Translate this page into:
In vitro evaluation of antifungal activity of some agricultural fungicides against two saprolegnoid fungi infecting cultured fish
⁎Corresponding author at: Botany and Microbiology Dept., Collage of Science, King Saud University, P.O. 2455, Riyadh 11451, Saudi Arabia. ashraf812@yahoo.com (Ashraf Abdel-Fattah Mostafa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Saprolegniasis often cause a significant economic losses to fish hatchery and intensive fish industry. The treatment of saprolegnoid diseases with malachite green seems to have harmful effect and is considered as a mutagenic and carcinogenic substance. The teratogenic and carcinogenic potential of malachite green increased the necessity to find an effective alternative treatment to control the disease.
Methods
In the present study, seven fungicides used in agriculture were investigated to control Saprolegnia parasitica and S. diclina in vitro. The antifungal activity of each fungicide was compared with that of malachite green as reference fungicide using food poisoning technique. Fish toxicity of the effective fungicides was performed to detect the most applicable fungicides in fish aquarium.
Results
Four of seven fungicides were found to be effective against the two saprolegnoid fungi. Azoxystrobin and metalaxyl were the most effective fungicides inhibited fungal growth of the two saprolegnoid fungi completely at concentration of 200 ppm and 250 ppm respectively while cyazofamid and etridiazole were moderately effective. Acute toxicity assay of the two effective fungicides showed metalaxyl was low toxic to fish with LC50 of 360 ppm while azoxystrobin was 225 ppm. Hence, it was confirmed that 200 and 250 ppm of azoxystrobin and metalaxyl which completely inhibited mycelial growth of the saprolegniod fungi could be safely used for saprolegniasis control.
Conclusion
These fungicides which proved to be effective and fishery safer may be applicable as a aquatic fungicides avoiding teratogenic and carcinogenic risk of malachite green.
Keywords
Saprolegniasis
Alternative fungicides
Carcinogenic
Azoxystrobin
Metalaxyl
1 Introduction
Fish are considered as a vital source of food in the basis of its high consumption, alimental and economic value to fish farmers. The main disease in fish hatchery responsible for reduction of yield and high mortality are saprolegniasis caused by saprolegnoid fungi (Saprolegnia parasitica and S. diclina) (Earle and Hintz, 2014). Saprolegniasis of fresh water fishes can be developed at all stages of fish's life cycle form egg, larva, fingerlings, fry to independent adult (Thoen et al., 2011; Lone and Manohar, 2018). Etiological agents of saprolegniasis cause a huge mortality of fish, damage of fish hatchery and destroy intensive fish cultivation industry (Fregeneda-Grandes et al., 2007; Faruk and Anka, 2017). Saprolegniasis can be controlled by application of malachite green and other chemical fungicides as formalin (Gieseker et al., 2006; Mostafa et al., 2020), hydrogen peroxide and copper sulfate (Sun et al., 2014; Tedesco et al., 2019). Malachite green was one of the cornerstones used in treatment of fish parasites (Wang et al., 2019) and proved to be effective fungicide on farmed fish (Paul et al., 2018). So it was extensively used by aquaculture industry all over the world for several years in the obscurity of authorized veterinary medicinal alternative. Despite the proven efficiency of malachite green in the prevention of saprolegnoid fungi outbreak, its repeated applications have proven to be carcinogenic, mutagenic and teratogenic (Pierrard et al., 2012; Jindal and Sinha, 2019). Therefore, it was obsoleted in fish hatchery intended for human consumption (Mitrowska et al., 2007). Formalin was widely used as an effective fungicide with concentration of 1000–2000 ppm for controlling fungal diseases of fish in fish hatchery (Tedesco et al., 2019). Formalin was also banned in several countries as it was reported to be harmful to the user's health, accumulated to fish flesh and polluted the environment (Leal et al., 2018). Several studies demonstrated the efficiency of hydrogen peroxide with concentration ranged from 1000 to 2500 ppm to prevent fungal growth and control fungal fish diseases in aquaculture with improving water quality through oxygen dissociation (Mitchell et al., 2009; Novakov et al., 2018). Overtreatment with hydrogen peroxide may be toxic and can further damage fish tissue resulting in recurring infection (Rach et al., 2004; Small, 2004). Recently, attempts with varying success and mostly in vitro, have been made to find potentially effective treatments against the saprolegnoid fungi. Chemicals, such as saprolmycins, oridamycins, pyceze, modified chitosans and peracetic acid, have been tested. One out of 15 azole antifungal agents used in agriculture namely agriazoles (clotrimazole), appeared to be sufficiently effective against Saprolegnia spp. (Warrilow et al., 2014). In addition, a treatment with peptides derived from pseudomonas protegenes (Wang and Zhang, 2017) and copper, silver and selenium nanoparticles (Kalatehjari et al., 2015; Sadeghi & Peery, 2018) may be a promising alternatives. Natural compounds, such as essential oils and medicinal plant extracts, may also have some success in controlling Saprolegnia (Caruana et al., 2012). Recently, attempts have been made to find potentially effective fungicides against the saprolegnoid fungi. So the main goal of the present study was achieved to evaluate and assess the capability of seven agricultural fungicides conventionally used in Oomycetes control in treating Saprolegnia parasitica and S. diclina infecting fish hatchery.
