Translate this page into:
In vitro study on antidiabetic and antihypertensive activities of ethanolic extract of propolis of Indonesian stingless bee Tetragonula sapiens
⁎Corresponding author at: Faculty of Pharmacy, Universitas Indonesia, Cluster of Health Sciences Building, Depok 16424, West Java, Indonesia. d.kartika@univpancasila.ac.id (Diah Kartika Pratami)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The stingless bee, Tetragonula sapiens, is one of the species producing propolis in Indonesia. To date, there is still limited information about the standard characteristics and biological activity of propolis. This study investigated the standardized extract of Indonesian propolis in terms of its potency for controlling diabetes and high blood pressure in vitro, in accordance with its standard parameters. Three types of extracts; i.e., ethanolic extract of propolis (EEP), spray-dry propolis without wax (SDPNW), and spray-dry propolis with wax (SDPW); were prepared from T. sapiens stingless bees collected in South Sulawesi, Indonesia. The ethanolic extractable, loss on drying, ash, and water content parameters of EEP, SDPNW, and SDPW met the natural extract requirement. There is no significant value for their residual solvent levels (<0.54%), heavy metals (the absence of As, Cd, Pb, and Hg contamination), or the absence of microbial contamination. ACE inhibition activity on captopril, EEP, SDPNW, and SDPW had IC50 values of 5.97, 115.06, 112.93, and 116.70 ppm, respectively. While the IC50 values of acarbose, SDPNW, EEP, and SDPW for antidiabetic activity of α-glucosidase inhibition were 53.34, 56.48, 60.09, and 68.28 ppm, respectively. The TFC and TPC content of SDPNW were higher than EEP and SDPW, with values of 1.59% and 1.93%, respectively. It can be said that Indonesian propolis extract meets the standards for an extract and has the potential to reduce diabetic and hypertensive diseases. Further preclinical and clinical studies are needed to develop standardized propolis extract as an anti-diabetic and antihypertensive agent.
Keywords
α-Glucosidase enzyme
Angiotensin converting enzyme
Antidiabetic
Antihypertensive
Propolis
Tetragonula sapiens
1 Introduction
Honey bees get propolis from the resinous secretions and leaf buds of plant species. These secretions are then combined with saliva and enzymatic secretions to produce propolis. Propolis has been found to show antimicrobial activity, and as such, honey bees use it to protect their larvae, honey stores, and comb from microbial infections. Moreover, it is used to block cracks, smooth out internal walls, and seal spaces in their hives (Bankova et al., 2021). Numerous studies have shown multiple biological activities of the propolis produced by Apis mellifera honeybees and stingless bees, including antimicrobial, antioxidant, anti-inflammatory, cardioprotective, and antiproliferative (Abdullah et al., 2020; Popova et al., 2022; Zullkiflee et al., 2022). Therefore, propolis has great potency to be used as an alternative complementary medicine for the potential treatment of metabolic syndrome, various acute and chronic diseases (Zulhendri et al., 2021).
In modern global societies, metabolic syndrome and chronic diseases show an increasing trend. In particular, our lifestyles result in an increase in cases of chronic diseases related to hypertension. Hypertension has led to many fatal failures, including heart attacks. In the correlation with the COVID-19 pandemic, it has been currently associated with the severity, acute respiratory distress syndrome, ICU care, and recovery progression of the patients (Xie et al., 2021). These phenomena are often seen for patients with the medical treatment using angiotensin-converting enzyme inhibitors (ACE-Is) or angiotensin-receptor blockers (ARBs) drugs. In fact, these kinds of drugs have been recommended for hypertension medication because of their genetic polymorphism that causes angiotensin-converting enzyme 2 (ACE2) to be more active. Although some synthetic ACE inhibitors are widely used, their chronic use has many side effects, such as coughing, renal failure, postural hypotension, and angioedema (Laurent, 2017). Several pathogenetic mechanisms have been proposed through the use of drugs that can regulate ACE2 expression (Fleming et al., 2021). Exploring ACE inhibitors from natural products has been extensively conducted in recent years because of their potential lower side effects.
In other hand, International Diabetes Federation reported that diabetes mellitus has become a global health issue and will continue to rise in both developed and developing countries until 2021 (Sun et al., 2022). Diabetes is strongly linked to worse outcomes, especially in people who don't have their disease under control (Fleming et al., 2021). Furthermore, poor glucose control and diabetes mellitus (DM) are another important risk factors for the COVID-19 result (Guo et al., 2020). Typically, antidiabetic drugs are used to control the work of alpha-glucosidase enzymes by stopping the intestine from absorbing as many monosaccharides (Sahlan et al., 2020). Nevertheless, the potential side effects of these synthetic drugs lead people to find a safer therapeutic approach using natural ingredients.
