Translate this page into:
In vivo antioxidant efficacy and therapeutic potential of Artemisia brevifolia leaves extract against CCl4-induced reproductive damages in male albino rats
⁎Corresponding authors. asmabinm@gmail.com (Asma Ashraf), mushahid@ksu.edu.sa (Shahid Mahboob)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The current study was aimed to investigate the alleviative effect of Artemisia brevifolia (A. brevifolia) plant extract against CCl4-induced testicular damage in male albino rats. Forty-eight male albino rats were categorized into eight equal experimental groups i.e., control, vehicle control, CCl4 (1 ml/kg), CCl4 + silymarin, CCl4 + A. brevifolia (150 mg/kg), CCl4 + A. brevifolia (300 mg/kg), A. brevifolia (150 mg/kg) and A. brevifolia (300 mg/kg). After 56 days, blood and testicular samples were collected, and antioxidant enzyme activity, lipid peroxidation, hormonal concentration, daily sperm production (DSP), and histomorphometry were analyzed. The animals treated with the A. brevifolia extract exhibited a significant (p < 0.05) increase in the catalase (CAT), peroxidase (POD), superoxide dismutase (SOD), glutathione reductase (GR) activity, and significant (p < 0.05) decrease in thiobarbituric acid reactive substances (TBARS) level, potentially damaged by CCl4. Significant (p < 0.05) restoration in luteinizing hormone (LH), follicle-stimulating hormone (FSH), testosterone concentrations, and DSP was observed in the A. brevifolia treated groups. Besides, CCl4 significantly (p < 0.05) dysregulated the lipid profile by increasing cholesterol, LDL, and triglycerides, while reducing HDL. The histopathological analysis evinced that CCl4 significantly (p < 0.05) damaged the testicular tissues. However, A. brevifolia treatment considerably abated the detrimental effects of CCl4 in rat testes. In conclusion, our results suggested the therapeutic role of A. brevifolia in oxidative stress-related disorders of testes.
Keywords
Artemisia brevifolia
Carbon tetrachloride
Oxidative stress
Testicular damage
Albino rat
Antioxidant enzymes
1 Introduction
Spermatogenesis in animals is severely affected by exposure to various environmental toxicants, causing abnormal sperm morphology, low sperm quality, and oligospermia (Aitken et al., 2004; Sharpe, 2010). These environmental toxicants mostly entered the environment due to industrial waste. After becoming part of the environment, such toxicants enter the terrestrial animals through contaminated air, foodstuff, and water (Aitken et al., 2004; Wirth and Mijal, 2010). These toxicants affect the endocrine system, which may cause irregularities in male reproductive systems (Benoff et al., 2009; Coutts et al., 2007).
Carbon tetrachloride (CCl4) is extensively used as a standard toxicant to stimulate chemical toxicity in testes (Samad et al., 2020). Cytochrome P450 enzymes are present in testes, which convert the CCl4 into a potentially toxic substance (Naz et al., 2014). Initially, CCl4 damages the tissues by producing trichloromethyl and trichloromethyl peroxyl radicals with the help of cytochrome P450 enzymes (Halliwell and Gutteridge, 2007). When trichloromethyl free radicals reach a very high level, they instigate oxidation in membrane protein, lipids and finally culminate in histopathological deteriorations (Abdel Moneim and El-Khadragy, 2013).
Some medicinal plant extracts contain antioxidants that become incorporated into the body and support the antioxidants by reducing free radicals and increasing the life duration of biological processes (Khan and Younus, 2011; Wang et al., 2019; Ijaz et al., 2020a). Such antioxidant-containing plant extracts have shown therapeutic potential against chemically induced damages (Bakar et al., 2019). Herbal remedies have been witnessed as a miracle for treating numerous diseases (Bari et al., 2020; Zhao et al., 2020). Artemisia is a widely distributed genus of family Asteraceae, comprising 20–500 descriptive species associated with Anthemideae (Kubitzki, 2007; Ling et al., 2006). Central Asia is regarded as the parental area home to many different genus Artemisia varieties, from where its other species originated and spread to the whole world. This encompasses herbs and shrubs, which mostly flourish in sustainable ecosystems. They are primarily used for food and ornamental purposes (Ling et al., 2006). A. brevifolia Wall. ex DC. (Asteraceae), locally known as Afsanteen, has been used extensively in ethnoveterinary medicines in Pakistan as an anthelmintic. However, the protective effects of A. brevifolia on the male reproductive system have not been investigated yet. Therefore, the current study was performed to evaluate the therapeutic properties of A. brevifolia to counter CCl4-induced testicular injury in male albino rats.