2 Materials and methods
2.1 Isolation of saprolegnoid strains
Two strains of Saprolegnia spp. (S. parasitica and S. diclina) were isolated from infected tilapia fish (Oreochromis niloticus) using sesame baiting technique. The fungal strains were purified, identified according to the methods described by Willoughby (1985) and Johnson et al. (2002) and preserved in PDA slant till used.
2.2 Preparation of zoospores suspensions
Zoospores suspensions of S. parasitica and S. diclina were prepared by the method described by Diéguez-Uribeondo et al. (1994). Colony discs (7 mm) of actively growing S. parasitica and S. diclina were immersed in flasks containing (50 ml) of sterilized aquarium water supplemented with antibiotic mixture and (0.1%) tween-20 to eliminate bacterial growth and facilitate zoospores separation from saprolgenoid mycelium. The flasks were incubated at 20 ± 2 °C for 18 hrs and emerging zoospores were harvested and counted using haemocytometer slide. The concentration of the isolated zoospores was diluted and adjusted to 1.2 × 105 (zoospores /ml) through measuring their absorbance at wave length (580 µm) using spectrophotometer.
2.3 Fungicides
Seven fungicides including azoxytrobin (Tazer, 25.0%), cyazofamid (Segway_O, 34.5%), etridiazole (Terramster_4EC, 44.3%), fluopicolide (Adorn, 39.5%), mefenoxam (Subdue_Maxx, 22.0%), Metalaxyl (Sebring_480FS, 44.08%) and Propamocarb (Banol, 66.5%) were screened for their antifungal activities against two saprolegnoid fungi using food poisoned technique in potato dextrose broth medium.
2.4 Antifungal assay of fungicides
Antifungal activities of these fungicides were evaluated on two saprolegnoid fungi (S. parasitica and S. diclina) using potato dextrose broth medium by food poisoning technique according to the method described by Bhagwat and Datar (2014). The fungicides were dissolved in 5 ml of methanol then mixed with 45 ml of sterile potato dextrose broth medium (PDB) to attain final concentration of 0.25 mg/ml of each fungicide. For control, 5 ml of methanol was added to PDB medium and 1.0 ml of each zoospores suspensions was inoculated to the treatment flasks and control sets. After the incubation of the flasks at 20 ± 2 °C for 7 days, their contents were filtered (Whatman No.1) and biomass of the gained mycelium was weighed after drying at 70 °C for three successive days till their weights remain constant. The inhibition of fungal growth correlated with fungicides treatment was calculated as percentage from the fungal mycelial growth in control
2.5 Fungicidal analysis of effective fungicides compared with reference fungicide (Malachite green).
Four of seven fungicides belonging to 4 different chemical categories were found to be effective against the two saprolegnoid fungi (Table 1). The chemo-physical properties of these effective fungicides were illustrated in Table 2 and their antifungal activities were conducted to evaluate their efficiency in controlling saprolegnoid fungi. To determine minimum inhibitory concentration (MIC) and minimal fungicidal concentration (MFC) of the effective fungicides, different concentrations of the concerned fungicides based on their active ingredients (0.0, 25, 50, 100, 150, 200, and 250 ppm) were prepared by dissolving their requisite amount in 5 ml of methanol, sterilized through Millipore filter (0.22 µm) then mixed with 45 ml of sterile potato dextrose broth medium to attain the final concentrations. To compare efficacy of agricultural fungicides with that of malachite green (reference fungicide) in controlling saprolegnoid fungi, different concentrations (0.0, 0.125, 0.250, 0.50, 1.0, 2.0 ppm) of malachite green were prepared by dissolving 1 mg in100 ml of methanol as a stock solution and their requisite amount were mixed with flasks containing 45 ml of potato dextrose broth. The flasks were inoculated with 1 ml of saprolegnoid zoospores suspensions and were incubated at 20 ± 2 °C for 7 days. After incubation, they were filtered and biomass of the saprolegnoid fungi were weighed after drying at 70 °C for three successive days. The fungicidal efficiency of fungicides were evaluated and their MIC & MFC were determined then compared with those of malachite green as reference fungicide with three replicates. Asterisked values (*) in the same column are significantly different at (P < 0.05) compared to control. Means followed by different letters in each column differ significantly at P ≤ 0.05. Data are the means of three replicates (n = 3) ± standard error.
