuis protect against hepatic damage and oxidative stress caused by Hg2+. Rev. Mex. Cienc. Farm.. 2010;41:30-35.
- [Google Scholar]
- Antioxidant and anti-inflammatory properties of an aqueous Cyanophyta extract derived from Arthrospira platensis: contribution to bioactivities by the non-phycocyanin aqueous fraction. J. Med. Food. 2015;18:535-541.
- [Google Scholar]
- Cellular effects of pyocyanin, a secreted virulence factor of Pseudomonas aeruginosa. Toxins. 2016;8:236.
- [CrossRef] [Google Scholar]
- Looking through the eyes of fungi: molecular genetics of photoreception. Mol. Microbiol.. 2007;64(5):15.
- [Google Scholar]
- Violacein synergistically increases 5-fluorouracil cytotoxity, induces apoptosis and inhibits Akt-mediated signal transduction in human colorectal cancer cells. Carcinogenesis. 2006;27:508-516.
- [Google Scholar]
- Antioxidant properties of violacein: possible relation on its biological function. Bioorg Med Chem.. 2006;14:8307-8313.
- [Google Scholar]
- A rapid method for the determination of microbial biomass by dry weight using a moisture analyser with an infrared heating source and an analytical balance. Lett. Appl. Microbiol.. 2010;50(3):283-288.
- [CrossRef] [Google Scholar]
- Impact of violacein-producing bacteria on survival and feeding of bacterivorous nanoflagellates. Appl. Environ. Microbiol.. 2004;70:1593-1599.
- [Google Scholar]
- Light effects on cell development and secondary metabolism in Monascus. J. Ind. Microbiol. Biotechnol.. 2005;32:103-108.
- [Google Scholar]
- Isolation of a psychrotrophic bacterium from the organic residue of a water tank keeping rainbow trout and antibacterial effect of violet pigment produced from the strain. Biochem. Eng.. 2003;J.12:79-86.
- [Google Scholar]
- Effects of light conditions on prodigiosin stability in the biocontrol bacterium Serratia marcescens strain B2. J. Gen. Plant Path.. 2004;6:367-370.
- [Google Scholar]
- Optimization of prodigiosin production by Serratia marcescens using crude glycerol and enhancing production using gamma radiation. Biotechnol. Rep,. 2017;14:47-53.
- [CrossRef] [Google Scholar]
- Industrial and environmental applications of halophilic microorganisms. Environ. Technol.. 2010;31(8–9):825-834.
- [CrossRef] [Google Scholar]
- Pyocyanin, a virulence factor produced by Pseudomonas aeruginosa, alters root development through reactive oxygen species and ethylene signaling in Arabidopsis. Mol. Plant Microbe Interact.. 2014;27(4):364-378.
- [CrossRef] [Google Scholar]
- Light-mediated changes in pigmentation of Pseudomonas aeruginosa cultures. J. Gen. Microbiol.. 1979;113:261-266.
- [Google Scholar]
- Carotenoid synthesis in Neurospora crassa. MethodsEnzymol.. 1985;110:253-267.
- [CrossRef] [Google Scholar]
- Biology and survival of extremely halophilic archaeon Haloarcula marismortui RR12 isolated from Mumbai salterns, India in response to salinity stress. Sci. Rep.. 2016;6:25642.
- [CrossRef] [Google Scholar]
- Reevaluation of the violacein biosynthetic pathway and its relationship to indolocarbazole biosynthesis. Chem Bio Chem.. 2006;7:1231-1240.
- [Google Scholar]
- Melanin photoprotection in the human retinal pigment epithelium and its correlation with light-induced cell apoptosis. Proc. Natl. Acad. Sci. U.S.A. 2005:8978-8983.
- [Google Scholar]
- Attenuation of diabetic complications by C-phycoerythrin in rats: antioxidant activity of C- phycoerythrin including copper -induced lipoprotein and serum oxidation. Br. J. Nutr.. 2009;102:102-109.
- [Google Scholar]
- Light influences pigment, biomass and morphology in Chaetomium cupreum – SS02 – a photo response study. Int. J. Curr. Microbiol. App. Sci.. 2014;3:53-64.
- [Google Scholar]
- Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J. Biosci Bioeng. 2010:346-350.
- [Google Scholar]
- Effect of temperature on prodigiosin synthesis in Serratia marcescens. Chinese. 2014;54(5):517-524.
- [Google Scholar]
- Effect of light on nonphotosynthetic microorganisms. Jpn. J. Microbiol.. 1975;19(5):387-393.
- [Google Scholar]
- Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta)? Phycologia. 1991;30:257-261.
- [Google Scholar]