2 Materials and methods
2.1 Collection and preparation of plant extract
A. brevifolia plant was collected from the Noori Top (Naran Vally, Mansehra District, KPK, Pakistan) in September 2018. The leaves of A. brevifolia were dehydrated under shade for 15 days and ground into powder. 1 kg of powder was dipped in the crude-methanol for 72 h and repeated process for two times before filtration was completed through Whatman (No. 1) filter and evaporation of methanol was conducted on a rotary evaporator in lower force at 40 °C. We stocked the obtained plant extract at 4 °C for subsequent analysis.
2.2 Experimental protocol
Mature male albino rats (160–180 g) were housed in the UAF Pharmacology department's animal house at 25 ± 2 °C. Rats were provided with tap water and a balanced diet. The study was approved by the ethical committee, University of Agriculture, Faisalabad, Pakistan (DGS No. 6477-80). Forty-eight mature male albino rats were divided into eight experimental groups comprising of 6 rats each. The first group was designated as the control group. The second group was administered with 1 ml/kg DMSO orally (Vehicle control), while the third group was administered with CCl4 dissolved in olive oil (1 ml/kg). The fourth group was treated with a 100 mg/kg dose of silymarin with CCl4, where the Fifth and sixth groups were served with the extract of A. brevifolia (150 mg/kg and 300 mg/kg) + CCl4 in order. However, groups VII and VIII were treated only with A. brevifolia extracts at the rate of 150 and 300 mg/kg, respectively. The dose of CCl4 was used following a previous study by Samad et al. (2019), while quantities of A. brevifolia were selected following the dose used by Ahsan et al. (2020). On the 56th day of trial, rats were given anesthesia by diethyl ether and then decapitated. Retro-orbital venous plexus was used for blood collection with the help of a fine needle, and serum was separated from the blood. On the 56th day of trial, rats were decapitated after anesthetization. Blood samples were collected in sterilized tubes, stored at 4 °C after centrifugation. Testes were removed, rinsed with the normal saline, homogenized by adding distilled water, and further centrifuged for approximately 15 min at 3000 rpm and stored at −20 °C.
2.3 Biochemical study
Chance and Maehly (1995) procedure was followed to estimate the peroxidase (POD) and catalase (CAT) activity. The method of Nishikimi et al. (1972) was pursued to determine the activity of superoxide dismutase (SOD). The action of glutathione reductase (GR) was assessed by the process of Factor et al. (1998). To carry out the Thiobarbituric acid reactive substances (TBARS), a modified scheme by Iqbal et al. (1996) was trailed.
2.4 Estimation of FSH, LH and testosterone concentrations
Follicle-stimulating hormone (FSH) and Luteinizing hormone (LH) concentrations were estimated by using specific kits from Bio-Vendor (Gunma, Japan). ELISA kits (Catalog# EK-311-15; Sensitivity 98%) were used to evaluate the testosterone hormone concentration from serum samples.
2.5 Daily sperm production
Daily sperm production was estimated using the method of Robb et al. (1978). The number of spermatids that were resilient to homogenization was divided by 6.3 for the calculation of DSP.
Where Y = number of spermatids present in homogenate6.3 = Entire days through spermatids remained in the seminiferous tubules epithelial part.
2.6 Analysis of lipid profile
The evaluation of cholesterol, triglycerides, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) was performed on blood plasma using commercially available kits (Diagnostic Product Corporation, Los Angeles, USA).
2.7 Histopathological examination
Fixation of the testicular tissues was accomplished in 10% formalin buffer, desiccated in alcohol, and fixed in paraffin wax blocks. Thin sections of 4–5 µm were sliced and fixed on a glass slide, stained with hematoxylin/eosin, and further studied under the light microscope (Nikon Labophot, Japan) at 400×.
