Translate this page into:
In-vitro phytopharmacological and anticancer activity of Loranthus Longiflorus Desv. Var. Falcatuskurz against the human lung cancer cells
⁎Corresponding author. hak3962@sch.ac.kr (H-J Kim)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In this study, we evaluated anticancer activity of methanol extract and purified compound from Loranthus Longiflorus against Human lung cancer cells (A-549), human cervical carcinoma (HeLa) and human hepatocellular carcinoma cell line (HepG2). Cytotoxicity of Loranthus Longiflorus was investigated using MTT assay. Doxorubicin was used as the positive control. MTT assay showed activity against all three tested cell lines. The cytotoxicity activities expressed as percentage of cell viability and the compound was effective on HepG2, HeLa and A-549 cell lines. Cytotoxicity of methanolic extract of LLF against A-549 cell line was measured and the IC50 value of methanol extract of LLF was 17 ± 1.5 µg/ml. In HeLa cell line, the IC50 value of methanol extract of LLF was16 ± 1.0 µg/ml. Cytotoxicity of methanolic extract of LLF against HepG2 cell line was also evaluated. IC50 value of Doxorubicin (standard) was 11 ± 1.0 µg/ml and IC50 value of methanol extract of LLF was 18 ± 0.5 µg/ml. Similarly cytotoxicity showed a significant activity of the compound LLF on HepG2, HeLa and A-549 cell lines. The IC50 values of compound were 13.5 ± 1.5 µg/ml, 15 ± 1.5 µg/ml, 17 ± 0.5 µg/ml against A-549, HeLa and HepG2 cell lines. The present findings confirmed cytotoxic effect of methanol extract and purified compound from Loranthus Longiflorus against A-549, HeLa and HepG2 cell lines.
Keywords
Medicinal plants
Herb
Anticancer
Cell lines
Cytotoxicity
1 Introduction
Anticancer drugs are derived from various medicinal plants and these drugs have various advantages. Also, microbial sources of anticancer molecules such as, doxorubicin and dactinomycin were derived from microorganisms and vinblastine, taxanes, topotecan, were derived from various medicinal plants. In recent years, more than 250,000 plants were screened to identify potent molecules and some of these molecules were pharmacologically and chemically investigated and characterized. Tropical rainforest has lot of potential and remains untapped and could be analyzed. Hence, the search of novel cytotoxic substances from microbial, plant, marine and natural sources continues with the scientific collaboration within their field among scientists worldwide (Cragg and Newman, 1999; Antonisamy et al., 2015; Balamurugan, 2015; Kannan and Agastian, 2015). Very few anticancer molecules were derived from natural sources because cancer involves highly complex symptoms and signs. Medicinal plants have various applications to treat infectious, skin disorders, parasitic, inflammatory, viral and parasitic diseases. These all are the one of the symptoms of cancer disease reported. Hence, medicinal plants can be used to treat these symptoms in cancer patients (Popoca et al., 1998; Rathi et al., 2015). In India, medicinal plants have been used among various populations to treat various diseases and have not been completely explored (Valsalam et al., 2019). Also, these group of people still used medicinal plants to treat various diseases and disorders.
Tumor cell grow rapidly and these uncontrolled growth is a common property of tumour cells. Medicinal plants have the property to control the growth of tumor cells. Hence analysis of cancer cell growth inhibiting mechanism is very useful to understand the anticancer property of medicinal plants. Analysis and identification of novel molecules with anti-tumor activity is useful for the development of anti-cancer drug (Kang et al., 2000). In the preset study, we have isolated and characterized pharmacological properties of compounds from medicinal plant, Loranthus longiflorus. Previously, Swamy and Tan (2000) characterized anticancer molecule from Nigella satia L. and this molecule showed inhibiting activity against hepatocellular carcinoma cell line (Hep G2). Cancer cause severe mortality among human population. There are number of cancer types have been reported and the most common cancers are liver, prostate, colorectal, lung and oesophagus types in men (Rajkumari et al., 2019; Gurusamy et al., 2019). In women population, cervical, colorectal, stomach, lung and breast cancer are very common. Lung cancer is the most common types of cancers in human being and causes second most causality in human population. It has been reported that cancer pose serious threat to human being and 1/3 of human population pose risk for developing any one types of cancer during their life time. Medicinal plants have various bioactivities to inhibit the growth of cancer cells. More than 80% of diseases can be effectively treated using herbal medicine (Etkin, 1981; Arokiyaraj et al., 2015).
