Translate this page into:
In vitro antimicrobial potency of Elettaria cardamomum ethanolic extract against multidrug resistant of food poisoning bacterial strains
⁎Corresponding author. asali@ksu.edu.sa (Ashraf Abdel-Fattah Mostafa)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
The antimicrobial resistance of food poisoning strains is considered a public health burden contributing to high mortality and morbidity rates globally. Furthermore, the severe side effects of chemotherapy represent a serious issue which necessitates finding safe sources for anticancer agents for adjuvant therapy to decrease harmful impact of chemotherapeutic drugs. Hence, the current study is conducted to evaluate the antibacterial efficiency of Elettaria cardamomum against multidrug resistant strains causing food poisoning outbreaks.
Methods
The resistance profile of the tested food poisoning bacterial pathogens was evaluated using disk diffusion assay. Moreover, the antibacterial efficiency of E. cardamomum solvent extracts was achieved using disk diffusion assay to determine the efficiency of these extracts against multidrug resistant strains. Cytotoxicity assay was achieved to assess the antiproliferative efficiency of Elettaria cardamomum solvent extracts against MCF7 breast cancer cell line.
Results
Methicillin resistant- Staphylococcus aureus (MRSA) strain exhibited resistance to all tested antibiotics except norfloxacin while E. coli strain showed susceptibility to all antibiotics used in the study. Furthermore, P. aeruginosa showed resistance to cefotaxime, trimethoprim + sulfamethoxazole, cefixime, ceftriaxone and cefaclor antibiotics while it was sensitive to norfloxacin antibiotic. In addition, S. aureus strain expressed resistance to cefatziodime, cefixime and ceftriaxone antibiotics. The ethanolic extract of Elettaria cardamomum showed the highest antibacterial activity against the concerned food poisoning bacterial strains recording zone diameters ranged from 11.91 ± 0.17 mm to 26.81 ± 0.24 mm. The ethanolic extract of Elettaria cardamomum showed MIC values of 0.25 and 0.50 mg/disc against S. aureus and E. coli strains recording inhibition zone diameters of 16.83 ± 0.14 and 12.34 ± 0.18 mm respectively. GC–MS analysis revealed that α-terpinyl acetate, 1,8-cineole and β‑pinene were the main phytochemical components of the Elettaria cardamomum ethanolic extract recording percentages of 41.24%, 28.14% and 5.98% respectively. In conclusion, the potent efficiency of E. cardamomum ethanolic extract against the tested food poisoning strains supports utilizing these extracts in bioformulation of safe and effective food preservatives avoiding side effects of chemical preservatives and controlling the problem of antibiotic resistance.
Keywords
Elettaria cardamomum
Food poisoning
Antibacterial
Multidrug resistance
Antiproliferative
Gas chromatography-mass spectrometry
- MIC
-
Minimum inhibitory concentration
- MBC
-
Minimum bactericidal concentration
- MHA
-
Mueller-Hinton Agar
- RT
-
Retention time
- M.W
-
Molecular weight
- GC–MS
-
Gas chromatography-Mass spectrometry
Abbreviations
1 Introduction
Food poisoning is considered as one of the most common cause of illness and death in developing countries (Abebe et al., 2020). Most epidemiological reports of food poisoning are associated with bacterial contamination, especially members of Gram-negative bacteria such as E. coli, S. typhi and P. aeruginosa (Holmes et al., 2021). In this regard, other Gram positive bacterial pathogens including Staphylococcus aureus and MRSA have been reported as causative agents of food borne illness (Rodríguez-Melcón et al., 2021). Antimicrobial resistance is a worldwide issue resulting in microbial tolerance to clinically relevant antibiotics, prompting the development of novel treatment options (Frieri et al., 2017). Furthermore, antibacterial resistance poses a serious threat to public health and well-being as the drug resistant bacterial pathogens are thought to cause 2 million illnesses and 23,000 deaths in the United States each year (Hossain et al., 2021). These ailments cost the United States an additional $20 billion in healthcare costs and $35 billion of the lost productivity (Abushaheen et al., 2020).