Fungicides
Mycelial dry weight (mg)
Mycelial growth inhibition %
S. diclina
S. parasitica
S. diclina
S. parasitica
Azoxystrobin
0.00* ± 0.0a
0.00* ± 0.0a
100.0
100.0
Cyazofamid
79.1* ± 0.7b
114.5* ± 0.3b
44.58
34.01
Etridiazole
86.7* ± 0.4c
126.3* ± 0.7c
39.24
27.21
Fluopicolide
132.4* ± 0.6d
153.1* ± 1.2d
7.22
11.78
Mefenoxam
129.6* ± 0.5d
155.2* ± 0.6d
9.18
10.55
Metalaxyl
0.00* ± 0.0a
0.00* ± 0.0a
100.0
100.0
Propamocarb
135.3* ± 0.4d
162.4* ± 0.8e
5.19
6.40
Control
142.7 ± 1.3
173.5 ± 0.5
-- --
-- --
Characteristics of fungicides
Fungicides
Chemical category
Azoxystrobin
Cyazofamid
Metalaxyl
Etridiazole
Trade name
Tazer
Segway,O
Sebring, 480FS
Terramster, 4EC
Supplier Co.
Nufarm-U.K
OHP-USA
Nufarm- USA
Chemtura - USA
Chemical name
methyl (E)-2-[2-[6-(2-cyanophenoxy)pyrimidin-4-yl]oxyphenyl]-3-methoxyprop-2-enoate
4-chloro-2-cyano-N,N-dimethyl-5-(4-methylphenyl)imidazole.1-sulfonamide
methyl 2-(N-(2-methoxyacetyl)2,6-dimethylanilino)propanoate
5-ethoxy-3-(trichloromethyl)-1,2,4-thiadiazole
Chemical formula
C22H17N3O5
C13H13ClN4O2S
C15H21NO4
C5H5Cl3N2OS
Molecular weight
403.39
324.79
279.33
247.53
Active ingredients %
25
34.5
44.08
44.3
Physical state
Liquid
Liquid
Liquid
Liquid
Solubility (in water)
Dispersed
Dispersed
Dispersed
Insoluble
(in organic solvent)
Soluble
Soluble
Soluble
Soluble
pH (1% solution)
6.8
6.2
6.34
4.6
Bioaccumulative potential
Biodegradable
Biodegradable
Biodegradable
Biodegradable
Acute fish toxicity
0.47 mg/L of 96 hrs exposure time
1.4 mg/L of 96 hrs exposure time
139 mg/L of 96 hrs exposure time
2.4 mg/L of 96 hrs exposure time
2.6 Acute fish toxicity test of the effective fungicides
Acute fungicides toxicity test was performed with fingerlings of tilapia fish (Oreochromis niloticus) using six concentrations of the both effective fungicides (azoxystrobin and metalaxyl). Stock solutions of azoxystrobin and metalaxyl were prepared with the concentrations of (0.0, 50, 100, 200, 300, 400 and 500 ppm). A total of 70 clinically normal tilapia fingerlings with average body weight (10 ± 0.5 gm) were used. These fish were held in seven glass aquaria of dimensions (30 × 50 × 80 cm) containing 60 L of water and equipped with an air supply and dechlorinated tap water in a rate of 10 fish/aquarium. Water temperature was maintained at 23 ± 2 °C and pH was adjusted to 7.0 ± 0.5 through the experiment period. Fish were acclimatized to lab. conditions one week before the experiment during which they were fed on commercial diet. Seven experimental fish groups were exposed to different concentrations of azoxystrobin and metalaxyl and kept for 96 hrs. in aerated water without fish feeding. Dead fish were discarded immediately from the glass aquarium to avoid water deterioration. The number of dead fish was estimated and their mortality percentages were calculated after 24, 48, 72 and 96 hrs. of exposure for each fungicide concentration respectively.