2.8 Statistical analysis
The obtained dataset was checked for normality before subjecting it to further data analysis. The final data was presented as Means ± SEM. For comparing various groups, we applied the one-way analysis of variance (ANOVA) followed by Tukey's test with the help of Minitab software. The level of significance was set at p < 0.05 during all analyses.
3 Results
3.1 Effect of A. brevifolia on the antioxidant-enzymes and lipid peroxidation
One of the leading objectives was to evaluate the antioxidant enzyme activities such as CAT, SOD, POD, and GR. These enzyme activities showed a significant reduction (p < 0.05) in the CCl4-administered group than in the control group. Treatment of A. brevifolia against CCl4 indicated significantly improved enzyme activities compared with the CCl4 treated group. Rats co-treated with A. brevifolia and CCl4 revealed a significant increase in SOD, POD, CAT, and GR activities compared to the CCl4 group (Table 1). The level of TBARS was substantially (p < 0.05) increased in the CCl4 administered group compared to the control group. There was a substantial (p < 0.05) reduction in TBARS level was witnessed in CCl4 + A. brevifolia treated rats than the CCl4 administered group. The A. brevifolia significantly decreased TBARS in the CCl4 treated group, with no remarkable difference from the control group. Values having dissimilar superscripts are significantly (p < 0.05) different.
Groups
CAT (U/mg protein)
SOD (U/mg protein)
POD (nmole/mg protein
GR (nM NADPH oxidized/min/mg tissue)
TBARS (nM/mg tissue)
Control
8.80 ± 0.19a
5.25 ± 0.10a
6.94 ± 0.11a
134.0 ± 3.79a
13.07 ± 0.13a
Vehicle control
8.39 ± 0.03a
5.07 ± 0.05a
6.78 ± 0.05a
124.3 ± 2.96a
13.86 ± 0.10a
CCl4
3.93 ± 0.13d
1.83 ± 0.08c
2.97 ± 0.06d
59.33 ± 3.38d
25.75 ± 0.18d
CCl4 + Silymarin
7.88 ± 0.09b
4.84 ± 0.07ab
6.18 ± 0.07ab
114.3 ± 2.96b
15.94 ± 0.15a
CCl4 + A. brevifolia (150 mg/kg)
6.80 ± 0.13c
4.23 ± 0.07b
5.87 ± 0.05b
92.66 ± 2.18ab
18.32 ± 0.40b
CCl4 + A. brevifolia (300 mg/kg)
7.84 ± 0.08a
5.21 ± 0.17a
6.46 ± 0.13a
116.0 ± 2.64b
16.15 ± 0.47ab
A. brevifolia (150 mg/kg)
7.99 ± 0.08a
5.01 ± 0.04a
6.59 ± 0.06a
124.7 ± 1.45a
14.28 ± 0.09a
A. brevifolia (300 mg/kg)
8.35 ± 0.08a
5.06 ± 0.15a
7.04 ± 0.09a
121.0 ± 3.79b
13.97 ± 0.11a
3.2 Effect of A. brevifolia on hormonal concentrations and DSP
FSH, LH, and testosterone concentrations were significantly (p < 0.05) decreased in the CCl4-treated group than in the control group. However, in the co-treated groups and A. brevifolia alone administered groups, the hormonal level was significantly (p < 0.05) improved in a dose-dependent fashion (Table 2). The CCl4 administered group showed a substantial (p < 0.05) reduction in DSP compared to the control group. However, the DSP illustrated similar trends in the control and vehicle control groups. Nonetheless, the DSP displayed restoration in the sperm counts in CCl4 + A. brevifolia treated group. Furthermore, A. brevifolia only treated groups showed normal level of DSP. Values having dissimilar superscripts are significantly (p < 0.05) different.