Medicinal plants have been used to extract various anticancer molecules. Active compounds extracted from Calendula officinalis have anticancer properties (Eva et al., 2006). Lung cancer is most commonly reported disease in male individuals and also reported in females cause more than 25% cancer death in the U.S alone (Cardenal, 1999). In every year more than 150,000 US citizens are suffered by Lung cancer and are very serious diseases throughout the world. Treatment of cancer is mainly performed using chemotherapy; however it provides half relief to the patients (Sandler, 2000). Also, herbal medicines are frequently used to treat cancer (Sadava, 2002). Alternative medicines, including Chinese medicines have been used to treat various cancers at various compositions. In the present study, Loranthus Longiflorus was subjected for anticancer secondary metabolites against human lung cancer cell line, human cervical carcinoma and human hepatocellular carcinoma cell line.
2 Materials and methods
2.1 Medicinal plant
In this study Loranthus longiflorus was collected and dried under shade for a week, followed by mechanical grinding. The powdered raw material was used for the preparation of compounds. Methanol was used for the extraction of phytochemicals by standard method and the compounds were purified by using Thin Layer Chromatography (Fabricant and Farnsworth, 2001; Phrompittayarat et al., 2007).
2.2 Preparation of Dulbecco's modified eagle medium (DMEM) medium
Suspended 13.3 g of DMEM medium in 900 mL double distilled water was transferred into a beaker and the temperature was maintained between 15 and 30 °C. Then sodium bicarbonate (3.0 g) was added and the pH of the solution was adjusted using 1 N NaOH/1N HCl after filtration. The final pH of the solution ranged between 7.60 and 8.20 after the addition of sodium bicarbonate. The filter sterilized medium was used for cell culture.
2.3 Balanced salt solution (BSS) preparation
BSS solution (1000 mL) was prepared by mixing 0.008 g sodium chloride, 0.4 g potassium chloride, 0.140 mg calcium chloride, 0.1 g magnesium chloride, 0.060 g disodium hydrogen phosphate, 0.060 g potassium dihydrogen phosphate, 1.0 g glucose and 0.350 g sodium hydrogen carbonate. Finally the solution was mixed and made up to 1 L using double distilled water.
2.4 Cell culture
In this study cancer cell lines such as; human cervical carcinoma (HeLa) and human hepatocellular carcinoma cell line (HepG2) were obtained from National Center for Cell Sciences (NCCS), Pune. These three cell lines were carefully maintained in DMEM medium and further 2 mM l-glutamine was supplemented. The concentration of BSS was adjusted to maintain 0.1 mM non essential amino acids, 1.5 g/L glucose, 1.5 g/L Na2CO3, 10 mM (4-(2-hydroxyethyl)-1-piperazineethane sulfonic acid) (HEPES), 2 mM l-glutamine, 1 mM sodium pyruvate and 10% fetal bovine serum. Penicillin and streptomycin were added at the concentration of 100 IU/100 µg and these were adjusted to 1 mL/L level. Then the cell lines were carefully maintained using 5% CO2 in a humidified CO2 chamber (Wahyu et al., 2013).
2.5 Morphological analysis of cell lines
The selected three cancer cell lines were cultured using cover slips and the density was 1 × 105 cells/cover slip. The cell lines were incubated with sample at various concentrations, finally fixed using acetic acid and ethanol solution at 1:3 (v/v) ratio. Then all cover slips were carefully mounted on clean slides for morphometric analysis. These results were subjected for the analysis of morphological changes. An inverted microscope (Nikon, Japan) was used for the analysis of cell morphology at 40× magnification.
2.6 Cytotoxicity analysis
The inhibitory effect of LLF (IC50) value was analyzed by performing MTT assay by standard method. All three cancer cell lines were cultured using microtitre plates to reach about 90% confluence after 48 h incubation. Then the used culture medium was carefully replaced with fresh medium containing anticancer compounds at various concentrations and incubated for about 48 h. Then the culture medium was separated and 3-(4, 5-dimethylthiozol-2-yl)-3, 5-diphenyl tetrazolium bromide (100 µL) was loaded and further kept for 4 h at 37 °C. Then DMSO (50 µL) was carefully added with the wells and this solubilized formazan crystals. Then the OD of the sample was read at 620 nm using a micro titre plate reader and the viability (%) was calculated.
2.7 Apoptosis and fluorescence microscopy
In this study, fluorescence microscope was used for the analysis of apoptosis. Acridine orange (AO) (100 mg/mL) and ethidium bromide (100 mg/mL) (EtBr) was prepared and mixed. From this mixture, 1 µL dye was taken with this 0.9 mL cancer cell lines were added on cover slips at aseptic conditions. After this pretreatment cancer cells were retained after washing with phosphate buffered saline (pH 7.2) and it was stained with staining solution (AO/EtBr). It was incubated for 120 s, further it was washed with PBS twice and viewed under a fluorescence microscope at 400× magnification. Likewise the cancer cell lines were kept on cover slip in a microtitre plant and treated the cells with sample for one day. Then 0.2% triton X-100 was added and incubated for 3 min with DAPI (10 μL) and mounted. The cells were finally observed using a fluorescent microscope.
