Translate this page into:
In vitro cytotoxicity in A549, Hepg2, MCF-7, and DLD-1 cancer cell lines and ADME/toxin analysis of a benzimidazole derivative
* Corresponding author E-mail address: senemakkoc@sdu.edu.tr (S. Akkoc)
-
Received: ,
Accepted: ,
Abstract
The development of novel, targeted, and low-toxicity therapeutic agents is crucial in addressing cancer, which remains a significant global health issue. Benzimidazole derivatives have drawn significant interest owing to their structural similarity to biological nucleotides and several biological roles, including anticancer activity. This study synthesized a benzimidazole derivative (se-182) and tested its anticancer activities on four cancer cell lines, i.e., A549 (lung carcinoma), MCF-7 (breast carcinoma), HepG2 (liver carcinoma), and DLD-1 (colorectal carcinoma) in vitro. Cisplatin served as a reference drug in the study. The se-182 exhibited significant cytotoxic effects that were dose-dependent across all assessed cancer cell lines. The se-182 exhibited high cytotoxic activity against A549 (IC50 = 15.80 μg/mL) and HepG2 cells (IC50 = 15.58 μg/mL). Computational models indicated superior blood-brain barrier permeability and moderate gastrointestinal absorption, whereas ADME studies showed that se-182 met significant drug-likeness requirements. The compound, with an IC50 of 1000 mg/kg, was categorized as class IV in the toxicity assessment. The se-182 exhibited significant dose-dependent cytotoxicity against HepG2 cells (IC50 = 15.58 µM) demonstrating a stronger effect in comparison with cisplatin (IC50 = 37.32 µM).
Keywords
A549
ADMET
Benzimidazole
Cytotoxicity
DLD-1
HepG2
1. Introduction
Cancer is a considerable global health burden, resulting in roughly 10 million fatalities in 2020, or to almost one in six deaths globally (International Agency for Research on Cancer, 2020). The anticancer medications available today are insufficient to address this grave issue (Léonard et al., 2011; Sanchez et al., 2011). For this reason, selective compounds that are easy to synthesize and have minimal toxicity continue to be a difficult issue in the fight against cancer (Kim and Eng, 2012). Benzimidazole and its derivatives, as members of a diverse family of nitrogen-containing heterocycles, have proved crucial in the development of several drugs (Baumann et al., 2011). Because of their structural relationship to naturally occurring nucleotides, benzimidazole derivatives may be comprehended from a biological perspective (Haque et al., 2012). Thus, they can communicate with the biopolymers of the biological system (Banerji and Pramanik, 2015). The benzimidazole nucleus is also present in the structure of vitamin B12 (Hsieh et al., 2019).
Benzimidazoles interact with biological systems’ biomolecules with ease because of their structural resemblance to purines. As such, they are an important pharmacophore in drug development and have a great deal of potential for use in medicinal chemistry (Fang et al., 2019; Shimomura et al., 2019). Hybrids based on benzimidazoles are likewise approved by the FDA or undergoing clinical studies. These include the clinical trials for Veliparib (in progress), Abemaciclib (approved), and Dovitinib (in progress) (Choi et al., 2018; Ghisoni et al., 2019; Kim, 2017). Medicinal chemists have developed several novel chemotherapy agents, one of which has a benzimidazole moiety, driven by the significant therapeutic potential of these compounds (Morais et al., 2017). The many biological activities shown by molecules with benzimidazole moiety have motivated researchers globally to synthesize several benzimidazole analogs (Brishty et al., 2021).
Anti-cancer medication capsules have reportedly been shown to hold more promise than conventional therapies (Montané et al., 2020). Significant advances in the field of metallo-medicines as anti-cancer agents have been spurred by the unintentional discovery of cisplatin as an anti-cancer chemotherapeutic agent. As a result, many researchers are working on metal-based chemotherapy (Wang et al., 2011). From then on, a plethora of promising metallo-medicines have been identified and are being utilized in clinics across the globe to treat cancer, beginning with carboplatin, oxaliplatin, and cisplatin (AlAjmi et al., 2018). However, their therapeutic applications are severely limited by their severe side effects and the resilience of cancer cells (Rehman and Khan, 2015). Developing viable substitutes is deemed essential to lessen the unmanageable treatment expenses and growing resistance. The experimentally developed benzimidazole derivative se-182 was tested for its cytotoxic and antiproliferative effects on HepG2, MCF-7, A549, and DLD-1 cancer cell lines in a laboratory setting. In addition, the absorption distribution metabolism excretion toxicity (ADMET) evaluation was performed on the synthesized compound.