Groups
FSH (ng/mL)
LH (ng/mL)
Testosterone (ng/mL)
DSP × 106/gram
Control
3.83 ± 0.08a
2.70 ± 0.04a
4.56 ± 0.05a
22.09 ± 0.29a
Vehicle control
3.74 ± 0.07a
2.57 ± 0.04a
4.47 ± 0.06a
21.00 ± 0.33a
CCl4
2.01 ± 0.06d
1.04 ± 0.04c
0.96 ± 0.10c
09.62 ± 0.14c
CCl4 + Silymarin
3.45 ± 0.06b
2.34 ± 0.04b
3.94 ± 0.04b
18.55 ± 0.25b
CCl4 + A. brevifolia(150 mg/kg)
3.24 ± 0.05c
2.37 ± 0.06b
4.04 ± 0.07b
17.33 ± 0.17b
CCl4 + A. brevifolia(300 mg/kg)
3.45 ± 0.03b
2.45 ± 0.06bc
4.39 ± 0.05a
17.89 ± 0.09b
A. brevifolia (150 mg/kg)
3.44 ± 0.05b
2.58 ± 0.02a
4.39 ± 0.02a
18.89 ± 0.09b
A. brevifolia (300 mg/kg)
3.63 ± 0.04a
2.66 ± 0.02a
4.46 ± 0.07a
18.88 ± 0.09b
3.3 Effect of A. brevifolia on lipid profile
The CCl4 exposure triggered a significant (p < 0.05) increase in the triglycerides and LDL levels as compared to the control group albino rats (Table 3). Similarly, we observed a significant (p < 0.05) rise in cholesterol level in the CCl4-intoxicated rats versus control rats. On the contrary, the HDL level displayed an opposite trend and exhibited a significant (p < 0.05) decrease in the CCl4-administered groups versus the control group. The rats treated with A. brevifolia and CCl4 (cotreated) showed a noticeable reduction in triglycerides, cholesterol and LDL, and an increase in HDL level than the CCl4 treated group. Values having dissimilar superscripts are significantly (p < 0.05) different.
Groups
HDL (mg dl−1)
LDL (mg dl−1)
Triglycerides (mg dl−1)
Cholesterol (mg dl−1)
Control
37.7 ± 1.24a
7.54 ± 0.54a
48.2 ± 2.02a
44.4 ± 1.75a
Vehicle control
36.8 ± 0.78a
7.66 ± 0.71a
49.4 ± 2.46a
45.6 ± 1.41a
CCl4
13.0 ± 0.94d
19.9 ± 1.08c
89.5 ± 2.94c
66.7 ± 2.22c
CCl4 + Silymarin
35.3 ± 1.10b
8.33 ± 0.63ab
54.3 ± 2.50b
46.4 ± 1.67b
CCl4 + A. brevifolia(150 mg/kg)
30.5 ± 0.61c
9.72 ± 0.36b
61.0 ± 1.26bc
49.8 ± 1.58b
CCl4 + A. brevifolia(300 mg/kg)
33.8 ± 1.05bc
9.24 ± 0.44b
55.4 ± 2.24b
47.7 ± 1.62b
A. brevifolia (150 mg/kg)
37.5 ± 0.90a
7.68 ± 0.59a
49.9 ± 2.44a
45.7 ± 1.59b
A. brevifolia (300 mg/kg)
38.5 ± 1.06a
7.36 ± 0.51a
47.3 ± 2.51a
44.12 ± 1.57b
3.4 Effect of A. brevifolia on histomorphometry of the testicular tissues