The antihypertensive and antidiabetic effects of propolis have been widely reported. Propolis has been shown in animal studies to reduce diabetes symptoms. Streptozotocin (STZ), alloxan, D-glucose, or fructose were used in vivo animal models to induce specific pathological changes in glucose metabolism leading to chronic hyperglycemia, a key factor associated with cardiovascular complications. In these studies, propolis administration reduced blood glucose rise and improved insulinemia while protecting pancreatic beta cells in chemically induced diabetes mellitus (Balica et al., 2021). Additionally, human clinical trials showed that reducing the symptoms of diabetes and pre-diabetes. There is a high mortality rate and a reduced quality of life associated with macrovascular or microvascular complications in diabetic patients. Propolis has been shown to have a significant protective effect in diabetes mellitus in several randomized controlled studies that showed it reduced fasting blood glucose (FBG) and glycosylated hemoglobin (HbA1C), which are important predictors of vascular complications, in diabetic patients when taken orally for at least two months (Balica et al., 2021). These properties are considered to attribute to the phenolic and flavonoid components contained in propolis (Mishima et al., 2005). The extract of propolis had high levels of phytochemical compounds, mostly phenolic derivatives. In vivo research suggests that it may also have antidiabetic properties. This may be because some of these constituents work synergistically (Usman et al., 2017). In the current COVID-19 pandemic condition, propolis is frequently used as an adjuvant as well because it could act as an immunostimulant to enhance the immune response and has been found to suppress COVID-19′s effects (Berretta et al., 2020). Propolis in standard medical procedures reportedly reduced hospital stays (Silveira et al., 2021).
The phytochemicals of organisms are varied depending on many factors, including their natural sources, geographic location, extraction method, and so on. The variety of these biochemical compounds could result in different biological activities in the obtained extract (Zulhendri et al., 2021). Pujirahayu et al., 2019 investigated the inhibitory effect of different triterpenes from Indonesian stingless bee propolis on alpha-glucosidase (cycloartenol, ambonic acid, mangiferonic acid, and ambolic acid). Mangiferonic acid from propolis had the largest inhibitory impact on alpha-glucosidase with an IC50 of 3.46 µM/mL (Pujirahayu et al., 2019). In order to get more comprehensive information on the potency of propolis extracted from Indonesia to be used in the healthcare system as a way to treat diabetes and high blood pressure, this study reported the activity of an ethanolic propolis extract (EEP) collected from the Indonesian stingless bee species Tetragonula sapiens. The standard protocol of EEP and spray-dried propolis extracts were prepared in the form of with and without wax and used in the study according to the regular protocol in Indonesia. The activities were examined in vivo on the ACE and α-glucosidase enzyme inhibitory activity, including other quality parameters.
2 Material and methods
2.1 Propolis source
The samples of propolis were obtained from the beehive of T. sapiens, taken from Luwu Utara district, South Sulawesi, Indonesia. The beehive that had taken its honey, cleaned bee pollen, and bee bread (a fermented mixture of bee pollen) was kept in the refrigerator until it was extracted. The types of beehives were a combination of smooth (taken from inside the hive) and rough (taken from outside the hive).
2.2 Extraction
The propolis was extracted according to previous research (Pratami et al., 2018). The propolis with the ratio 1:5 m/V was macerated using ethanol under stirring at 300 rpm for 8 h. The yield was calculated using the dry weight of the extract and soaked samples. The drug extract ratio (DER) native, or the ratio of the mass of the starting material (herbal substance) to the mass of the resulting native extract, was calculated as the ratio of a herbal substance to a native herbal preparation (herbal preparation). To produce spray-dry propolis non-wax (SDPNW), the filtrate of EEP was microencapsulated with maltodextrin (MD) and gum arabic (GA) (Pratami et al., 2020). A mini spray dryer (Buchi B290, Flawil, Switzerland) was used for the experiment. To produce spray-dry propolis with wax (SDPW), the filtrate of EEP was made by using the propolis solution without keeping the propolis extract overnight in a refrigerator.
2.3 Characterization of the propolis extract
2.3.1 Physico-Chemical tests
The physico-chemical characteristics of the propolis extracts were determined using specific and non-specific parameters (Bankova et al., 2019; Pratami et al., 2021). The specific parameters were the organoleptic test, i.e., appearance, color, smell, and taste, and the determination of the content of matter soluble in 96% ethanol and water. The nonspecific parameters were measured, including the amount of ash, water, heavy metals, residual solvents, and microbial assay.
2.3.2 Phytochemical Screening
The identification of chemical compound groups from samples of propolis extract (EEP, SDPNW, and SDPW) was carried out according to the Farnsworth method (See Table 1).
Phytochemical Screening
Test
Observations (Indicating a Positive Test)
Detection of alkaloid
Dragendroff’s test
A reddish-brown precipitate
Mayer’s test
A creamy white/yellow precipitate
Detection of flavonoid
Isoamyl alcohol test
The upper layer appears colour
Detection of saponins
Foam test
Persistent foam for 10 min.
Detection of tannins
Gelatin test
A white precipitate
FeCl3′s test
Blue-green colour
Stiasny’s test
Obtaining a precipitate indicates the presence of tannin catechism.
Stiasny’s and FeCl3′s test
A blue-black staining indicates the presence of gallic tannin
Detection of essential oils
Petroleum ether test
Aromatic smell
Detection of steroids
Liebermann-Burchard’s test
Blue or green color
Detection of terpenoids
Liebermann-Burchard’s test
Crimson color
Detection of quinone
NaOH test
Intensive red color
Detection of coumarin
NaOH test
Blue-green fluorescence from under the
UV light
2.3.3 Heavy metal contamination test
Hg, Cd, As, and Pb were the representative heavy metal parameters required by the National Agency of Drug and Food Control (NA-DFC or BPOM) in Indonesia. The test was performed using atomic absorption spectrophotometry (Shimadzu AA-6300, Kyoto Prefecture, Japan) (Directorate General of Pharmaceutical and Medical Devices, 2017).