2. Materials and Methods
2.1 Synthesis of a N-Fenilbenzimidazol
N-Phenyl-o-phenylenediamine (5 g; 27.17 mmol) was mixed with a 20% excess of N,N-dimethylformamide dimethylacetal (3.89 g; 32.61 mmol). To enable the elimination of MeOH and HNMe2, it was heated for three hours in a water bath. The remaining yellow oily residue was distilled off in vacuo.
2.2 Synthesis of 1-Phenyl-3-naphthalenomethylbenzimidazolium chloride, se-182
The palladium metal complex of this substance was published in the article, but the NMR information of this substance was not provided (Akkoç et al., 2014). In 5 milliliters of dimethyl ether (DMF), 1.15 gr of 1-chloromethylnaphthalene (6.52 mmol) was combined with 1.27 gr of N-phenylbenzimidazole (6.55 mmol) and stirred at 60°C for 18 hours. The salt was precipitated using 15 mL of diethyl ether when the reaction liquid cooled. The salt was subsequently filtered and vacuum dried. The se-182 product underwent crystallization purification with a 1:2 ratio of ethyl alcohol to diethyl ether (Fig. 1).
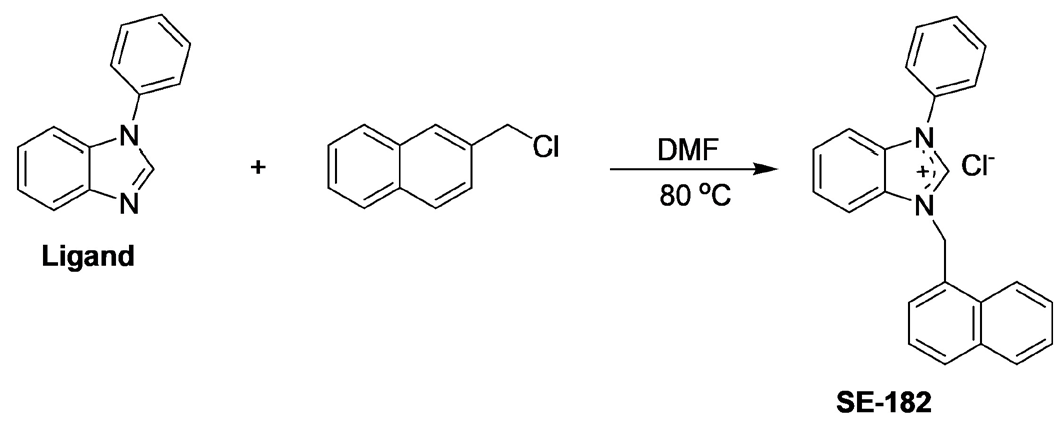
- Synthesis scheme of a benzimidazolium salt.
Yield: 93%, m.p.: 220-221°C. IR ν(CN)= 1595.98 cm-1. 1H NMR (300 MHz, CDCl3), δ: 6.65 (s, 2H, NCH2C10H7); 7.28-7.89 (m, 16H, Ar-H); 11.70 (s, 1H, 2-CH). 13C NMR (75.47 MHz, CDCl3), δ: 49.6 (NCH2C10H7); 122.8, 124.9, 125.4, 126.5, 127.5, 127.7, 128.1, 128.4, 129.1, 130.2, 130.7, 130.9, 131.3, 131.7, 133, and 133.9 (Ar-C); 143.4 (2-CH).
2.3 Diphenyltetrazolium bromide (MTT) test analysis of growth inhibition
The cytotoxicity of the benzimidazole derivative se-182 was assessed in four lines, i.e., HepG2, A549, MCF-7, and DLD-1 utilizing a typical MTT reduction test. The cell lines were obtained from the American Type Culture Collection (ATCC) in the United States and maintained in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and 1% GlutaMAX. In contrast, Michigan Cancer Foundation-7 (MCF-7) cells were cultured in Roswell Park Memorial Institute (RPMI) medium instead of DMEM. The selection of cell culture medium was based on the distinct needs of each cell line to ensure maximum development and physiological relevance. The DMEM and RPMI media are extensively used, and each has been formulated to address specific cellular requirements. The literature indicates that DMEM has been used for the cultivation of HepG2, A549, and DLD-1 cells (Akkoc, 2024). Conversely, RPMI was used for the growth of MCF-7 cells. Consequently, these two media were used in cell culture in our study.