We observed a significant (p < 0.05) decline in the numbers of spermatogonia, primary and secondary spermatocytes, and spermatids recorded in the CCl4-treated group as compared to the control group (Table 3). On the other hand, the spermatogonia, primary and secondary spermatocytes, and spermatids displayed significantly (p < 0.05) improved numbers in both the CCl4 + A. brevifolia co-treated groups. Similar was the case in the only A. brevifolia treated group, as compared to CCl4 administrated group as displayed in Fig. 1(A-H) and Table 4. Interstitial spaces and Tubular lumen showed considerable enlargement while the tunica-propria, seminiferous tubules diameter, and epithelial height were significantly (p < 0.05) decreased in the CCl4-treated group as compared to the control group (Table 5). We observed a noticeable restoration in the A. brevifolia + CCl4 (co-treated) histological parameters and the only A. brevifolia treated groups compared with the CCl4 treated group as indicated in Fig. 1 and Table 5. Values having dissimilar superscripts are significantly (p < 0.05) different. Values having dissimilar superscripts are significantly (p < 0.05) different.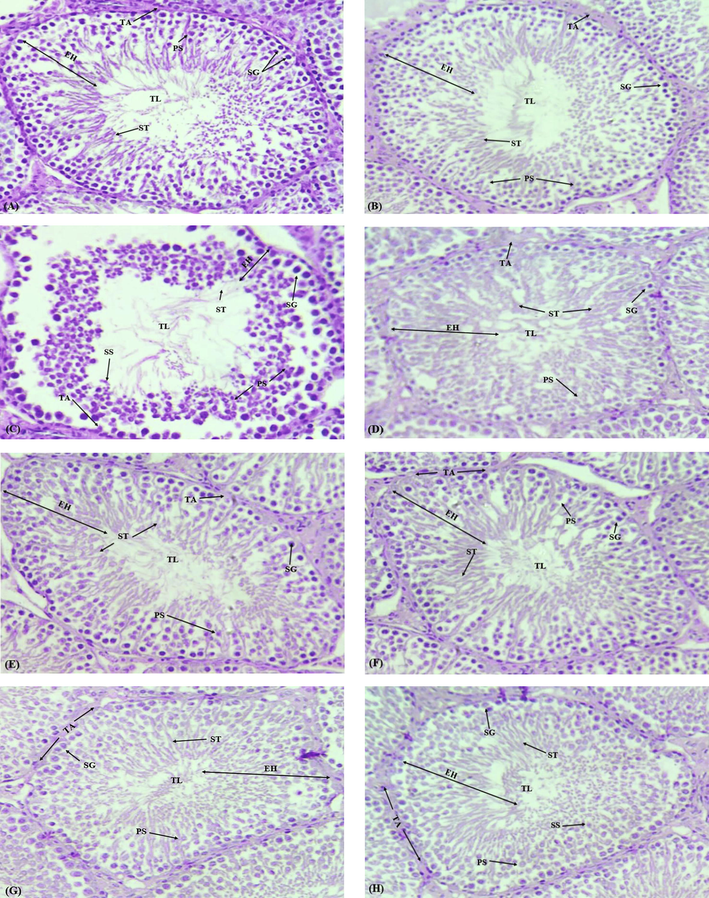
Histopathological photographs of the control and treated groups from A) Control group exhibiting the dense and healthy germinal epithelium and the slight luminal area comprising spermatozoa. B) Vehicle-control. C) CCl4 administered group showing empty lumen and deterioration of epithelial layer and interstitial-spaces D) CCl4 + Silymarin group exhibiting recovery in ST E) CCl4 + A. brevifolia (150 mg/kg) group showing recovered the tubular-lumen loaded with spermatids, germinal epithelium, and reestablishment of collapsed interstitial spaces. F) CCl4 + A. brevifolia (300 mg/kg). More recovery than group E. G) Shows A. brevifolia (150 mg/kg) group. H) shows A. brevifolia (300 mg/kg) group exposing dense STs with fewer interstitial spaces and standard spermatogenesis with the packed lumen. Epithelial-height (EH), Tubular lumen (TL), Spermatids (ST), Primary-spermatocytes (PS), Tunica Propria (TP), Secondary-spermatocytes (SS), Spermatogonia (SG).