2.7 Statistical analysis.
The experiments were set up in three replicates for each treatment and the data were reported as mean ± SE (standard error).The data were also analyzed statistically with GraphPad Prism 5.0 (GraphPad Software,Inc., La Jolla, CA, USA) using One-way analysis of variance (ANOVA) and differences among the means were determined for significance at P ≤ 0.05.
3 Results
3.1 Antifungal assay of fungicides
Seven fungicides conventionally used in agricultural field were screened for their antifungal activities at concentration of (0.25 mg/ml) against two saprolegnoid fungi (Table 1). Among these, four fungicides (azoxystrobin, cyazofamid, etridiazole and metalaxyl) were found to be effective against (S. diclina and S. parasitica) while the other three fungicides (fluopicolide, mefenoxam and propamocarb) showed a slight inhibitory effect at the same concentration. Both of azoxystrobin and metalaxyl were the most effective fungicides in suppressing mycelial growth of the saprolegnoid fungi followed by cyazofamid and etridiazole. Azoxystrobin and metalaxyl showed a highest antifungal activities as mycelial growth of the concerned fungi were absolutely inhibited at 0.25 mg/ml while cyazofamid and etridiazole showed a moderate activities in suppressing their mycelial growth at the same concentration. Cyazofamid reduced mycelial growth of S. diclina and S. parasitica to 44.58 and 34.01% while etridiazole retarded their mycelial growth to 39.24 and 27.21% respectively.
Hence, the chemo-physical properties of the four effective fungicides were illustrated in Table 2 and were also chosen for further antifungal investigations. The MIC and MFC of these effective fungicides were employed by food poisoning technique to assess their fungistatic and fungicidal properties in comparison with malachite green as a reference fungicide.
3.2 Fungicidal analysis of effective fungicides compared with reference fungicide (Malachite green)
Saprolegnia diclina was more sensitive to malachite green as its mycelial growth was inhibited to 55.77% at concentration of 0.5 ppm while mycelial growth of S. parasitica was inhibited to 50.29% at the same concentration respectively. The fungicidal effect of malachite green was obvious at concentration of 0.25 ppm and increased in proportion to malachite green concentrations reached to maximum at concentration of 1 ppm inhibiting completely mycelial growth of the two saprolegnoid fungi (Table 3). Malachite green was strongly effective against S. diclina and S. parasitica with MIC of 0.125 & 0.25 ppm respectively while minimal fungicidal concentrations (MFC) of both fungal strains was 1 ppm. The concentration effect of the effective fungicides on the mycelial growth of the saprolegnoid fungi is shown by plotting their concentrations against mycelial growth of the saprolegnoid fungi (Fig. 1). Growth inhibitions of saprolegnoid fungi were increased with fungicides concentrations reached to maximum at 200 ppm and 250 ppm for azoxystrobin and metalaxyl while a higher concentration was required for cyazofamid and etridiazole respectively to attain the same effect. Moreover, results reported in Table 4 revealed that azoxystrobin was strongly effective fungicide and showed fungicidal and fungistatic activities against S. diclina and S. parasitica reducing their mycelial growth to 70.88 and 55.64% with MIC of 150 ppm while their growth was completely inhibited with MFC of 200 ppm respectively. Moreover, metalaxyl also showed a strong antifungal activity against the saprolegnoid fungi inhibiting mycelial growth of S. diclina and S. parasitica to 83.08 and 60.23% with MIC of 200 ppm and completely suppressed their mycelial growth with MFC of 250 ppm respectively. In contrast, antifungal activities of cyazofamid and etridiazole were confirmed to be moderately effective against the saprolgnoid fungi with MIC of 250 ppm and higher concentrations were required to suppress completely their mycelial growth. All mycelial growth inhibition of the effective fungicides were reported to be significant for all treatments at the level of (P < 0.05). Asterisked values (*) in the same column are significantly different at (P < 0.05) compared to control (0 ppm). Means followed by different letters in each column differ significantly at P ≤ 0.05. Data are the means of three replicates (n = 3) ± standard error. Means followed by different letters in each column differ significantly at P ≤ 0.05. Data are the means of three replicates (n = 3) ± standard error.