The synthesized compound (se-182) was dissolved in 0.5% dimethyl sulfoxide (DMSO) and administered to the cells. Similarly, the negative control cells were subjected to DMSO at the same ratio. Cisplatin was dissolved in phosphate-buffered saline. Cells sown at a density of 5 × 103 cells/well were subjected to treatment with cisplatin and se-182 for 72 hours, at concentrations varying from 25 to 200 μM (Akkoc and Muhammed, 2024). The same conditions were used to cultivate cells that contained cisplatin (positive control drug). The treated cells’ old media were swapped out for new media after a 72-hr incubation period. Each well was filled with 5 mg/mL of MTT reagent in DMEM, and the cells were then incubated for a further 2-3 hours at 37°C. Following the treatment, each well received 200 μL of DMSO after the supernatants were properly removed. Using a multiwall plate reader, absorbance was measured at 590 nm. Drug sensitivity was reported as the drug dose needed to lower cell viability by 50% (IC50).
2.4 ADME and toxicity (ADMET) properties
Utilizing the ChemBioDraw Ultra 14.0 program, the SMILES (Simplified Molecular Input Line Entry System) notation for the synthetic chemical se-182, C12=CC=CC=C1N(C3=CC=CC=C3)C=[N+]2CC4=CC=CC5=C4C=CC=C5.[Cl-], was produced. The ADMET (Absorption, Distribution, Metabolism, Excretion, and Toxicity) characteristics were computed using this molecular model. The SwissADME webpage (Daina et al., 2017) was used to compute ADME characteristics, whilst the Protox 3.0 webserver was employed to assess toxicity properties (Banerjee et al., 2018).
3. Results
3.1 Cytotoxic activity studies
A compound (se-182) and cisplatin were evaluated for their cytotoxicity at 200, 100, 50, and 25 µM concentrations in tested cancer cell lines for 72 hours with an MTT assay. The findings on anticancer efficacy show that se-182 may markedly reduce the viability of all four cell types in a dose-dependent manner. The DLD-1 cell line demonstrated increased viability. The findings have been shown in Table 1 and Fig. 2.
| Compounds | IC50 (µM) | |||
|---|---|---|---|---|
| MCF-7 | A549 | HepG2 | DLD-1 | |
| se-182 | 32.73 | 15.80 | 15.58 | 65.89 |
| Cisplatin | 31.29 | 9.879 | 37.32 | N.T.* |
N.T.*: Not tested

- Cell viability assays for A549, HepG2, MCF-7, and DLD-1 cells. The graphs indicate the rate of viability for relevant cells after 72-hour exposure to different compounds (se-182 and cisplatin) at varying concentrations (25, 50, 100, 200 µM).
The MTT test utilized 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide to assess cell viability. This test employs mitochondrial dehydrogenase, found in metabolically active cells, to convert yellow MTT into a purple formazan product (Banerji et al., 2013). After treatment with various concentrations of se-182, HepG2 and A549 cells were the most affected cells compared to DLD-1 and MCF-7. Based on the statistical difference between the tested sample concentration and the positive control, cisplatin caused a 50% inhibition (IC50) on the treated cell line’s viability. The viability of A549 cells treated with se-182 was higher than those treated with cisplatin. The se-182 (15.58 µM, p < 0.01) was more potent than cisplatin (37.32 µM) in affecting the viability of HepG2 cell line and exhibited a higher cytotoxic effect with a lower IC50 value. Similarly, se-182 had a cytotoxic effect (65.89 µM, p < 0.01) against the DLD-1 cell line (Table 1). Leica Inverted microscope images of MCF-7 cells have been given below (Figs. 3-6).

- Zero-hour images of negative control cells and cells treated with different concentrations (200-25 µM) of compound se-182.

- Twenty-four-hour images of negative control cells and cells treated with different concentrations (200-25 µM) of compound se-182.