Groups
Spermatogonia
(n)Primary spermatocytes
(n)Secondary spermatocytes
(n)Spermatids
(n)
Control
39.00 ± 1.15b
33.33 ± 2.02a
30.66 ± 1.76a
41.66 ± 0.88a
Vehicle control
36.66 ± 3.53c
31.33 ± 2.73ac
28.33 ± 3.48ab
40.66 ± 2.18a
CCl4
26.00 ± 1.52d
19.66 ± 2.33d
17.33 ± 1.76d
23.66 ± 0.88d
CCl4 + Silymarin
34.33 ± 0.33c
31.00 ± 0.57ac
27.66 ± 0.66b
35.66 ± 1.33c
CCl4 + A. brevifolia (150 mg/kg)
31.66 ± 0.33 cd
29.00 ± 1.52c
24.66 ± 0.88bd
34.66 ± 0.33c
CCl4 + A. brevifolia (300 mg/kg)
34.33 ± 0.33c
30.66 ± 0.66ac
28.33 ± 0.33ab
36.00 ± 1.00c
A. brevifolia (150 mg/kg)
41.00 ± 1.00b
35.33 ± 0.33ab
32.33 ± 1.76ac
42.66 ± 1.45a
A. brevifolia (300 mg/kg)
44.66 ± 0.33a
39.00 ± 1.73b
36.33 ± 1.45c
47.00 ± 1.52b
Groups
Interstitial spaces (µm)
Tunica propria (µm)
ST diameter
(µm)ST epithelial height (µm)
Tubular lumen (µm)
Control
7.62 ± 0.39 a
22.89 ± 0.45a
178.52 ± 0.59a
72.79 ± 0.57a
9.74 ± 0.22a
Vehicle control
6.76 ± 0.23b
21.28 ± 0.04a
176.65 ± 0.39a
72.13 ± 0.54a
9.55 ± 0.33a
CCl4
11.3 ± 0.70d
12.31 ± 0.76d
166.55 ± 0.99d
20.06 ± 0.42d
60.9 ± 0.77d
CCl4 + Silymarin
9.25 ± 0.23c
17.52 ± 0.76ab
177.67 ± 0.16a
51.4 ± 0.52c
17.3 ± 0.63c
CCl4 + A. brevifolia (150 mg/kg)
9.86 ± 0.22c
15.54 ± 0.34c
176.03 ± 0.38a
49.36 ± 0.99c
21.2 ± 1.29c
CCl4 + A. brevifolia (300 mg/kg)
7.84 ± 0.07a
18.57 ± 0.3ac
178.28 ± 0.59a
52.68 ± 0.47c
17.0 ± 1.32c
A. brevifolia (150 mg/kg)
7.75 ± 0.29a
26.63 ± 0.36b
180.46 ± 0.58b
74.54 ± 0.31ab
12.0 ± 0.96a
A. brevifolia (300 mg/kg)
7.45 ± 0.40a
27.46 ± 0.89b
183.12 ± 0.34c
77.08 ± 0.10b
12.6 ± 0.54a
4 Discussion
The mammalian cells have developed different non-enzymatic and enzymatic processes that could be managed through free radicals. The antioxidant defense system becomes deficient in response to reactive oxygen species (ROS) during oxidative stress situations (Halliwell and Gutteridge, 2007; Ijaz et al., 2020b). The CAT, POD, SOD, and GR activities decrease due to increased ROS production (Abdel-Moneim et al., 2011). The antioxidants generally proceed in a systematic pathway in a bid to cope with oxidative stress-mediated damages. The present study showed that CCl4 decreased the CAT, POD, SOD, and GR enzyme activities and elevated the TBARS level. Surplus level of H2O2 in testes due to CCl4 instigated the tissue damage and suppressed the antioxidant enzymes (Ojo et al., 2016). The A. brevifolia extract scavenged the free radicals and restored enzymatic abilities to trim down the oxidative stress by CCl4. Bioactive compounds in A. brevifolia (as in other species of the same genus) could be a primary reason for decreasing free radicals. Our results are consistent with Shah and Khan (2017), who documented the protective role of Jurenia dolomiaea on rat testes. The A. brevifolia may shield the testes from oxidative injury by decreasing the free radicals, which corroborates low TBARS level and increased antioxidant enzyme activity.
CCl4, as a potentially harmful compound, decreased the concentration of FSH, LH, testosterone, and DSP. The present study was initiated by keeping in view the previous observations that testicular atrophy, germinal layer degeneration, reduced testosterone levels, and gonadotropins could be caused by CCl4 intoxication (Khan and Ahmed, 2009). LH encourages Leydig cells to produce testosterone, and on the other hand, FSH promotes spermatogenesis (Jaffat et al., 2014). Therefore, the CCl4 intoxication in testicular tissues could be attributed to the toxic effect of CCl4 by decreasing FSH, LH, and testosterone concentration. The CCl4 could also disturb the suprachiasmatic hypothalamic nucleus (SCN), which retards the release of gonadotrophins from the pituitary gland (Khan et al., 2011). Increased FSH, LH, testosterone, and DSP levels due to A. brevifolia extract could be credited to its immediate effect on SCN, pituitary gland, or gonadal status. The extract of A. brevifolia can effectively prevent the CCl4 induced effect on hormone and DSP.