2.3.4 Microbial contamination test
Total Plate Count (TPC) and Yeast Mold Number (YMN) were selected to analyze the microbial contamination of the extract. The measurement was carried out for at least three replications. A similar procedure was carried out to determine the YMN by using potato dextrose agar (PDA) media (Directorate General of Pharmaceutical and Medical Devices, 2017).
2.4 Determination of total phenolic and flavonoid content
The total phenolics (TPC) and flavonoid content (TFC) of propolis extracts (EEP, SDPNW, and SDPW) were analyzed according to the Pharmacopoeias Formularies Herbal Medicine Edition II 2017 (Directorate General of Pharmaceutical and Medical Devices, 2017). The TPC was presented in Gallic acid equivalent (mg GAE/g) and TFC was presented in Quercetin Eqivalent (mg QE/g).
2.5 α-Glucosidase enzyme inhibition activity test
The α-glucosidase inhibition test of the propolis extract samples (EEP, SDPNW, and SDPW) was measured based on the previous research with slight modifications (Alaribe et al., 2021). The decreasing p-nitrophenol concentration produced by the α-glucosidase enzyme was measured (Table 2). Here, the absorbance was measured with a Microplate Reader ELx800 (Biotek, USA) at λ 405 nm to find the IC50 of the extract compared to acarbose, which was used as a positive control. The IC50 was found by putting the concentration data on the (X) axis and the percentage of inhibition on the (Y) axis of the equation y = a + bx. A1 = acarbose; A0 = Blank acarbose; ESP1 = EEP, SDPNW, and SDPW samples; ESP0 = Blank EEP, SDPNW, and SDPW; C = Control; B = Blank.
Reagent
Volume (µL)
A1
A0
ESP1
ESP0
B
C
Sample
50
50
50
50
–
–
DMSO
–
–
–
–
50
50
Phosphate Buffer pH 7.0
450
450
450
450
450
450
p-nitrophenyl-α-D-glucopyranoside 10 Mm
250
250
250
250
250
250
Incubation for 5 min at 37 °C
α-glucosidase solution 0.030 U/Ml
250
–
250
–
–
250
Phosphate Buffer pH 7.0
–
250
–
250
250
–
Incubation for 20 min at 37 °C
Sodium carbonate 0.2 M
1000
1000
1000
1000
1000
1000
Measure the absorbance with a Microplate Reader 96 well EL × 800 at λ 405 nm
2.6 ACE inhibition activity test
The ACE inhibition activity was determined using methods described in previous research with modifications (Osés et al., 2020). Each of the samples (EEP, SPNW, and SDPW) was dissolved in a phosphate buffer solution containing 300 mM NaCl at pH 8.3 with captopril as the control. Furthermore, a blank solution and a series of EEP, SDPNW, and SDPW (concentrations of 25 to 200 ppm) were made. Furthermore, about 50 µL of the sample solution and 50 µL of 5 mM of a substrate, hippuryl-histidyl-leucine were added into each well in a 96-well microplate. After incubating at 37 0C for 15 min, 50 µL of a 4 mU/mL ACE solution was inserted, and the mixture was incubated at 37 0C for 30 min. Afterwards, 200 µL of 1 M hydrochloric acid was added, extracted with 1.5 mL of ethyl acetate, and centrifuged at 4000 rpm for 15 min. Then, 1.0 mL of the supernatant was pipetted into a different test tube and left at room temperature for 45 min to evaporate. After it was dried, 3.0 mL of distilled water was used to dissolve it, and the absorption was measured at a wavelength of 228 nm. The absorption obtained was converted into a standard curve of the comparison series solution for hippuric acid to calculate the concentration. The obtained hippuric acid was used to calculate the percentage of inhibition (%), and the % was used to determine the IC50 (Osés et al., 2020).
3 Results and discussion
3.1 Extraction
Fig. 1 shows the appearances of the obtained extracts of EEP, SDPNW, and SDPW. The former had a liquid form because it contained more solvent than 10%. The EEP obtained was thickened using a water bath; hence, the yield and DER native could be determined as shown in Table 3. The macerated EEP consisted of 800 mg/mL of raw propolis from T. sapiens, obtained by comparing the extract with the raw material used. The DER is the ratio of the quantity of the herbal substance used to the quantity of the herbal preparation produced. The ratio of herbal substance to native herbal preparation is known as DER native. The ratio of the herbal substance to the non-native extract, including the native extract and any solvent residues or additional excipients, is known as the herbal substance to non-native extract mass ratio. The starting herbal substance, the extraction solvent, the manufacturing process, and the manufacturing equipment are the main factors that affect the quantity and composition of the native extract and the DERnative.
Propolis extract samples. (a) EEP; (b) SDPNW; (c) SDPW.
Extract
Weight of Raw material (g)
Final extract (g)
Der-native
(g)
Yield (%)
EEP
1,600.00
80.32
19.92
5.02
SDPNW
22.02
16.59
1.32
75.35
SDPW
22.06
17.34
1.27
78.60
The ethanolic propolis extract was microencapsulated to improve the physical characteristics of propolis. Since this technique is commonly used in commercial products, we determined its effect on the quality of the extract. In this study, the yield of microencapsulation reached its maximum value at an inlet temperature of spray-dried of 120 °C, flow rate of 30%, and a propolis: MD-GA ratio of 1:1 (Pratami et al., 2020).