- Forty-eight-hour images of negative control cells and cells treated with different concentrations (200-25 µM) of compound se-182.

- Seventy-two-hour images of negative control cells and cells treated with different concentrations (200-25 µM) of compound se-182.
3.2 ADME and toxicity (ADMET) properties
In pharmacology and pharmacokinetics, the acronym ADME stands for absorption, distribution, metabolism, and excretion. A medicine’s long-term effects on the body may be measured by looking at these four letters, which stand for descriptors. As a result, the drug development sector relies heavily on ADME parameter determination for novel compounds. Physicochemical, lipophilicity, solubility, pharmacokinetic, and drug-likeness characteristics make up these measures. The SwissADME web server was used to determine these markers (Table 2).
| Physicochemical properties | Drug likeness properties | |||
|---|---|---|---|---|
| Properties | Value | Requirement | Value | Compatible |
| Lipinski’s rule | ||||
| Molecular formula | C24H19ClN2 | MW ≤ 500 | 370.87 |
No MLOGP >4.15 |
| Molecular weight (MW, g/mol) | 370.87 | M LOGP ≤ 4.15 | 5.20 | |
| Number of heavy atoms | 27 | HBA Atoms ≤ 10 | 0 | |
| Number of aromatic heavy atoms (AHA) | 25 | HBD Atoms ≤ 5 | 0 | |
| Number of rotatable bonds (RB) | 3 | Ghose’s rule | ||
| Number of H-bond acceptors (HBA) | 0 | 160 ≤ MW ≤ 480 | 370.87 | No |
| Number of H-bond donors (HBD) | 0 | -0.4 ≤ WLOGP ≤ 5.6 | 2.12 | |
| Molar refractivity (MR) | 114.71 | 40 ≤ MR ≤ 130 | 114.71 | |
| TPSA (Å2) | 8.81 | 20 ≤ atoms ≤ 70 | 46 | |
| Lipophilicity | Veber’s rule | |||
| Log Po/w (iLOGP) | -2.47 | RB ≤ 10 | 3 | Yes |
| Log Po/w (XLOGP3) | 6.87 | TPSA ≤ 140 | 8.81 | |
| Log Po/w (WLOGP) | 2.12 | Egan’s Rule | ||
| Log Po/w (MLOGP) | 5.20 | WLOGP ≤ 5.88 | 2.12 | Yes |
| Consensus Log Po/w | 3.26 | TPSA ≤ 131.6 | 8.81 | |
| Pharmacokinetics | Muegge’s Rule | |||
| GI absorption | High | 200 ≤ MW ≤ 600 | 370.87 |
No XLOGP3 > 5 |
| BBB permeant | Yes | -2 ≤ XLOGP3 ≤ 5 | 6.87 | |
| P-gp substrate | Yes | TPSA ≤ 150 | 8.81 | |
| CYP1A2 inhibitor | Yes | Number of rings ≤ 7 | 5 | |
| CYP2C19 inhibitor | No | Number of carbon > 4 | 24 | |
| CYP2C9 inhibitor | No | Number of heteroatoms > 1 | 2 | |
| CYP2D6 inhibitor | No | RB ≤ 15 | 3 | |
| CYP3A4 inhibitor | No | HBA ≤ 10 | 0 | |
| Log Kp (skin permeation, cm/s) | -3.68 | HBD ≤ 5 | 0 | |
| Water solubility | Predicted toxicity properties | |||
| Log S (ESOL) Class | -6.95 Moderately soluble | IC50 (mg/kg) | 1000 | |
| Log S (Ali) Class | -6.87 Moderately soluble | Toxicity class (1 to 6, worst to best) | 4 | |
| Log S (SILICOS-IT) Class | -8.35 Poorly soluble | |||
MW: Molecular weight, HBA: Hydrogen bond acceptors, HBD: Hydrogen bond donors, TPSA: Topological Polar Surface Area, RB: Rotatable bonds, WLOGP: Water Logarithm of the Octanol-Water Partition Coefficient (LogP), GI: Gastrointestinal, BBB: Blood-Brain Barrier
When assessing potential novel drugs using ADME metrics, drug-likeness characteristics are crucial. Companies are considering some guidelines for potential new drugs. Pharmacia (Egan), Bayer (Muegge), Pfizer (Lipinski), Amgen (Ghose), and GSK (Veber) are the firms and regulations in question. The most prominent of these rules is the rule of five proposed by Lipinski. Molecular weight, MLOGP, and the number of hydrogen bond acceptors and donors are among the physicochemical and lipophilicity properties of compounds that are considered in Lipinski’s rule of five (Lipinski et al., 2012). Substances must have a molecular weight value that is between 150 and 500 Da. The MLOGP number needs to be < 4.15. It requires < 5 hydrogen bond donor atoms and < 10 hydrogen bond acceptor atoms. In accordance with Lipinski’s Rule of Five, the synthesized molecule se-182 exhibited a molecular weight < 500, a hydrogen bond acceptor count < 10, a hydrogen bond donor count x 5, and an MLOGP of ∼ 4.15. It meets all requirements except for MLOGP.