Malachite green conc. (ppm)
Mycelial dry weight (mg)
Mycelial growth inhibition %
S. diclina
S. parasitica
S. diclina
S. parasitica
0.00
141.3 ± 1.1a
173.6 ± 0.4a
0.00
0.00
0.125
119.7* ± 0.8b
158.3* ± 0.6b
15.29
8.81
0.250
103.4* ± 0.5c
137.8* ± 0.9c
26.82
20.62
0.50
62.5* ± 0.6d
86.3* ± 0.5d
55.77
50.29
1.00
0.00* ± 0.0e
0.00* ± 0.0e
100.0
100.0
2.00
0.00* ± 0.0e
0.00* ± 0.0e
100.0
100.0
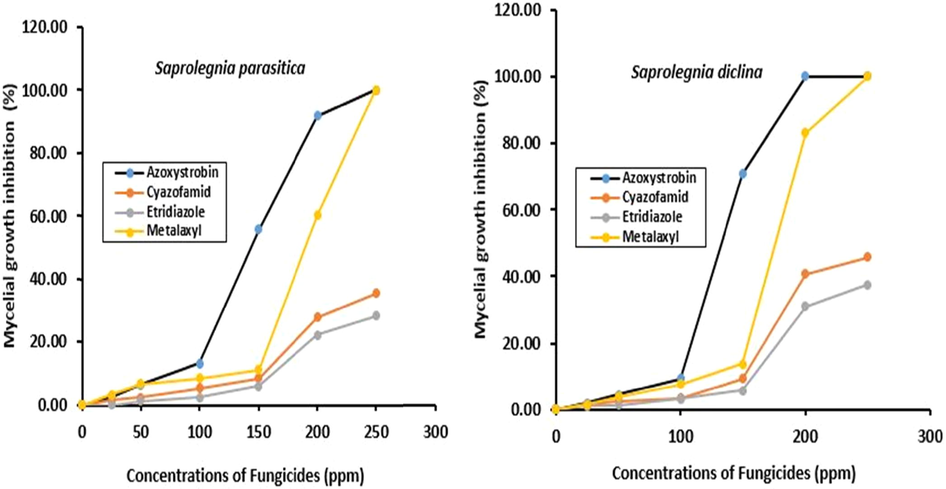
Effect of different concentrations of fungicides on mycelial growth of Saprolegnia parasitica and S. diclina.
Fungicides
Conc. (ppm)
Mycelial dry weight (mg)
Mycelial growth inhibition (%)
S. diclina
S. parasitica
S. diclina
S. parasitica
Azoxystrobin
0.00
146.3 ± 0.8a
177.2 ± 0.6a
0.00
0.00
25
143.5 ± 1.2a
172.5 ± 0.4a
1.91
2.65
50
139.7 ± 0.2b
165.9 ± 0.5b
4.51
6.38
100
132.7 ± 0.5c
153.7 ± 0.7c
9.30
13.26
150
42.6 ± 0.7d
78.6 ± 1.3d
70.88
55.64
200
0.00 ± 0.0e
14.5 ± 0.0e
100.0
91.82
250
0.00 ± 0.0e
0.00 ± 0.0f
100.0
100.0
Cyazofamid
0.00
139.5 ± 0.6a
172.6 ± 0.2a
0.00
0.00
25
137.4 ± 0.9a
170.1 ± 0.5a
1.51
1.45
50
135.8 ± 1.4a
168.3 ± 0.6a
2.65
2.49
100
134.7 ± 0.7a
163.4 ± 0.4b
3.44
5.33
150
126.3 ± 0.5b
157.9 ± 0.7c
9.46
8.52
200
82.7 ± 1.1c
124.7 ± 1.5d
40.72
27.75
250
75.7 ± 1.3d
111.3 ± 1.8e
45.74
35.52
Etridiazole
0.00
137.0 ± 0.2a
175.4 ± 0.7a
0.00
0.00
25
135.4 ± 0.7a
175.3 ± 0.6a
1.17
0.00
50
135.1 ± 0.4a
173.1 ± 0.3a
1.39
1.31
100
132.6 ± 0.6a
171.3 ± 0.5a
3.21
2.34
150
129.2 ± 0.8b
165.1* ± 0.9b
5.69
5.87
200
94.6 ± 1.7c
136.5* ± 1.3c
30.95
22.18
250
85.7 ± 1.4d
125.7* ± 1.6d
37.45
28.34
Metalaxyl
0.00
143.6 ± 1.3a
168.7 ± 0.5a
0.00
0.00
25
141.4 ± 0.8a