The present investigation illustrated an overall escalation of the cholesterol, triglycerides, and LDL levels, whereas HDL levels exhibited a reduction in CCl4-administered rats. Lipids play a vital role in cell and organ systems' structural and functional stability (Rawi et al., 2012). The higher levels of lipids, such as cholesterol, triglycerides, and LDL, result in a disordered condition called hyperlipidemia, which generates toxicity in testicular tissues (Mehta et al., 2003). Moreover, the excess plasma level of cholesterol and HDL level reduction results in spermatogenic damages and reduced testosterone concentration culminating in male infertility (Ibrahim et al., 2012). The experimental groups administered with A. brevifolia + CCl4 showed an improved lipid profile as compared to CCl4 treated group.
CCl4 administration invoked a remarkable decrease in the germ cells at various stages, whereas we observed an improved cell count in the A. brevifolia administered groups. Degeneration of germ cells, seminiferous tubules, increased interstitium, and tubular lumen was evident in the CCl4-administered group. The proposed presence of flavonoids in genus artemisia may help recover the CCl4 mediated injuries (Sajid et al., 2016). Similar curative effects were reported by the treatment of Carissa opaca plant extracts against CCl4 caused injuries in testes (Sahreen et al., 2013). In conclusion, our study detected that A. brevifolia extract effectively protected the male albino rats from the CCl4 induced testicular toxicity by restoring the testicular histological alterations. The use of A. brevifolia substantially improved the germinal epithelial height, augmented the tunica propria thickness, and increased the diameter of seminiferous tubules.
5 Conclusion
In conclusion, our findings propose that A. brevifolia has illustrated curative properties to counter the disturbances in antioxidant enzyme activity and hormonal dysregulation, impairments in the architecture of seminiferous tubules. A. brevifolia entails antioxidant and androgenic properties to deal with the sterility issues in animals and humans, instigated by oxidative stress. The protective effects of A. brevifolia plant extract on testes may be attributed to its antioxidant and androgenic nature.
Acknowledgements
The Author MUI acknowledges the University of Agriculture, Faisalabad, Pakistan for the provision of technical facilities. “The authors (SM and KAG) express their sincere appreciation to the Researchers Supporting Project number (RSP-2021/93), King Saud University, Riyadh, Saudi Arabia.”
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The redox status in rats treated with flaxseed oil and lead-induced hepatotoxicity. Biol. Trace Elem. Res.. 2011;143(1):457-467.
- [Google Scholar]
- The potential effects of pomegranate (Punica granatum) juice on carbon tetrachloride-induced nephrotoxicity in rats. J. Physiol. Biochem.. 2013;69(3):359-370.
- [Google Scholar]
- Methanol extract of Artemisia brevifolia as a curative agent against CCl4 induced nephrotoxicity in albino rats. J. King Saud Univ. Sci.. 2020;32(7):3072-3078.
- [Google Scholar]
- Assessment of ginger extract and ginger nanoparticles protective activity against acetaminophen-induced hepatotoxicity and nephrotoxicity in rats. Pak. Vet. J.. 2019;39(4):479-486.
- [Google Scholar]
- Anti-hyperglycemic efficacy of derris ovalifolia in alloxan-induced diabetic wister rats. Pak. Vet. J.. 2020;40(1):108-112.
- [Google Scholar]
- Cadmium concentrations in blood and seminal plasma: correlations with sperm number and motility in three male populations (infertility patients, artificial insemination donors, and unselected volunteers) Mol. Med.. 2009;15(7-8):248-262.
- [Google Scholar]
- Environmental toxicant-induced germ cell apoptosis in the human fetal testis. Hum. Reprod.. 2007;22(11):2912-2918.
- [Google Scholar]
- Disruption of redox homeostasis in the transforming growth factoralpha/c-myc transgenic mouse model of accelerated hepatocarcinogenesis. J. Biol. Chem.. 1998;273(25):15846-15853.
- [Google Scholar]
- Free Radicals in Biology and Medicine. USA: Oxford University Press; 2007.
- Selenium-enriched probiotics improves murine male fertility compromised by high fat diet. Biol. Trace Elem. Res.. 2012;147(1-3):251-260.
- [Google Scholar]
- Methanolic extract of Fraxinus xanthoxyloides attenuates cisplatin-induced reproductive toxicity in male albino rats. Pak. Vet. J.. 2020;40(4):216-220.