3 Results
The cytotoxicity effect of methanol extract and isolated compound from LLF were studied using cancer cell lines HeLa, A-549, and HepG2. The degree of cytotoxicity of these test compounds towards the cell lines were determined using MTT assay. The cytotoxic activity was expressed as percentage of cell viability in HepG2, HeLa and A-549 cell lines compared with the control (Fig. 1). The cytotoxicity of methanol extracts of LLF on HepG2, HeLa and A-549 cell lines were studied using MTT assay. Cytotoxicity of methanolic extract of LLF against A-549 cell line was measured. IC50 value of Doxorubicin (standard) is 9.5 ± 1.0 µg/ml and IC50 value of methanol extract of LLF is 17 ± 1.5 µg/ml (Fig. 2).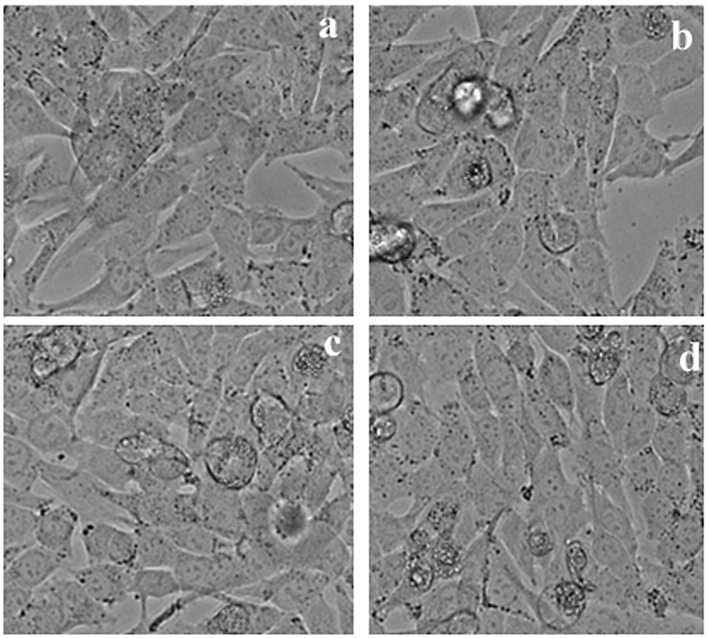
Different concentrations of methanol extract from LLF on A549 cells.
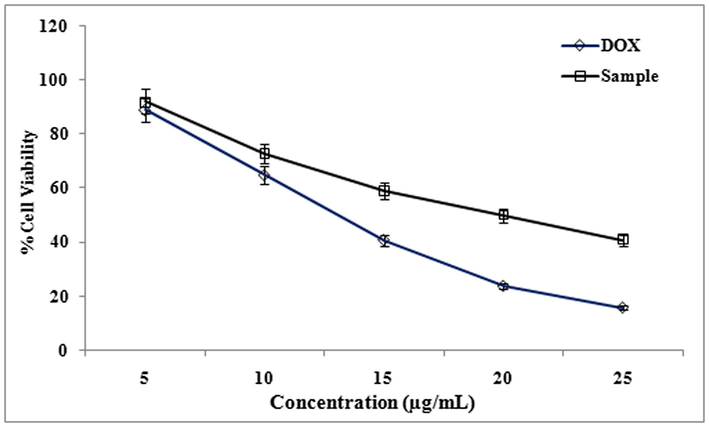
Effect of different concentrations (5 to 25 µg/ml) of methanol extract from LLF on A549 cells. The values are mean of three different experiments.