Our previous research found sesamin, curcumin, 8-epi-helenalin, and kushenol F in propolis free wax identified by Ultra-performance Liquid Chromatography with Time-of-Flight Mass Spectrometry (UPLC-TOF-MS) using the MSE mode (Pratami et al., 2018). In previous research, Indonesian propolis wax contains anti-microbial marker compounds such as thymol, curcumene, tetraline, and E-p-coumaric acid, which have been found to show anti-candida potency (Mahadewi et al., 2018). The propolis wax is a by-product of the purification process that has not been used further because its polyphenolic content is generally lower than that of pure propolis. However, the polyphenol content in wax propolis has been shown to be effective in inhibiting Candidiasis vaginalis (Farida et al., 2020).
3.2 Phytochemical Screening
Phytochemical screening was conducted to initially evaluate the potential secondary metabolites contained in the propolis extracts (Table 4). Generally, all propolis extracts qualitatively contain alkaloids, flavonoids, saponins, tannins, quinones, steroids, triterpenoids, essential oils, and coumarins. Herein, the EEP showed the strongest color intensity than two other extracts. Those secondary metabolites have been known to have different biological activities, and as such, their variety should affect the activity of the propolis extracts. Note: (+++) indicates a positive result with stronger color intensity, (++) indicates a positive result with weaker color intensity, and (-) indicates a negative result.
No
Phytochemical Screening
Results
EEP
SDPNW
SDPW
1
Alkaloids
+++
++
++
2
Flavonoids
+++
++
++
3
Saponins
+++
++
++
4
Tannins
++
+
+
Galat tannins
++
+
+
Katekat tannins
++
+
+
5
Quinones
++
+
+
6
Steroids
+++
++
++
7
Triterpenoids
+++
++
++
8
Essential oils
+++
++
++
9
Coumarins
+++
++
++
Flavonoids are one of the types of compounds that have the activity to form chelate complexes on the active site of ACE (Yang et al., 2021). Phenolics are also reported to increase the inhibitory potential of ACE. These activities are due to some functional groups of those compounds, i.e., hydroxyl and carboxyl groups, that can act as hydrogen bond donors or acceptors (Ranilla et al., 2010). It is supported by the fact that replacing their hydrogen bond atom donors and acceptors in the form of Zn phenolic and flavonoid compounds could decrease their inhibitory ability (Al Shukor et al., 2013). Considering the phytochemical profile test, the propolis extracts should have the potency to inhibit ACE and alpha glucosidase.
3.3 Specific quality parameter determination test
The EEP sample was obtained as a thick, brownish-yellow extract. This property is due to the extract still containing up to 10% solvent. The EEP has a typical odor of propolis and a bitter taste. Those properties and low water solubility limit the application of EEP in pharmaceutical products (Pratami et al., 2020). Improving its character to be accepted by consumers without significantly changing its efficacy is required. One of the common methods for conducting microencapsulation using the spray drying method could be used. A water-soluble encapsulation matrix is predicted to protect bioactive substances and even increase dose potency (Maroof et al., 2020). In this study, SDPNW and SDPW showed a quite more interesting color (light brown powders) than EEP, with the typical odor and taste of propolis but less of its bitter and unpleasant taste. The spray-dry technique resulted in dried propolis with a water content of 5.11 ± 0.01 and 5.12 ± 0.02 for SDPNW and SDPW, respectively. The result was similar to the previous research (Pratami et al., 2021). Microencapsulation technology could help overcome the bitter and unpleasant taste of propolis, as well as its low water solubility and easy loss of antioxidant activity and phenolic compounds.
Table 5 shows the solubility of the propolis extracts in water and ethanol. In this context, our extracts fulfill the standard requirements of the regulation standard for extract in Materia Medica Indonesia. Spray-drying with microencapsulation significantly improved the solubility of the extracts in both solvents. Extractives are extraneous components that can be separated from the insoluble cell wall material by their solubility in water or neutral organic solvents. To remove different types of extractives, different polar solvents are required. As a result, extractives are frequently classified based on the type of solvent (e.g., ethanol-soluble extractives) (Bankova et al., 2021). Secondary metabolites extracted in water as the solvent were observed to be saponins and flavonoids because they were mostly polar compounds. Meanwhile, saponins, flavonoids, steroids, and triterpenoids were found in the ethanol extractant. As a universal solvent, ethanol can attract polar, semipolar, and nonpolar compound; hence, a great variety of compound can be extracted in this solvent (Bankova et al., 2021). Meanwhile, water is a polar solvent and tends to dissolve polar compounds. Description: *based on Materia Medica Indonesia. The data were presented in mean ± SD, n = 3.
Determination
EEP
SDPNW
SDPW
Standard Requirements*
Ethanol-Soluble Extract Content (%)
24.44 ± 0.73
83.99 ± 0.03
78.76 ± 0.04
Not less than 16%
Water-Soluble Extract Content (%)
7.96 ± 0.05
89.92 ± 0.05
88.52 ± 0.07
Not less than 4%
In addition, some specific parameters were examined for any propolis samples (Bankova et al., 2019). The content of matter soluble in ethanol was found to be 70% (balsam content), which is acceptable as suggested by the International Commission (IHC), which requires a minimum value of 45%. For comparison, Brazilian legislation determines a minimum of 35% ethanol extractable substances and a maximum of 25% wax (Bankova et al., 2019).