163.3 ± 0.4a
1.53
3.20
50
138.2 ± 0.4b
157.4 ± 0.7b
3.76
6.70
100
132.6 ± 0.5c
154.5 ± 0.9c
7.59
8.42
150
123.7 ± 0.7d
149.8 ± 0.6d
13.86
11.20
200
24.3 ± 0.3e
67.1 ± 1.2e
83.08
60.23
250
0.00 ± 0.0f
0.00 ± 0.0f
100.0
100.0
3.3 Acute fish toxicity test of the effective fungicides:
On the other hand, the acute toxicity of the two effective fungicides in tilapia fish was performed by exposing fish in aquaria containing different concentrations of azoxystrobin and metalaxyl for 96 hrs. Results indicated the variable susceptibility of the exposed tilapia fish to the tested fungicides. All fish were died at 400 ppm of azoxystrobin after 48hrs of exposure while 60% of fish moralities were recorded at the same concentration for metalaxyl fungicide. Moreover, 20, 40, and 70% of accumulative mortalities were observed at concentrations of 100, 200 and 300 ppm of azoxystrobin exposure for 96hrs respectively while no fish mortality was recorded at 50 ppm. On the other hand, no fish mortalities was attained at 50 and 100 ppm and only 10 and 40% of mortalities were detected at 200 and 300 ppm of metalaxyl exposure for 96 hrs respectively. Median lethal concentration of acute toxicity assay for the effective fungicides were shown in Fig. 2. According to plotted graph of fungicides toxicity assay, the lethal concentration causing 50% of fish mortalities after 96 hrs of exposure (LC50) was recorded at 225 ppm for azoxystrobin while metalaxyl was recorded at 360 ppm respectively.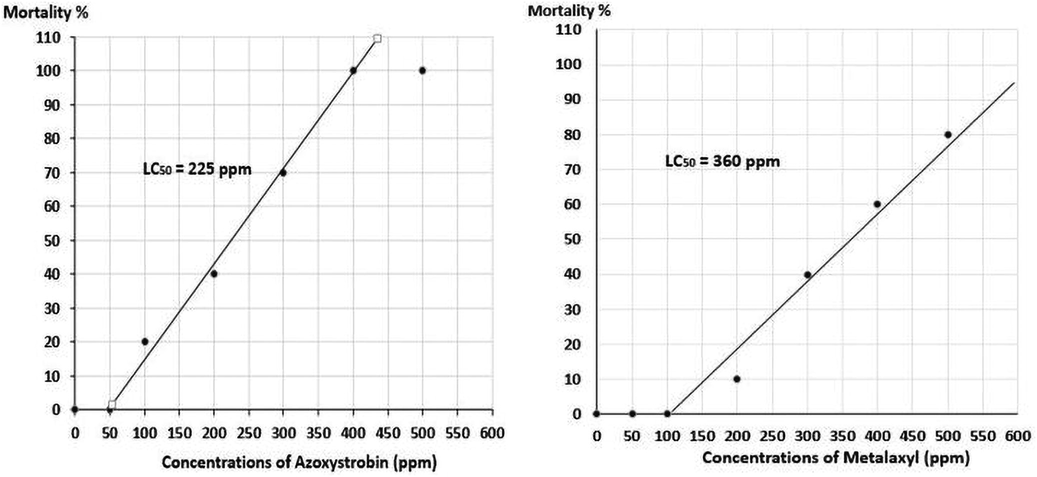
Acute toxicity of the effective fungicides on tilapia fish during their exposure for 96 hrs.