Cytotoxicity of methanolic extract of LLF against HeLa cell line was studied. IC50 value of Doxorubicin (standard) was 13 ± 1.0 µg/ml, IC50 value of methanol extract of LLF is16 ± 1.0 µg/ml. Cytotoxicity of methanolic extract of LLF against HepG2 cell line was also evaluated. IC50 value of Doxorubicin (standard) is 11 ± 1.0 µg/ml and IC50 value of methanol extract of LLF is18 ± 0.5 µg/ml. Similarly cytotoxicity showed a significant activity of the compound isolated from LLF on HepG2, HeLa and A-549 cell lines in MTT assay (Fig. 3). Cytotoxicity of compound against A-549 cell line with IC50 value of Doxorubicin (standard) was 9.5 ± 1.0 µg/ml, IC50 value of compound was13.5 ± 1.5 µg/ml (Fig. 4). Cytotoxicity of compound against HeLa cell line with IC50 value of Doxorubicin (standard) was 13 ± 1.0 µg/ml and IC50 value of compound was15 ± 1.5 µg/ml (Fig. 6). Cytotoxicity of compound against HepG2 cell line with IC50 value of Doxorubicin (standard) was 11 ± 1.0 µg/ml, IC50 value of compound was 17 ± 0.5 µg/ml (Fig. 7) The results showed that LLF methanol extract and isolated compound with different concentrations tested were varied widely and it was estimated that in concentrations between 5 µg/ml and 25 µg/ml. HeLa is more inhibited by the compound when compared to A-549 and HepG2 cell lines (Figs. 8–10).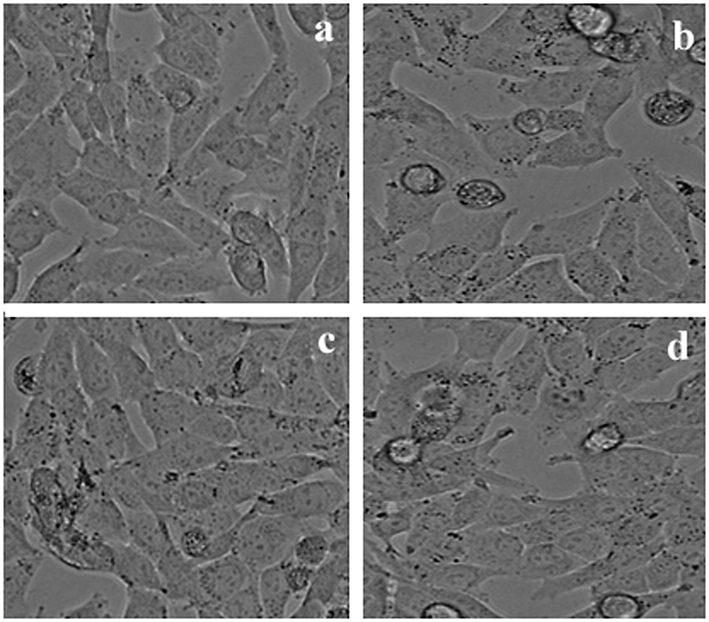
Different concentrations of Compound from LLF on A549 cells.
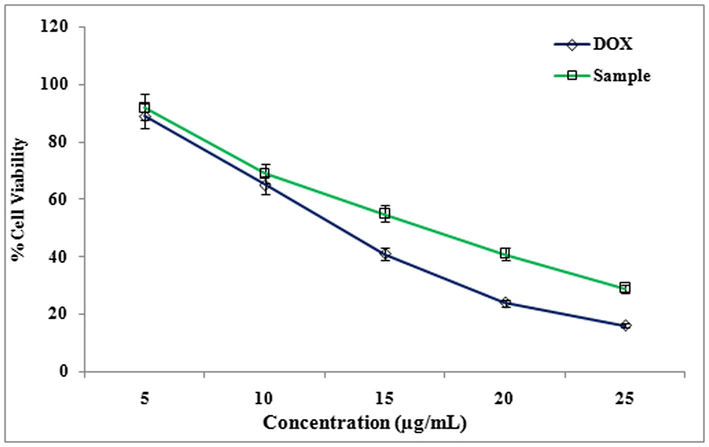
Effect of different concentrations (5 to 25 µg/ml) of Compound from LLF on A549 cells. The values are mean of three different experiments.
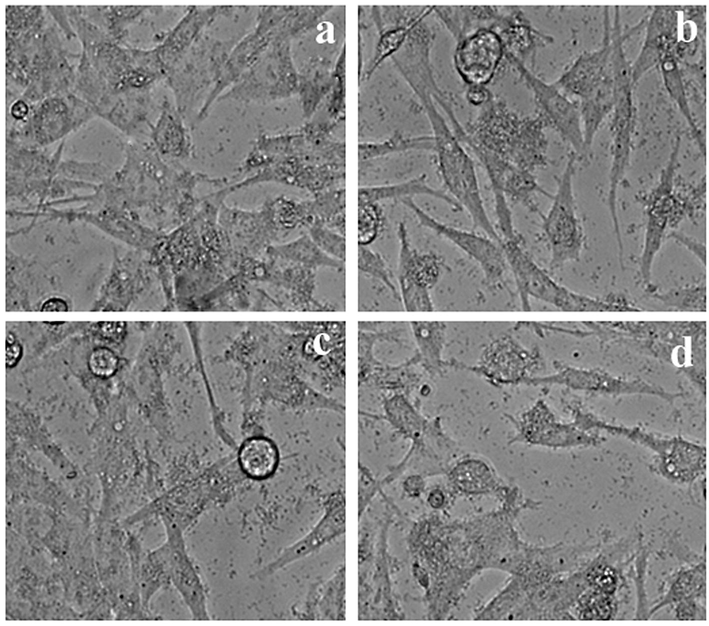
Different concentrations of Methanol extract from LLF on HeLa cells.