Toxicity properties of compound se-182 were calculated using the Protox-II web server. The IC50 values of toxic doses are typically expressed in mg/kg of body mass. The IC50 is the exposure threshold at which 50% of the test subjects succumb. Toxicological categories are delineated using the extensively used Global Harmonized System (GHS) for the categorization and labeling of substances. The class was rated first to sixth, signifying was worst to best. When the toxicity properties of compound se-182 were examined in Table 2, it was observed that the IC50 value was 1000 mg/kg, while the toxicity class was fourth.
The boiled-egg model is important for gastric absorption. In the model, substances with white patches are more easily absorbed than those without. The region in yellow represents the fraction of the total mass that is able to cross the blood-brain barrier (BBB). The yellow section of the model represents chemicals that have breached the BBB and may have therapeutic potential for disorders affecting the central nervous system (CNS). By comparing the synthesized molecule se-182 to the boiled egg model, we can see that it falls within the yellow zone, suggesting that it may have use in treating CNS problems.
Before a medicine is formulated for use in the pharmaceutical or clinical trial fields, two fundamental criteria are examined for each drug: HIA and CNS absorption (Majid et al., 2022). Substances must traverse the BBB to influence the CNS. Concurrently, drugs that are inert inside the CNS must be limited to prevent any detrimental effects on it. Consequently, the capacity to surmount this obstacle is vital (Hassan et al., 2022). Reduced BBB permeability and HIA in isolated flavones point to a low risk of detrimental effects on the CNS. Fig. 7 displays the EGG-BOILED curve. This method facilitates the evaluation of a drug’s capacity to traverse the BBB and its HIA. The methodology comprises two primary regions: the HIA and the BBB penetration area (yolk). If any component is inside the gray area, it indicates insufficient HIA or BBB penetration.

- Boiled-egg model. PGP: P-glycoprotein..
4. Discussion
Continued effort to discover novel treatments for cancer that exhibit enhanced efficacy and reduced adverse effects is a significant concern in global health. The diverse biological activities and attractive pharmacological properties of benzoimidazole derivatives render them ideal materials for the development of anticancer drugs. These heterocyclic compounds have significant diversity and potential as anticancer agents owing to their cytotoxic, antioxidant, and antibacterial properties. This work was aimed at synthesizing and evaluating the anticancer activity of a novel benzimidazole molecule (se-182) in comparison to cisplatin, a recognized agent for inducing apoptosis in cancer cells, as a reference standard. Cisplatin was selected as the standard due to its status as a well-established chemotherapeutic agent for cancer treatment. Cisplatin may serve as a standard for evaluating our synthetic compound due to its well-characterized mechanism of action, primarily involving DNA binding and the subsequent initiation of apoptosis. This strategy allows for the comparison of present medicines with potential future developments.
Many chemicals have been synthesized to find new compounds with potent anticancer effects to progress the area of medicine. To combine a variety of biological activities with various groups and create potent anticancer candidates, we synthesized a benzimidazole conjugate. As a classic anticancer chemotherapeutic reference utilized in various research analyzing the anticancer properties of benzimidazole, we chose cisplatin here. Based on its mode of action, which is linked to inducing apoptosis in cancer cells, cisplatin was selected (Boot et al., 2018).