4 Discussion
Saprolegnasis control is mainly based on the application of chemical fungicides (as malachite green, formalin, hydrogen peroxide and copper oxysulphate). Despite of their proven efficiency, malachite green was proved to be carcinogenic (Sudova et al., 2008; Bilandžić et al., 2012), formalin was reported to be harmful to user's health (Allegra et al., 2019) and repeated application of hydrogen peroxide may damage fish tissue resulting in recurring infection (Henriksen et al., 2013). So, seven fungicides were chosen for their approved efficiency against oomycetes in agricultural field. The fungicides were screened at 0.25 mg/ml in vitro to evaluate their activities for saparolegnasis control. Assay showed that, four fungicides provided a significant inhibition of mycelial growth of saprolegnoid fungi and their sensitivity to a given fungicide varied greatly. Azoxystrobin showed complete suppression on mycelial growth of S. diclina and S. parasitica at 200 ppm followed metalaxyl which appeared to retard their mycelial growth completely at 250 ppm. These results were in accordance with that of Bartlett et al., (2002) and Hu et al., (2013) who reported that azoxystrobin has antisaprolegnia activity with concentration of 0.212 mg/ml against saprolegnia mycelial growth. On the other hand, cyazofamid and etridiazole were found to be moderately effective against S. diclina and S. parasitica in suppressing their mycelial growth and a higher concentrations more than 250 ppm were required to be effective. Saprolegnia diclina was more sensitive to four effective fungicides and malachite green compared with S. parasitica. The fungicidal effect of malachite green was obvious already at the concentration of 0.25 ppm against the saprolegnoid fungi and increased linearly to 100% inhibition at the concentration of 1 ppm (MIC100), similar to Warrilow et al. (2014). The study of MIC and MFC of the effective fungicides compared with reference fungicide (malachite green) are necessary to evaluate their efficiency in suppressing mycelial growth of the saprolegnoid fungi. Azoxystrobin was strongly effective against the saprolgnoid fungi and its MIC with MFC were comparatively lower than that of metalaxyl. However, malachite green was the most effective fungitoxicant suppressing growth of saprolegnoid fungi than that of azoxystrobin and metalaxl as mycelial growth of the two saprolegnoid fungi were completely inhibited at 1 ppm of malachite green while a higher concentration up to 200 and 250 ppm were required for azoxystrobin and metalaxyl to attain the same effect respectively. According to acute toxicity assay, metalaxyl fungicide showed low toxicity to fish with LC50 value of 360 ppm while azoxystrobin was 225 ppm. Considering the low toxicity of these fungicides, it was concluded that 250 and 200 ppm of metalaxyl and azoxystrobin which completely inhibited the mycelial growth of the two saprolegnoid fungi could be safely used for saprolegniasis control through immersion of the diseased fish in aquaria containing the previous concentrations of the effective fungicides for 30 min.
5 Conclusions
The present study indicates that application of some fungicides conventionally used for oomycetes control, may be effective in controlling saprolegnasis. Azoxystrobin and metalaxyl may be used as an attractive alternative to teratogenic malachite green as they exhibited a wider margin of safety where their acute fish toxicity assay was low recording 0.225 and 0.360 mgL−1 of 96 hrs exposure time respectively. However, field studies will be required before practical use of these fungicides in fish aquaculture.
Acknowledgement
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group No. (RGP-1438-090).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Formaldehyde Exposure and Acute Myeloid Leukemia: A Review of the Literature. Medicina. 2019;55(10):638.
- [Google Scholar]
- Antifungal activity of herbal extracts against plant pathogenic fungi. Arch. Phytopathol. Plant prot.. 2014;47(8):959-965.
- [Google Scholar]
- Malachite green residues in farmed fish in Croatia. Food Control. 2012;26(2):393-396.
- [Google Scholar]
- The efficacy of selected plant extracts and bioflavonoids in controlling infections of Saprolegnia australis (Saprolegniales; Oomycetes) Aquaculture. 2012;358:146-154.
- [Google Scholar]
- Repeated zoospore emergence in Saprolegnia parasitica. Mycol. Res.. 1994;98:810-815.
- [Google Scholar]
- New approaches for controlling Saprolegnia parasitica, the causal agent of a devastating fish disease. Trop. Life Sci. Res.. 2014;25(2):101-109.
- [Google Scholar]
- An overview of diseases in fish hatcheries and nurseries. Fundam. Appl. Agric.. 2017;2(3):311.
- [Google Scholar]
- Fungi isolated from cultured eggs, alevins and broodfish of brown trout in a hatchery affected by saprolegniosis. J. Fish Biol.. 2007;71(2):510-518.
- [Google Scholar]
- Formalin treatment to reduce mortality associated with Saprolegnia parasitica in rainbow trout, Oncorhynchus mykiss. Aquaculture. 2006;253(1-4):120-129.
- [Google Scholar]
- Effect of hydrogen peroxide on immersion challenge of rainbow trout fry with Flavobacterium psychrophilum. PLoS ONE. 2013;8(4)
- [Google Scholar]
- In Vitro Screening of Fungicidal Chemicals for Antifungal Activity against Saprolegnia: FUNGICIDAL CHEMICALS FOR ANTI-SAPROLEGNIA ACTIVITY. J. World Aquacult. Soc.. 2013;44(4):528-535.