3.4 Non-Specific quality parameter Determination test
Table 6 shows a non-specific quality parameter for the propolis extracts. The EEP had the highest total ash content of the three. The value is still aligned with the standard regulation of Materia Medica Indonesia, which requests a maximum value of 16%. Nevertheless, it has not reached the International Honey Commission (IHC), which requests a maximum value of 5% (Falcão et al., 2013). The total ash content indicates the content of inorganic compounds. Furthermore, other non-specific quality parameters like water content (5.29% ± 0.10), loss on drying (8.04% ± 1.81), total ash content (6.88% ± 0.43), water-soluble ash content (2.72% ± 0.24), acid-insoluble ash content (0.08% ± 0.01), and residual solvent (0.54%) were in line with the requirement. The data were presented in mean ± SD, n = 3.
Non-Specific Quality Parameter
EEP
SDPNW
SDPW
Requirement
Total ash content (%)
6.88 ± 0.43
0.65 ± 0.00
0.80 ± 0.00
≤16%
Acid-insoluble ash content (%)
0.08 ± 0.01
0.06 ± 0.01
0.00 ± 0.00
≤0.10%
Water content (%)
5.29 ± 0.10
5.11 ± 0.01
5.12 ± 0.01
≤10%
Loss on drying (%)
8.04 ± 1.81
3.88 ± 0.02
3.65 ± 0.03
≤10%
Residual solvent (%)
0.54 ± 0.00
0.00 ± 0.00
0.00 ± 0.00
≤1%
The non-specific parameters of spray dry propolis samples SDPNW and SDPW were lower than EEP. The ash content is a measure of inorganic impurities in the extract (typically sand, nickel, aluminum, silicon, sodium, and other physiologic minerals) and also heavy metal (Hg, Cd, As, and Pb) contamination, which can cause different kinds of problems (Bankova et al., 2019). The goal of figuring out the ash content is to get an idea of the internal and external mineral content from the beginning of the process until the extracts are made. This is done by heating the extract until organic compounds and their derivatives are formed, and then evaporating it until only mineral and inorganic elements are left. The total ash content of each propolis extract indicates that the extract still contained some minerals, while the acid-insoluble ash content indicates the presence of residual and or other impurities.
The loss on drying implies the amount of water and volatile compounds in the extracts. This parameter is determined by drying the sample in an oven at 105 °C and calculating the weight loss using the gravimetric method. At this temperature, water and volatile compounds like essential oils and thermolabile compounds are exhausted. The content of these components should be well controlled since they affect the stability of the extract against microbe contamination. In this research, the applied extraction method could provide the propolis extracts with the characteristics that fulfill the Indonesian regulation standard (about 10%) (The Indonesian Food and Drug Authority, 2019).
3.5 Metal content
Indonesian standard regulations concern four metals in products for foods and drugs. The metals include Hg, Cd, As, and Pb content with values of less than 0.50 ppm, 0.30 ppm, 5 ppm, and 10 ppm, respectively (The Indonesian Food and Drug Authority, 2019). Therefore, all the propolis extracts examined fulfill the standard requirements. The plants and soil around stingless beehives, where bees collect resins, buds, pollen, nectar, and minerals, may reveal the main paths of heavy metals in the propolis. Since stingless bees may gather wax, resin, nectar, and minerals from plants and soil over a distance of a few hundred meters from their colonies, the low heavy metal level in propolis reflects low heavy metal pollution in plants, soil, and the atmosphere in the area where the propolis was obtained. This supported the idea that heavy metal levels can be utilized to monitor environmental contamination, as beehives in densely populated areas with substantial industrial or agricultural activity have high heavy metal contents (Abdullah et al., 2020).
3.6 Microbial contamination
Determination of microbial contamination of EEP obtained a TPC of ≤ 101 colonies/g and YMN of 5.4 × 101. Meanwhile, The SDPNW and SDPW obtained 1 × 10 CFU/g TPC and YMN. These results fulfilled the requirements of Indonesian regulation (BPOM Regulation Number 32 of 2019), where TPC (≤1 × 104 CFU/g) and YMN (≤1 × 103 CFU/g) (The Indonesian Food and Drug Authority, 2019). TPC is one of the hygiene and sanitation process indicators used to suspect the microbiological quality of a food product. The TPC must be kept as small as possible, despite the fact that these microbes are not harmful to human health. However, under certain conditions, they can transform into harmful microbes. Meanwhile, Candida and mold count plate are parameters that indicate the number of yeast molds present in a sample. Mold is a group of multicellular filamentous fungi. Filament is a distinguishing morphological feature of fungi that displays cotton-like fibrous colonies in order to differentiate them from yeast. Therefore, it is necessary to ensure that the raw materials used are clean (Directorate General of Pharmaceutical and Medical Devices, 2017).
3.7 Determination of TFC and TPC
Table 7 shows the total flavonoid and phenolic content of the EEP. The total flavonoid content of microencapsulated propolis powder without wax and with wax was 0.31%±0.01 and 1.59%±0.01, respectively. Herein, quercetin served as the reference standard, and AlCl3 was added as a shear reagent during the colorimetric process to determine the amount of total flavonoid content. This is because AlCl3 forms a complex with a flavone or flavonol group, a keto group on C-4, and a hydroxy group on C-3 or C-5 (Pratami et al., 2018). Meanwhile, Folin-Ciocalteu was used to measure TPC. Phenolic compounds react with the Folin reagent to form a blue complex. Gallic acid is a natural and stable phenolic that can react with the Folin-Ciocalteu reagent to form a stable blue molybdenum-tungsten complex. UV–VIS spectrophotometers can detect the product, and color intensity is proportional to phenolic ion concentration (Pratami et al., 2018). These results showed that the highest total flavonoid content was in the sample of microencapsulated propolis powder without wax compared to microencapsulated propolis powder with wax and EEP. The encapsulation technique could prevent the loss of chemical compounds in propolis. The process conditions, however, have a significant impact on how well an encapsulation process works. The data were presented in mean ± SD, n = 3.