There is increasing evidence that benzimidazole-based drugs possess anticancer potential, and our findings are similar. Numerous benzimidazole compounds have shown encouraging anticancer properties in recent studies using various cancer cell lines. It has several biological characteristics, such as cytotoxic, antioxidant, and antibacterial effects (Gök et al., 2019; Nile et al., 2013). The MTT assay was used to assess the cancer cells’ vitality following their in vitro exposure to a benzimidazole derivative (se-182). In comparison to cisplatin, a well-known anticancer drug, the current results demonstrated promising antiproliferative capability against HepG2 and MCF-7 cells at IC50 values. In our study, se-182, which was obtained from benzimidazole, was synthesized to investigate its potential cytotoxic effect on various human cancer cell lines and to reveal its potential roles in cytotoxic effect. Four distinct human cancer cell lines were used in an in vitro cytotoxicity study, and the tested chemical demonstrated potent cytotoxic action against A549 and HepG2 with IC50 values of 15.80 μM and 15.58 μM, respectively. Several benzimidazole compounds have been identified as important anticancer medicines in recent years. Human lung adenocarcinoma A549 cell lines were used to evaluate the in vitro anticancer activity of a variety of substituted benzimidazole derivatives in both normoxic and hypoxic conditions (Błaszczak-Świątkiewicz et al., 2014). When tested on rat adrenal medulla cell line (PC12) and human liver carcinoma cell line (HepG2) pheochromocytoma, Nofal et al. (2014) discovered that benzimidazole-thiazole compounds exhibited exceptional anticancer activities (IC50 = 0.518 and 0.578 mM, respectively). The antitumor efficacy of pyrimidine-benzimidazole compounds was reported by Shao et al. (2014). These compounds were produced and tested against the MCF-7, MGC-803, EC-9706, and SMMC-7721 cancer cell lines. When tested against cancer cell lines, the chemical demonstrated outstanding anticancer activity with IC50 values of 1.40, 1.07, 2.79, and 19.28 µM, respectively. The antitumor activity of benzimidazole-pyrazole hybrids was assessed by Sivaramakarthikeyan et al. (2020) against a range of cancer cell lines. The majority of the synthetic substances exhibited strong anticancer properties. Compound 61 exhibited strong anticancer properties against AsPC1, MRCS, and SW1900, with IC50 values of 32.9, 32.8, and 80.0 µM, in that order.
Mavvaji et al. (2024) also prepared molecules containing benzimidazole nuclei and tested them against lung (A549) and liver (HepG2) cancer cell lines. They found that their three synthesized molecules showed antiproliferative activity against both cancer cell lines. They found that the compound containing 4-nitrobenzyl groups exhibited high cytotoxic effect with IC50 values of 8.59 μM for HepG2 and 13.08 μM for A549 compared to the other two compounds containing benzyl group and 4-methylbenzyl group. Akkoc (2024) synthesized 1-phenyl-3-(2-hydroxyethyl)benzimidazolium bromide compound and tested it against A549, HepG2 and DLD-1 cell lines. In the MTT tests, the synthesized compound showed a significant cytotoxic effect against A549 cancer cell line with an IC50 value of 15.68 µM, but was ineffective against HepG2 and DLD-1 cancer cell lines. In this study, we found that the compound we synthesized demonstrated antiproliferative effects against all four cancer cell lines (MCF-7, DLD-1, A549, HepG2) tested.,
Anticancer drugs derived from benzimidazoles represent a rapidly growing field of investigation, and this study contributes significant new insights to this domain. Our novel benzimidazole derivative (se-182) exhibited significant antiproliferative activities against HepG2 and A549 cells, as well as other cancer cell lines. This chemical has significant anticancer potential, and these findings align with the existing literature on benzimidazole derivatives.
5. Conclusion
A benzimidazole derivative, se-182, was prepared with good yield. HepG2, MCF-7, A549, and DLD-1 were used as cell lines for anticancer studies. Different concentrations of the related compound (200-25 μg/mL) were tested against these cell lines and their cytotoxic effects were evaluated in terms of IC50 values. Compound se-182 showed cytotoxic effects against all cell lines. In particular, it had a high cytotoxic effect against HepG2 and A549 cancer cell lines with IC50 values of 15.58 and 15.80 µM, respectively.
Acknowledgments
SA would like to thank the Suleyman Demirel University Research Fund (TSG-2024-9516) for their financial support.