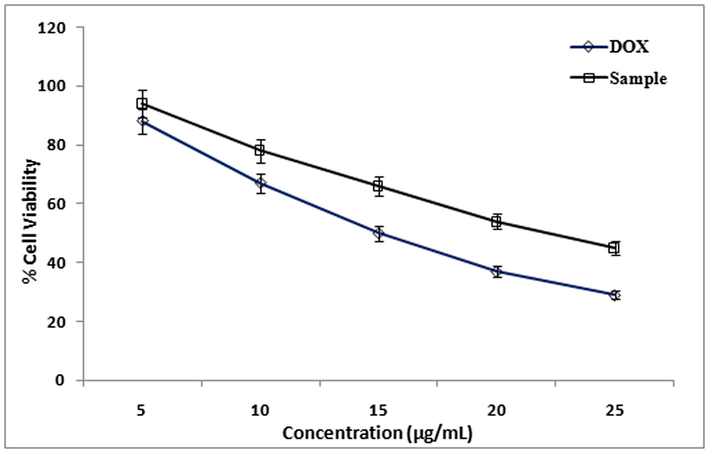
Effect of different concentrations (5 to 25 µg/ml) of Methanol extract from LLF on HeLa cells. The values are mean of three different experiments.
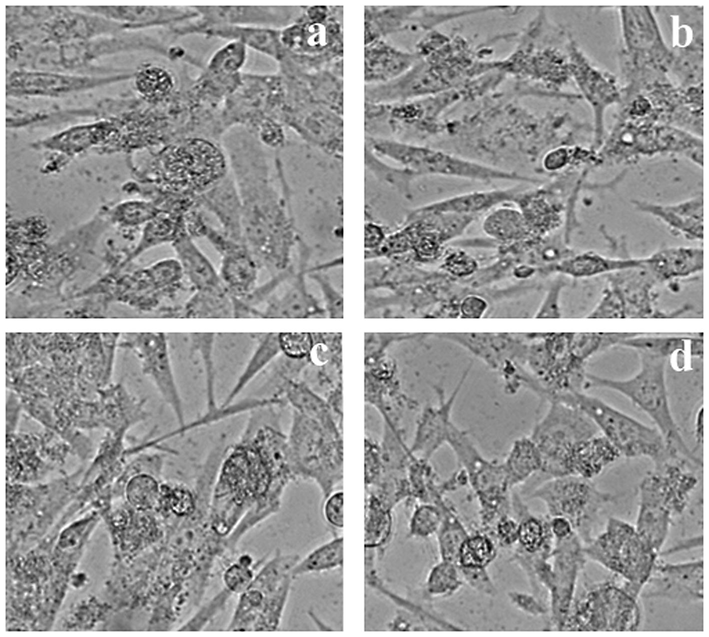
Different concentrations of Compound from LLF on HeLa cells.
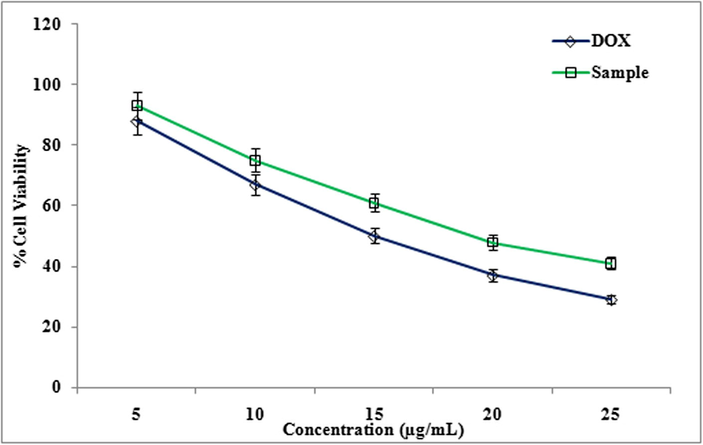
Effect of different concentrations (5 to 25 µg/ml) of Compound from LLF on HeLa cells. The values are mean of three different experiments.