Samples
Total flavonoid content (%)
Total phenolic content (mg GAE/g)
EEP
1.46 ± 0.0200
193.000 ± 1.5400
SDPNW
1.59 ± 0.0058
98.0821 ± 0.0465
SDPW
0.31 ± 0.0058
83.9082 ± 0.0442
Phenolic and flavonoid compounds are reported as the main substances inhibiting α-glucosidase and ACE (Yang et al., 2021). Phytochemical screening indicates the high phenolics content of the propolis extracts. Here, polyphenols are expected to be a major compound because they are widely distributed and chemically diverse secondary metabolites produced by plants at various stages of development. Polyphenols have an aromatic ring and hydroxyl functional groups. Flavonoids are the most abundant C6-C3-C6 phenolics. Among them are chalcones, flavones, flavonols, flavanones, isoflavonoids, anthocyanidins, and flavanols (catechins and tannins). Simple phenols, phenolic acids, coumarins, xanthones, stilbenes, and lignins are non-flavonoids. Two types of phenolic acids are C6-C1 benzoic acid and C6-C3 cinnamic acid. Propolis's chemical composition contains phenolics from various classes, including glycoside phenolic compounds, complicating their analysis (Bankova et al., 2019). Propolis contains 5% organic compounds, which are primarily made up of carboxylic acids, terpenoids, steroids, hydrocarbons, sugars, alkaloids, flavonoids, phenols, vitamins, amino acids, ketones, proteins, and other substances. Zullkiflee et al summarized flavonoids, polyphenols, carboxylic acids, quercetins, fatty acids, cinnamic acid, esters, and terpenoids as the most significant biologically active components in propolis. These include pinocembrin, galangin, carboxylic acids, caffeic acid, caffeic acid phenethyl ester (CAPE), saponin, phorbol, naringenin, gallic acid, naringin, benzoic acids, amino acids, apigenin, coumaric acid, steroids, vitamins, reducing sugar, and essential oils (Zullkiflee et al., 2022).
3.8 α-Glucosidase enzyme inhibition activity test
In vitro hypoglycemic effect of Indonesian propolis extract by α-glucosidase inhibition is shown in Fig. 2. The inhibition activity test used acarbose as a positive control, and it was found that it gave an IC50 of 53.36 ppm against an active compound inhibiting the α-glucosidase enzyme. Meanwhile, the enzyme inhibition activities of EEP, SDPNW, and SDPW gave IC50 values of 60.15, 56.48, and 68.22 ppm, respectively. Among the extracts, the SDPNW showed the highest α-glucosidase enzyme inhibition activity and a comparative result with acarbose (Fig. 2). It is expected that the quite significant phytochemicals, particularly TPC and TFC, contained in SDPNW are responsible for this kind of activity.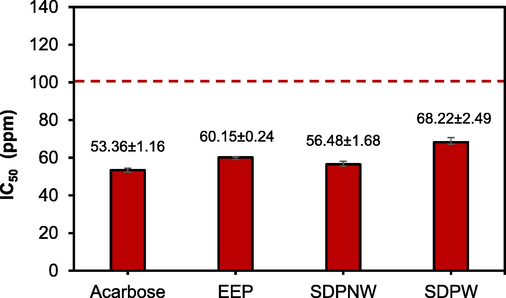
The IC50 of the propolis extracts inhibition of α-glucosidase enzymes.
The IC50 of SDPNW was greater than that of EEP and SDPW. This is because the total flavonoid content of SDPNW was greater than that of EEP and SDPW, with flavonoid contents of 1.59% > 1.46% > 0.31%, respectively. Flavonoids contained in propolis samples could reduce blood glucose levels through their ability to decrease glucose absorption. This is because they work synergistically with the action mechanisms of other anti-diabetic drugs (Gao et al., 2018). Research shows that flavonoid compounds can lower blood sugar levels, increase insulin sensitivity, and inhibit the action of the α-glucosidase enzyme (Al-Ishaq et al., 2019). Since α-glucosidase enzymes are involved in the digestion of carbohydrates, inhibiting them would cause a delay in the absorption of glucose and a reduction in postprandial hyperglycemia (Kerbab et al., 2019). In conclusion, propolis samples (SDPNW, EEP, and SDPW) have the potential to be antidiabetic.
Nigerian propolis (NP) has been reported to inhibit α-glucosidase enzymes with an IC50 value of 51.1 ± 3.5 ppm. Several active compounds including 3,8-dihydroxy-9-methoxy-pterocarpan and 8-prenylnaringenin have shown strong IC50 inhibitors of α-glucosidase with values lower than those of acarbose (IC50 = 5.0 and 5.2 g/mL, respectively) (Alaribe et al., 2021). In this study, the Indonesian propolis showed different activities compared with that, showing that the variation in the botanical source and phytogeographic region affect the potential activities of propolis. These lead to a variety in the chemical composition contained in the propolis, which affects its α-glucosidase inhibitory activity (Pujirahayu et al., 2019). For example, the research from Pujirahayu et al. discovered that the five cycloartane-type triterpenes isolated as the primary substances in T. sapiens propolis have strong anti α-glucosidase inhibitory activity. It was found that the functional groups of triterpenes of the cycloartane type significantly affected inhibition. Comparing the five isolated compounds' structures and inhibitory effects on α-glucosidase showed that the functional groups on cycloartane-type triterpenes had a significant impact on the inhibition.