- [Google Scholar]
- Malachite Green Induced Ultrastructural Corneal Lesions in Cyprinus carpio and Its Amelioration Using Emblica officinalis. Bull. Environ. Contam. Toxicol.. 2019;102(3):377-384.
- [Google Scholar]
- Johnson, T.W., Seymour, R.L., Padgett, D.E., 2002. Biology and systematics of the Saprolegniaceae. Digital book, available at: http://ilumina-dlib.org.
- Assessment of antifungal effects of copper nanoparticles on the growth of the fungus Saprolegnia sp. on white fish (Rutilus frisii kutum) eggs. Egypt. J. Aquat. Res.. 2015;41(4):303-306.
- [Google Scholar]
- Use of formalin in intensive aquaculture: properties, application and effects on fish and water quality. Rev. Aquacult.. 2018;10(2):281-295.
- [Google Scholar]
- Saprolegnia parasitica, A Lethal Oomycete Pathogen: Demands to be Controlled. J. Infect. Mol. Biol.. 2018;6(2)
- [Google Scholar]
- The effect of hydrogen peroxide on the hatch rate of channel catfish eggs and on Saprolegnia spp. infesting channel catfish eggs. N. Am. J. Aquacult.. 2009;71:276-280.
- [Google Scholar]
- The effects of cooking on residues of malachite green and leucomalachite green in carp muscles. Anal. Chim. Acta. 2007;586(1-2):420-425.
- [Google Scholar]
- Anti-saprolegnia potency of some plant extracts against Saprolegnia diclina, the causative agent of saprolengiasis. Saudi J. Biol. Sci.. 2020;27(6):1482-1487.
- [Google Scholar]
- Comparison of the Efficacy of Hydrogen Peroxide and Salt for Control of Fungal Infections on Brown Trout (Salmo trutta) Eggs. Acta Sci. Vet.. 2018;46(1):5.
- [Google Scholar]
- Assessment of intra-specific variability in Saprolegnia parasitica populations of aquaculture facilities in British Columbia, Canada. Dis. Aquat. Organ. 2018;128(3):235-248.
- [Google Scholar]
- Malachite green toxicity assessed on Asian catfish primary cultures of peripheral blood mononuclear cells by a proteomic analysis. Aquat. Toxicol.. 2012;114-115:142-152.
- [Google Scholar]
- Efficacy of hydrogen peroxide to control saprolegniasis on channel catfish (Ictalurus punctatus) eggs. Aquaculture. 2004;238(1-4):135-142.
- [Google Scholar]
- Evaluation of toxicity and lethal concentration (LC50) of silver and selenium nanoparticle in different life stages of the fish Tenualosa ilish (Hamilton 1822) Oceanogr. Fish J.. 2018;7(5):1-5.
- [Google Scholar]
- Accounting for Water Temperature during Hydrogen Peroxide Treatment of Channel Catfish Eggs. N. Am. J. Aquacult.. 2004;66(2):162-164.
- [Google Scholar]
- Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a reviewNegative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Vet. Med.. 2008;52(No. 12):527-539.
- [Google Scholar]
- The efficacy of copper sulfate in controlling infection of Saprolegnia parasitica. J. World Aquacult. Soc.. 2014;45(2):220-225.
- [Google Scholar]
- In vitro activity of chemicals and commercial products against Saprolegnia parasitica and Saprolegnia delica strains. J. Fish Dis.. 2019;42(2):237-248.
- [Google Scholar]
- Pathogenicity of Saprolegnia spp. to Atlantic salmon, Salmo salar L., eggs. J. Fish Dis.. 2011;34:601-608.
- [Google Scholar]
- Isolation and identification of Saprolegnia parasitica from grass carp and screening of sensitive drugs. J. Biotech Res.. 2019;10:230-238.
- [Google Scholar]
- In Vitro Inhibition of Saprolegnia sp. by an Antifungal Peptide from Pseudomonas protegensXL03. N. Am. J. Aquacult.. 2017;79(2):168-175.
- [Google Scholar]
- Clotrimazole as a Potent Agent for Treating the Oomycete Fish Pathogen Saprolegnia parasitica through Inhibition of Sterol 14α-Demethylase (CYP51) Appl. Environ. Microbiol.. 2014;80(19):6154-6166.
- [Google Scholar]
- Rapid preliminary screening of Saprolegnia on fish. J. Fish Dis.. 1985;8(5):473-476.
- [Google Scholar]