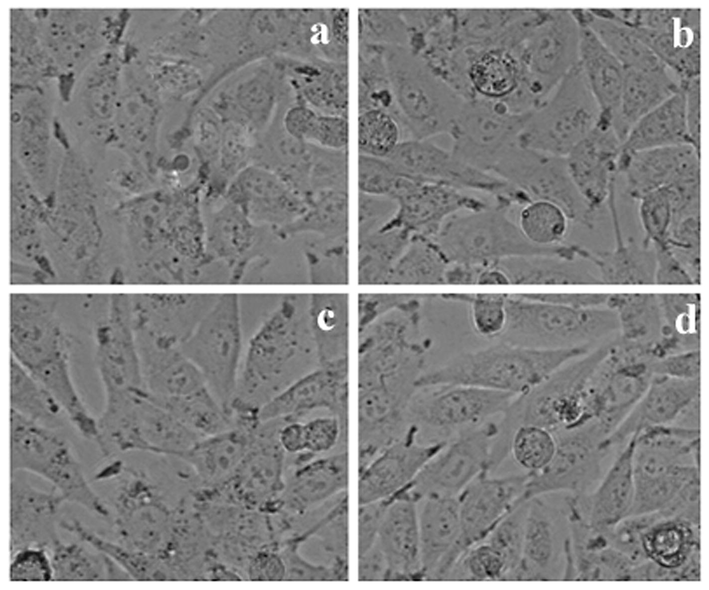
Different concentrations of Methanol extract from LLF on HepG2 cells.
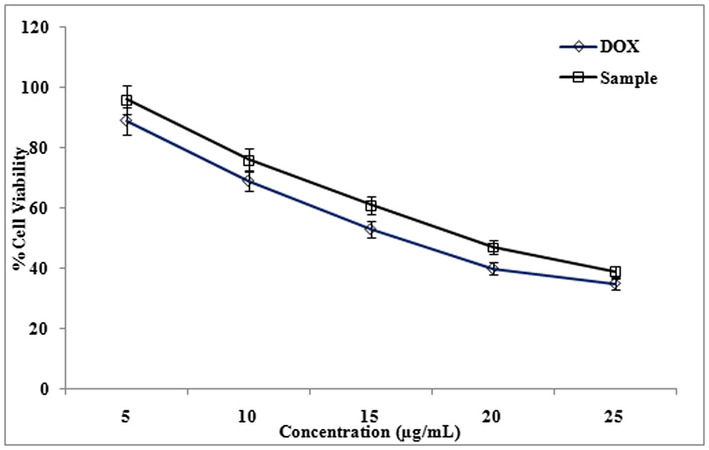
Effect of different concentrations (5 to 25 µg/ml) of Methanol extract from LLF on HepG2 cells. The values are mean of three different experiments.
4 Discussion
Medicinal plants constitute valuable bioactive secondary metabolites to treat various diseases and disorders. These herbs are easily available and relatively cheap than other sources. Medicinal plants have structural diversity with novel secondary metabolites (Amos et al., 2003). Hence, there is an increasing interest in the discovery of novel lead molecules and scientists are working closely towards validation of various traditional medicinal plants (Ameer, 2011). Many active compounds have been extracted from medicinal herbs. Loranthaceae is one of such families has been used for the extraction of various secondary metabolites (Calvin and Wilson, 2006). The therapeutic properties of from the member, Loranthaceae were greatly recognized. These medicinal plants have various biological properties, including, antitumor activity, headache treatment, immunomodulatory, anti-inflammatory, antioxidant, antiviral, antipyretic and antidiabetic activities (Iwalokun et al., 2011). In our study, the cytotoxicity effect of methanol extract and isolated compound from LLF were studied using HeLa, A-549, and HepG2 cell lines. Cytotoxicity of methanolic extract of LLF against A-549 cell line was measured. IC50 value of Doxorubicin (standard) is 9.5 ± 1.0 µg/ml. In a study, Dai et al. (2014) used various concentrations of myricanone against A549 cells for anticancer activity. Results revealed that myricanone induced potential inhibitory activity on A549 cells and the IC50 value was reported as 3.22 µg/ml. Cytotoxicity of compound against A-549 cell line with IC50 value of Doxorubicin (standard) is 9.5 ± 1.0 µg/ml, IC 50 value of compound is13.5 ± 1.5 µg/ml. In a study, Dai et al. (2014) used various concentrations of myricanone against A549 cells for anticancer activity. Results revealed that myricanone induced potential inhibitory activity on A549 cells and the IC50 value was reported as 3.22 µg/ml. Cytotoxicity of compound against HeLa cell line with IC 50 value of Doxorubicin (standard) is 13 ± 1.0 µg/ml, IC 50 value of compound is15 ± 1.5 µg/ml. HeLa is more inhibited when compared to A-549 and HepG2 cell lines. Recently, Chothiphirat et al. (2019) used Vatica diospyroides Symington extract for its anticancer potential against HeLa and SiHa cells. The bioactive compounds were extracted with various solvent and acetone extract showed activity against HeLa and SiHa cells. After the cells treated with extracts, morphological changes were observed due to the activity of hytochemicals. Acetone extract of this plant induced apoptosis in SiHa and HeLa cell lines.