3.9 ACE (Angiotensin converting Enzyme) inhibition test
The standard curve for hippuric acid was made to obtain a linear equation y = 0.0494x + 0.2472 (R2 = 0.9983) to calculate the concentration of the test and control solutions. An ACE inhibition activity test of the samples was conducted using captopril as a positive control. Captopril and the samples could inhibit the formation of hippuric acid from the breakdown reaction between Hippuryl-L-Histidyl-L-Leucine (HHL) and ACE substrates. Meanwhile, the hippuric acid formed was measured for absorption using a UV–Vis spectrophotometer at a maximum wavelength of 228.0 nm. The IC50 of captopril as a positive control and propolis samples against ACE are shown in Fig. 3.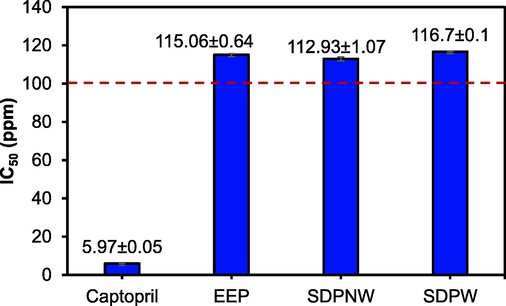
IC50 of the propolis extracts against ACE.
ACE regulates hypertension and related diseases by indirectly raising blood pressure. ACE inhibition is used as an important hypertension therapeutic approach. Indonesian propolis inhibited ACE completely at concentrations of 334.71 ppm (EEP), 366.49 ppm (SDNPW), and 360.82 ppm (SDNPW). Osés SM et al (2020) studied the ACE inhibitory activity of 10,000 ppm European propolis samples and found it ranged from 78 to 95% (Osés et al., 2020). Gargouri et al (2019) found that Tunisian propolis inhibits ACE by 90% (Gargouri et al., 2019).
Propolis had a significantly higher percentage of ACE-inhibitory activity than other natural products (Terminalia chebula fruit, branches of Piper umbellatum, and Adhatoda vasica Nees), allowing for effective hypertension control, and lowering the risk of cardiovascular disease (Kumar et al., 2011; Osés et al., 2020). Captopril-related antihypertensive drugs are common ACE inhibitors. Synthetic ACE inhibitors cause skin rashes, coughing, and angioedema. Natural products that inhibit ACE should be studied. Some studies have focused on flavonoids, which inhibit ACE and may treat hypertension. According to Osés et al (2020), flavanols, in conjunction with other propolis components and p-coumaric acid, were responsible for potential antihypertensive activity (Osés et al., 2020).
4 Conclusion
Propolis extracted from the stingless bee T. sapiens collected from South Sulawesi, Indonesia, produced by Tetragonula sapiens—ethanolic extract of propolis (EEP), spray-dry propolis without wax (SDPNW), and spray-dry propolis with wax (SDPW)—has been evaluated for its potential activity as an antidiabetic and antihypertensive. All the standardized extracts showed activity for inhibiting the α-glucosidase enzyme and the Angiotensin Converting Enzyme (ACE), respectively. The specific and non-specific quality evaluations demonstrate that the propolis extracts satisfy the standards for extract quality according to the Indonesian Pharmacopoeia's Formularies for Herbal Medicine and Propolis Standard Research. This result could be one of the guidelines for the Indonesian National Standard (SNI) of the accepted characteristics of propolis. Further pharmacodynamic studies must be done in order to investigate the potency of propolis in preventing diabetes and controlling high blood pressure in in vivo tests.
Acknowledgment
The authors would like to thank the National Research and Innovation Agency for laboratory equipment facilities, as well as researchers and technicians from Research Center for Vaccine and Drugs, Research Organization for Health, National Research and Innovation Agency (BRIN), for their support and assistance in the implementation research and also ELSA BRIN. This project was supported by Researchers Supporting Project number (RSP2023R230) King Saud University, Riyadh, Saudi Arabia.
Funding
This research was funded through the PUTI Q1 research scheme awarded by Universitas Indonesia to SF, grant number NKB-438/UN2.RST/HKP.05.00/2022.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Phytochemicals, mineral contents, antioxidants, and antimicrobial activities of propolis produced by Brunei stingless bees Geniotrigona thoracica, Heterotrigona itama, and Tetrigona binghami. Saudi J. Biol. Sci.. 2020;27:2902-2911.
- [CrossRef] [Google Scholar]
- Angiotensin-converting enzyme inhibitory effects by plant phenolic compounds: a study of structure activity relationships. J. Agric. Food Chem.. 2013;61:11832-11839.
- [Google Scholar]
- Nigerian propolis: chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res.. 2021;35:3095-3099.
- [CrossRef] [Google Scholar]
- Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules. 2019;9:1-35.
- [Google Scholar]
- Potential role of propolis in the prevention and treatment of metabolic diseases. Plants. 2021;10:1-14.
- [CrossRef] [Google Scholar]
- Standard methods for Apis mellifera propolis research. J. Apic. Res.. 2019;58:1-49.
- [CrossRef] [Google Scholar]
- Propolis and its potential against SARS-CoV-2 infection mechanisms and COVID-19 disease. Biomed. Pharmacother.. 2020;131:1-14.