- [Google Scholar]
- Casticin alleviates testicular and spermatological damage induced by cisplatin in rats. Pak. Vet. J.. 2020;40(2):234-238.
- [Google Scholar]
- Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Rep.. 1996;2(6):385-391.
- [Google Scholar]
- Protective effect of Allium ampeloprasum against toxicity induced by CCL4 in male white rats. J. Sci. Eng. Res.. 2014;5(10):825-828.
- [Google Scholar]
- Protective effects of Digera muricata (L.) mart. on testis against oxidative stress of carbon tetrachloride in rat. Food Chem. Toxicol.. 2009;47(6):1393-1399.
- [Google Scholar]
- Evaluation of antioxidant and fertility effects of Digera muricata in male rats. Afr. J. Pharm. Pharmacol.. 2011;5(6):688-699.
- [Google Scholar]
- Prevention of CCl(4)- induced oxidative damage in adrenal gland by Digera muricata extract in rat. Pak. J. Pharm. Sci.. 2011;24(4):469-473.
- [Google Scholar]
- Kubitzki, K., 2007. The families and genera of vascular plants. Vol VIII. Flowering plants. Eudicots. Asterales (Eds.) J W Kadereit and C Jeffrey Springer-Verlag Berlin Heidelberg, pp. 358.
- Ling, Y.R., Humphries, C.J., Shultz, L., 2006. Flora of China, Vol. 20 (Asteraceae), Editorial Committee (Eds.), Science Press and Missouri Botanical Garden Press, Beiging, - St. Louis. 2006.
- Effect of fruits of Moringa oleifera on the lipid profile of normal and hypercholesterolaemic rabbits. J. Ethnopharmacol.. 2003;86(2-3):191-195.
- [Google Scholar]
- Pistacia chinensis: A potent ameliorator of CCl4 induced lung and thyroid toxicity in rat model. BioMed Res. Int.. 2014;2014:1-13.
- [Google Scholar]
- The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophy. Res. Commun.. 1972;46(2):849-854.
- [Google Scholar]
- Protective influence of Ficus asperifolia Miq leaf extract on carbon tetrachloride (CCl4)-induced testicular toxicity in rats. J. Appl. Pharm.. 2016;6(6):37-41.
- [Google Scholar]
- Hazardous effects of acrylamide on immature male and female rats. Afr. J. Pharm. Pharmacol.. 2012;6(18):1367-1386.
- [Google Scholar]
- Daily sperm production and epididymal sperm reserves of pubertal and adult rats. J. Reprod. Fertil.. 1978;54(1):103-107.
- [Google Scholar]
- Effect of Carissa opaca leaves extract on lipid peroxidation, antioxidant activity and reproductive hormones in male rats. Lipids Health Dis.. 2013;12:90.
- [Google Scholar]
- Proficiencies of Artemisia scoparia against CCl4 induced DNA damages and renal toxicity in rat. BMC Complement. Altern. Med.. 2016;16(1):149.
- [Google Scholar]
- Methanolic extract of Nepeta paulsenii as an ameliorative agent against CCl4 induced testicular damage in male albino rats. J. King Saud Univ. Sci.. 2020;32(1):1168-1174.
- [Google Scholar]
- Increase of glutathione, testosterone and antioxidant effects of Jurenia dolomiaea on CCl4 induced testicular toxicity in rat. BMC Complement. Altern. Med.. 2017;17(1):1-9.
- [Google Scholar]
- Environmental/lifestyle effects on spermatogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci.. 2010;365(1546):1697-1712.
- [Google Scholar]
- Protective effects of baicalein against cadmium-induced oxidative stress in rat testes. Pak. Vet. J.. 2019;39(2):216-220.
- [Google Scholar]
- Adverse effects of low level heavy metal exposure on male reproductive function. Syst. Biol. Reprod. Med.. 2010;56(2):147-167.
- [Google Scholar]
- Regulating activity of polysaccharides from Portulaca oleracea L. on dendritic cells of mice immunized against foot-and-mouth disease. Pak. Vet. J.. 2020;40(1):7-12.
- [Google Scholar]