In our study, cytotoxicity of methanolic extract of LLF against HepG2 cell line was also evaluated. IC50 value of Doxorubicin (standard) is 11 ± 1.0 µg/ml and IC50 value of methanol extract of LLF is18 ± 0.5 µg/ml. In a study, Al-Faifi et al (2017) evaluated genotoxic activity and cytotoxic activity of Euphorbia triaculeata extract on prostate cell line (PC-3), breast cancer cell line (MCF-7), normal breast epithelial cell line (MCF-10A) and human hepatocellular carcinoma cell line (HEPG2). And, this plant showed activity against MCF-7 cell lines and marginal effect was reported on other used cell lines. In recent years, the biochemical property of medicinal plants from the family, Loranthaceae has been studied. From these medicinal plants the phytochemicals such as, flavonoids, lectins, alkaloids, polypeptides, arginine, polysaccharides, terpenoids and ssteroids. These phytochemicals show various biological activity including, cytotoxicity (Badr et al., 2013). The medicinal plant, L. ferrugineus showed cytotoxic and antiviral properties. It was reported that the aqueous extract from the plant showed blood pressure regulating activity. The phytochemical composition of L. ferrugineus revealed the presence of condensed tannins and flavonoids. Also, three flavonol compounds were characterized from the organic solvent faction of L. ferrugineus and also flavonol glycoside 4″-O-acetylquercitrin identified from this plant (Lohézic-Le Dévéhat et al., 2002). L. ferrugineus was reported for anticancer and antioxidant properties. This medicinal plant also has more quantities of lectin, which was known for anticancer activity. Also, various medicines were isolated and characterized from this medicinal plant. The phytochemical composition of this medicinal plant showed potent anticancer activity (Rostock et al., 2005; Badr et al., 2013; Ameer, 2011). Lohézic-Le Dévéhat et al. (2002) studied the anticancer cytotoxic properties of L. ferrugineus and reported cytotoxic effect against cancer cells.
5 Conclusions
The present finding confirmed cytotoxic effect of medicinal plant, Loranthus Longiflorus on Human Lung cancer cells (A-549), HeLa and HepG2 cells. Base on our findings, further characterization of the anticancer activities of Loranthus Longiflorus and structural characterization of active biomolecules is highly warranted.
Acknowledgements
The authors thank Loyola college for the support for the performance of the work. Authors extent their sincere appreciation to the Researchers Supporting Project (RSP-2019/108) King Saud University Riyadh Saudi Arabia. The authors Hak-Jae Kim thank the support received from Soochunhyang University research fund for this research work.
Declaration of Competing Interest
The authors of the manuscript entitled “In-vitro phytopharmacological and anticancer activity of Loranthus Longiflorus Desv. Var. Falcatuskurz against the human lung cancer cells”declared no conflict in this manuscript and publications.
References
- Evaluation of cytotoxic and genotoxic effects of Euphorbia triaculeata Forssk. extract. Asian Pacific J. Cancer Prevention: APJCP. 2017;18(3):771.
- [Google Scholar]
- Characterization of Loranthus ferrugineus cardiovascular activites. an ethnopharmacological and phytochemical investigation. Germany: LAP LAMBERT Academic Publishing; 2011. p. :288.
- Hypotensive activity of the ethanol extract of pavetta crassipes leaves. Biol. Pharm. Bull.. 2003;26(12):1674-1680.
- [Google Scholar]
- Anti-diarrhoeal activity of friedelin isolated from Azima tetracantha Lam. in Wistar rats.South Ind. J. Biol. Sci.. 2015;1:34-37.
- [Google Scholar]
- Green synthesis of Silver nanoparticles using aqueous extract of Taraxacum officinale and its antimicrobial activity. South Indian J. Biol. Sci.. 2015;2:115-118.
- [Google Scholar]
- Loranthin: a new polyhydroxylated flavanocoumarin from plicosepalus acacia with significant free radical scavenging and antimicrobial activity. Phytochem. Lett.. 2013;6(1):113-117.