- [Google Scholar]
- Pharmacopoeias Formularies Herbal Medicine (II. ed.). Jakarta: Ministry of Health Republic Indonesia; 2017.
- A proposal for physicochemical standards and antioxidant activity of Portuguese propolis. J. Am. oil Chem. Soc.. 2013;90:1729-1741.
- [Google Scholar]
- The beneficial effect of Indonesian propolis wax from Tetragonula sp. as a therapy in limited vaginal candidiasis patients. Saudi J. Biol. Sci.. 2020;27:142-146.
- [CrossRef] [Google Scholar]
- An overview of COVID-19 in people with diabetes: pathophysiology and considerations in the inpatient setting. Diabet. Med.. 2021;38:1-11.
- [Google Scholar]
- Serum antioxidant parameters are significantly increased in patients with type 2 diabetes mellitus after consumption of Chinese propolis: a randomized controlled trial based on fasting serum glucose level. Diabetes Ther.. 2018;9:101-111.
- [Google Scholar]
- Evaluation of bioactive compounds and biological activities of Tunisian propolis. Lwt. 2019;111:328-336.
- [Google Scholar]
- Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes. Metab. Res. Rev.. 2020;36:1-11.
- [Google Scholar]
- Halimium halimi folium: From the chemical and functional characterization to a nutraceutical ingredient design. Planta Med.. 2019;85:1024-1033.
- [Google Scholar]
- α-glucosidase inhibitors from plants: a natural approach to treat diabetes. Pharmacogn. Rev.. 2011;5:19-29.
- [Google Scholar]
- Selection of discrimination marker from various propolis for mapping and identify anti Candida albicans activity. AIP Conf. Proc.. 2018;1933
- [CrossRef] [Google Scholar]
- Microencapsulation of propolis by spray drying: a review. Dry. Technol.. 2020;40:1083-1102.
- [CrossRef] [Google Scholar]
- Antihypertensive effects of Brazilian propolis: identification of caffeoylquinic acids as constituents involved in the hypotension in spontaneously hypertensive rats. Biol. Pharm. Bull.. 2005;28:1909-1914.
- [Google Scholar]
- Phenolic profile, antioxidant capacities and enzymatic inhibitory activities of propolis from different geographical areas: needs for analytical harmonization. Antioxidants. 2020;9:1-16.
- [CrossRef] [Google Scholar]
- Chemical profile and antioxidant capacity of propolis from Tetragonula, Lepidotrigona, Lisotrigona, and Homotrigona stingless bee species in Vietnam. Molecules. 2022;27:1-12.
- [CrossRef] [Google Scholar]
- Phytochemical profile and sntioxidant activity of propolis ethanolic extract from Tetragonula bee. Pharmacogn. J.. 2018;10:73-80.
- [CrossRef] [Google Scholar]
- Microencapsulation optimization of propolis ethanolic extract from Tetragonula spp using response surface methodology. Int. J. App. Pharm. 2020;12:197-206.
- [CrossRef] [Google Scholar]
- Standardization and antioxidant activity of propolis extract for SARS-CoV2 infection therapy. J. Ilmu Kefarmasian Indones.. 2021;19:272-280.
- [Google Scholar]
- α-Glucosidase inhibitory activity of cycloartane-type triterpenes isolated from Indonesian stingless bee propolis and their structure–activity relationship. Pharmaceuticals. 2019;12:1-12.
- [CrossRef] [Google Scholar]
- Phenolic compounds, antioxidant activity and in vitro inhibitory potential against key enzymes relevant for hyperglycemia and hypertension of commonly used medicinal plants, herbs and spices in Latin America. Bioresour. Technol.. 2010;101:4676-4689.
- [CrossRef] [Google Scholar]
- The Effects of stingless bee (Tetragonula biroi) honey on streptozotocin-induced diabetes mellitus in rats. Saudi J. Biol. Sci.. 2020;27:225-230.
- [CrossRef] [Google Scholar]
- Efficacy of Brazilian green propolis (EPP-AF®) as an adjunct treatment for hospitalized COVID-19 patients: a randomized, controlled clinical trial. Biomed. Pharmacother.. 2021;138:111526
- [Google Scholar]
- IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract.. 2022;183:109119
- [Google Scholar]
- The Indonesian Food and Drug Authority, 2019. Indonesian FDA Decree No 32 Year 2019 about Requirements for Safety and Quality of Traditional Medicines, The Indonesian Food and Drug Authority, Jakarta.
- LC-MS analysis and effects of Malaysian propolis on insulin, glucagon, pancreas and oxidative stress status in streptozotocin-induced diabetic rats. J. Med. Biomed. Res.. 2017;16:15-27.
- [Google Scholar]
- Metabolic syndrome and COVID-19 mortality among adult black patients in New Orleans. Diabetes Care. 2021;44:188-193.
- [Google Scholar]
- Comparative study of inhibition mechanisms of structurally different flavonoid compounds on α-glucosidase and synergistic effect with acarbose. Food Chem.. 2021;347:129056
- [Google Scholar]
- Propolis in metabolic syndrome and its associated chronic diseases: a narrative review. Antioxidants. 2021;10:1-20.
- [CrossRef] [Google Scholar]
- Propolis: its role and efficacy in human health and diseases. Molecules. 2022;27:1-20.
- [CrossRef] [Google Scholar]