CRediT authorship contribution statement
Esra Bilici: Investigation, Formal analysis, Methodology, Writing - Original drafts, Writing - review and editing, Senem Akkoc: Investigation, Funding Acquisition, Methodology, Formal analysis, Resources.
Declaration of competing interest
The authors declare that they have no known competing interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
The authors confirm that there was no use of artificial intelligence (AI)-assisted technology for assisting in the writing or editing of the manuscript and no images were manipulated using AI.
References
- Cytotoxic activity properties of a benzimidazolium salt against lung, liver, and colon cancer cell lines. J. Indian Chem. Soc.. 2024;101:101445. https://doi.org/10.1016/j.jics.2024.101445
- [Google Scholar]
- Catalytic activities in the direct C5 arylation of novel palladium N-heterocyclic carbene complexes containing benzimidazol-2-ylidene nucleus. Inorganica Chim. Acta. 2014;413:221-230. https://doi.org/10.1016/j.ica.2014.01.015
- [Google Scholar]
- Synthesis, biological application, and computational study of a thymol-based molecule. J. Biologically Active Prod. Nat.. 2024;14:35-50. https://doi.org/10.1080/22311866.2024.2318578
- [Google Scholar]
- Design, synthesis, and biological evaluation of benzimidazole-derived biocompatible copper(II) and zinc(II) complexes as anticancer chemotherapeutics. Int. J. Mol. Sci.. 2018;19:1492. https://doi.org/10.3390/ijms19051492
- [Google Scholar]
- ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res.. 2018;46:W257-W263. https://doi.org/10.1093/nar/gky318
- [Google Scholar]
- Synthesis and cytotoxicity studies of 1-propenyl-1,3-dihydro-benzimidazol-2-one. J. Chem. Biol.. 2015;8:73-78. https://doi.org/10.1007/s12154-015-0130-8
- [Google Scholar]
- Potent anticancer activity of cystine-based dipeptides and their interaction with serum albumins. Chem Cent J. 2013;7:91. https://doi.org/10.1186/1752-153X-7-91
- [Google Scholar]
- An overview of the key routes to the best selling 5-membered ring heterocyclic pharmaceuticals. Beilstein J. Org. Chem.. 2011;7:442-495. https://doi.org/10.3762/bjoc.7.57
- [Google Scholar]
- Biological approach of anticancer activity of new benzimidazole derivatives. Pharmacol. Rep.. 2014;66:100-106. https://doi.org/10.1016/j.pharep.2014.01.001
- [Google Scholar]
- In-depth characterization of the cisplatin mutational signature in human cell lines and in esophageal and liver tumors. Genome Res.. 2018;28:654-665. https://doi.org/10.1101/gr.230219.117
- [Google Scholar]
- A Comprehensive account on recent progress in pharmacological activities of benzimidazole derivatives. Front. Pharmacol.. 2021;12:762807. https://doi.org/10.3389/fphar.2021.762807
- [Google Scholar]
- Phase II study of dovitinib in patients with castration-resistant prostate cancer (KCSG-GU11-05) Cancer Res. Treat.. 2018;50:1252-1259. https://doi.org/10.4143/crt.2017.438
- [Google Scholar]
- SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep.. 2017;7 https://doi.org/10.1038/srep42717
- [Google Scholar]
- Synthesis and evaluation of tetrahydroisoquinoline-benzimidazole hybrids as multifunctional agents for the treatment of Alzheimer’s disease. Eur. J. Med. Chem.. 2019;167:133-145. https://doi.org/10.1016/j.ejmech.2019.02.008
- [Google Scholar]
- Veliparib: A new therapeutic option in ovarian cancer? Future Oncol. 2019;15:1975-1987. https://doi.org/10.2217/fon-2018-0883
- [Google Scholar]
- In vitro antimicrobial studies of naphthalen-1-ylmethyl substituted silver N-heterocyclic carbene complexes. Arab. J. Chem.. 2019;12:2513-2518. https://doi.org/10.1016/j.arabjc.2015.04.019
- [Google Scholar]
- Design, synthesis and structural studies of meta-xylyl linked bis-benzimidazolium salts: Potential anticancer agents against ‘human colon cancer’. Chem. Cent. J.. 2012;6 https://doi.org/10.1186/1752-153x-6-68
- [Google Scholar]
- A comprehensive in silico exploration of pharmacological properties, bioactivities, molecular docking, and anticancer potential of vieloplain F from xylopia vielana targeting B-Raf kinase. Molecules. 2022;27:917. https://doi.org/10.3390/molecules27030917
- [Google Scholar]
- Design and synthesis of benzimidazole-chalcone derivatives as potential anticancer agents. Molecules. 2019;24:3259. https://doi.org/10.3390/molecules24183259
- [Google Scholar]
- International Agency for Research on Cancer (IARC), Global Cancer Observatory. Available from: https://gco.iarc.fr/.