- [Google Scholar]
- Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Ind. J. Biol. Sci.. 2015;1:47-51.
- [Google Scholar]
- Comparative morphology of epicortical roots in old and new world Loranthaceae with reference to root types, origin, patterns of longitudinal extension and potential for clonal growth. Flora-Morphol., Distrib., Funct. Ecol. Plants. 2006;201(1):51-64.
- [Google Scholar]
- Randomized phase III study of gemcitabine-cisplatin versus etoposide-cisplatin in the treatment of locally advanced or metastatic nonsmall-cell lung cancer. J. Clin. Oncol.. 1999;17(1):12-18.
- [Google Scholar]
- Anticancer potential of fruit extracts from vatica diospyroides Symington type SS and their effect on program cell death of cervical cancer cell lines. Sci. World J.. 2019;2019:9.
- [Google Scholar]
- Discovery and development of antineoplasic agents from natural sources. Cancer Invest.. 1999;17:153-163.
- [Google Scholar]
- In vitro anticancer activity of myricanone in human lung adenocarcinoma A549 cells. Chemotherapy. 2014;60(2):81-87.
- [Google Scholar]
- A Hausa herbal pharmacopoeia: biomedical evaluation of commonly used plant medicines. J. Ethnopharmacol.. 1981;4:75-98.
- [Google Scholar]
- A new extract of the plant calendula officinalis produces a dual in-vitro effect: cytotoxic anti-tumor activity and lymphocyte activation. BMC Cancer. 2006;6(1):119.
- [Google Scholar]
- The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect.. 2001;109:69-75.
- [Google Scholar]
- Environmental friendly synthesis of TiO2-ZnO nanocomposite catalyst and silver nanomaterials for the enhanced production of biodiesel from Ulva lactuca seaweed and potential antimicrobial properties against the microbial pathogens. J. Photochem. Photobiol. B: Biol.. 2019;193:118-130.
- [Google Scholar]
- Evaluation of the possible mechanisms of antihypertensive activity of loranthus micranthus: an African mistletoe. Biochem. Res. Int. 2011
- [Google Scholar]
- Antiproliferative effect of alkaloids from Sedum sarmentosum on murine and human hepatoma cell line. J. Ethnopharmacol.. 2000;70:177-182.
- [Google Scholar]
- In vitro regeneration of a rare antidiabetic plant Epaltes divaricata L. South Ind. J. Biol. Sci.. 2015;1:52-59.
- [Google Scholar]
- Antiviral and cytotoxic activities of some indonesian plants. Fitoterapia. 2002;73(5):400-405.
- [Google Scholar]
- Comparison of various extraction methods of Bacopa monnier. Naresuan Univ. J.. 2007;15(1):29-34.
- [Google Scholar]
- Cytotoxic activity of selected plants used as antitumorals in Mexican traditional medicine. J. Ethnopharmacol.. 1998;59(3):173-177.
- [Google Scholar]
- Synthesis of titanium oxide nanoparticles using Aloe barbadensis mill andevaluation of its antibiofilm potential against Pseudomonas aeruginosa PAO1. J. Photochem. Photobiol., B: Biol. 2019
- [CrossRef] [Google Scholar]
- Hepatoprotective activity of ethanolic extract of Alysicarpus vaginalis against nitrobenzene-induced hepatic damage in rats. South Ind. J. Biol. Sci.. 2015;1:60-65.
- [Google Scholar]
- Anticancer activity of a lectin-rich mistletoe extract injected intratumorally into human pancreatic cancer xenografts. Anticancer Res.. 2005;25(3B):1969-1975.
- [Google Scholar]
- Effects of four Chinese herbal extracts on drug-sensitive and multidrug-resistant small-cell lung carcinoma cells. Cancer Chemother. Pharmacol.. 2002;49(4):261-266.
- [Google Scholar]
- Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non-small-cell lung cancer. J. Clin. Oncol.. 2000;18(1):122-130.
- [Google Scholar]
- Cytotoxic and immunopotentiating effects of ethanolic extract of Nigella satia L. seeds. J. Ethnopharmacol.. 2000;70:1-7.
- [Google Scholar]
- Biosynthesis of silver and gold nanoparticles using Musa acuminata colla flower and its pharmaceutical activity against bacteria and anticancer efficacy. J. Photochem. Photobiol., B: Biol. 2019 j.jphotobiol.2019.111670
- [Google Scholar]
- Antioxidant, anticancer and apoptosis-inducing effects of Piper extracts in HeLa cells. J. Exp. Integr. Med.. 2013;3:225-230.
- [Google Scholar]