- The current state of targeted agents in rectal cancer. Int J Surg Oncol. 2012;2012:406830. https://doi.org/10.1155/2012/406830
- [Google Scholar]
- Abemaciclib: First global approval. Drugs. 2017;77:2063-2070. https://doi.org/10.1007/s40265-017-0840-z
- [Google Scholar]
- Whole-cell based hybrid materials for green energy production, environmental remediation and smart cell-therapy. Chem. Soc. Rev.. 2011;40:860-885. https://doi.org/10.1039/c0cs00024h
- [Google Scholar]
- Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev.. 2001;46:3-26. https://doi.org/10.1016/s0169-409x(00)00129-0
- [Google Scholar]
- An extensive pharmacological evaluation of new anti-cancer triterpenoid (nummularic acid) from ipomoea batatas through in vitro, in silico, and in vivo studies. Molecules. 2022;27:2474. https://doi.org/10.3390/molecules27082474
- [Google Scholar]
- Investigation of the cytotoxic activity, DFT calculation, and docking studies newly synthesized 1,3-disubstituted benzimidazolium chlorides on human liver cancer, lung cancer, and normal embryonic kidney cell lines. Biochem. Biophys. Res. Commun.. 2024;741:151024. https://doi.org/10.1016/j.bbrc.2024.151024
- [Google Scholar]
- Encapsulation for cancer therapy. Molecules. 2020;25:1605. https://doi.org/10.3390/molecules25071605
- [Google Scholar]
- Synthesis and biological evaluation of novel 2‐aryl benzimidazoles as chemotherapeutic agents. J. Heterocyclic Chem.. 2017;54:255-267. https://doi.org/10.1002/jhet.2575
- [Google Scholar]
- In vitro evaluation of selected benzimidazole derivatives as an antioxidant and xanthine oxidase inhibitors. Chem. Biol. Drug Des.. 2013;82:290-295. https://doi.org/10.1111/cbdd.12141
- [Google Scholar]
- Synthesis of some new benzimidazole–thiazole derivatives as anticancer agents. J. Heterocyclic Chem.. 2014;51:1797-1806. https://doi.org/10.1002/jhet.1886
- [Google Scholar]
- Understanding the interaction between human serum albumin and anti-bacterial/ anti-cancer compounds. Curr. Pharm. Des.. 2015;21:1785-1799. https://doi.org/10.2174/1381612821666150304161201
- [Google Scholar]
- Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev.. 2011;40:696. https://doi.org/10.1039/c0cs00136h
- [Google Scholar]
- Synthesis and biological evaluation of novel pyrimidine–benzimidazol hybrids as potential anticancer agents. Bioorg. Med. Chem. Lett.. 2014;24:3877-3881. https://doi.org/10.1016/j.bmcl.2014.06.050
- [Google Scholar]
- Drug library screen reveals benzimidazole derivatives as selective cytotoxic agents for KRAS-mutant lung cancer. Cancer Lett.. 2019;451:11-22. https://doi.org/10.1016/j.canlet.2019.03.002
- [Google Scholar]
- Molecular hybrids integrated with benzimidazole and pyrazole structural motifs: Design, synthesis, biological evaluation, and molecular docking studies. ACS Omega. 2020;5:10089-10098. https://doi.org/10.1021/acsomega.0c00630
- [Google Scholar]
- Preparation and characterization of amino-linked heterocyclic carbene palladium, gold, and silver complexes and their use as anticancer agents that act by triggering apoptotic cell death. J. Med. Chem.. 2011;54:5245-5249. https://doi.org/10.1021/jm101096x
- [Google Scholar]