In this regard, the multidrug-resistant Salmonella was reported to cause significant number of outbreaks in the U.S (Kumar et al., 2019). On the other hand, the bacterial resistance of P. aeruginosa strain represents a significant issue owing to the low permeability of its cell wall, the capability of expressing different resistance mechanisms as overexpression of efflux pumps and porin deletions (Drusano et al., 2021). High incidence of multidrug resistant P. aeruginosa strain has been reported in the last few decades causing significant number of mortalities and morbidities globally (Al-Orphaly et al., 2021). Furthermore, S. aureus is a Gram positive bacterial strain causing food poisoning illness which can be disseminated through contaminated food (Algammal et al., 2020). Ingestion of food contagioned with toxins of S. aureus strain poses staphylococcal food poisoning syndrome resulting in number of symptoms after 1–6 hrs including nausea, gastroenteritis, diarrhea, vomiting and abdominal pain (Bencardino et al., 2021). Methicillin-resistant S. aureus (MRSA) has been reported as a prominent nosocomial bacterial pathogen that causes hospitalized infections and has also been associated to foodborne illness (Algammal et al., 2020). Furthermore, MRSA has recently been added to the World Health Organization's list of high-priority drug-resistant pathogens (Dweba et al., 2018). MRSA strains were isolated as contaminants in the food production chain, prompting new research into their transmission among food-producing animals (Tomao et al., 2020). It was discovered that these microbes were found primarily in dairy products such as cheese and milk, as well as meat products such as chicken, rabbit, beef, pork, and turkey (Serra et al., 2021). Escherichia coli, on the other hand, is a Gram negative bacterial strain that is normally found as a commensal bacterium in the gut normal flora of human being and other animals (Drider 2021). The pathogenic strains of E. coli cause severe food poisoning infections resulting in incidence of severe diarrhea (Gourama 2020). The diarrheal syndrome of E. coli infection occurs when the usual net absorptive condition of water and electrolyte absorption is switched to secretion (Butt et al., 2020). Annually, roughly 1.7 billion cases of food poisoning related with diarrheal illnesses are reported worldwide (Bernard and Nicholson 2022). In this sense, diarrhea is the second leading cause of mortality among children under the age of five years (Solomon et al., 2020). Prevention of food deterioration and their causative agent is commonly accomplished by utilizing chemical agents in food preservation (Chaudhari et al., 2020). On the other hand, the frequent use of chemical preservatives was proved to possess harmful side effects on human health resulting from their accumulation in food chain (Hembrom et al., 2020). Furthermore, the overuse of these chemical agents in food preservation results in acquisition of antimicrobial resistance (Rodrigues et al., 2020). Consequently, the high incidence of multi-drug resistant bacterial strains induced by the overuse of antibiotics make it difficult to treat food poisoning illness (Ge et al., 2022). Recently, the use of plant extracts as natural food preservatives has received a great attention due to their safety, effectiveness and easy biodegradability (Jafarzadeh et al., 2020). Plant extracts were reported to be potential source of antimicrobial agents owing to their constituents of phenolic compounds, flavonoids and tannins (Yassin et al., 2021; Yassin et al., 2022a). Elettaria cardamomum belongs to Zingiberaceae family and commonly known as green or true cardamom (Ashokkumar et al., 2020). The cardamom essential oil and other recorded bioactive ingredients in cardamom capsules contribute to their unique aroma, nutraceutical and pharmaceutical characteristics (Ozdal et al., 2021). Few studies were performed on the antimicrobial activity of cardamom extracts against multidrug resistant microbes that cause food poisoning illness. In addition, the proven side effects of chemical food preservatives on human health besides the widespread of antimicrobial resistance necessitates the fabrication of natural and safe food preservatives. Hence, the objective of the current study is to detect the antibacterial potency of different E. cardamomum solvent extracts against the common etiological agents of food poisoning illness such as S. aureus, MRSA, E. coli, P. aeruginosa and S. typhimurium.
2 Materials and methods
2.1 Antibacterial susceptibility testing
Five bacterial strains namely, Methicillin-resistant S. aureus (ATCC 43300), S. aureus (ATCC 29213), P. aeruginosa (ATCC 9027), S. typhimurium (ATCC 14023),and E. coli (ATCC 25922) were assayed for their susceptibility to different antibiotics. Firstly, freshly prepared bacterial suspension was attained using sterile saline solution (0.85%). Briefly, the bacterial colonies were picked up using sterile loop, immersed in the prepared saline solution and the turbidity was adjusted using 0.5 McFarland standard to attain a viable cell count of 1.0×108 cfu/mL. Sterile Mueller-Hinton Agar (MHA) plates were seeded with 0.5 mL of the prepared bacterial suspension. The antibiotic disks used in current study were purchased from MASTDISCS (Mast Group Ltd., Mast House, Merseyside, U.K.). Antibiotic disks namely, cefaclor (CFC), cefatziodime (CAZ), cefixime (CFM), cefotaxime (CTX), ceftriaxone (CRO), norfloxacin (NOR) and trimethoprim + sulfamethoxazole (TS) of the concentrations 30, 30, 5, 30, 30, 10 and 25 µg/disk respectively were placed over the seeded plates. The plates were incubated at 37 °C for 24 hrs and sensitivity results were interpreted as stated by the clinical and laboratory standards institute (CLSI 2003) to detect the resistance profile of different tested strains.
2.2 Preparation of E. Cardamomum extracts
Elettaria cardamomum seeds were purchased from a local market in Riyadh, Saudi Arabia. The collected plant materials were identified by the herbarium staff of the Botany and Microbiology Department, College of Science, King Saud University. For the extraction of active phytochemicals, four solvents (water, ethanol, ethyl acetate and n-hexane) were utilized in the current study. Firstly, E. cardamomum seeds were washed with sterile distilled water after being surface disinfected with 0.5% sodium hypochlorite. The seeds were blinded using a mechanical blender after they had dried completely, and 50 g of the homogenized powder were soaked in 200 mL of various solvents. The flasks were incubated at 25 °C overnight with a magnetic stirrer, then centrifuged at 9000 rpm for 10 min to remove plant residues before being filtered with Whatman filter paper no. 1 to obtain clean filtrates. Finally, using a rotatory evaporator, the clear filtrates were concentrated and stored at 4 °C until use. The extract yields of various solvents were estimated using the formula below:
Extract yield = (R/S) × 100; Where R is the weight of the plant extract residue and S is the raw plant sample weight (Yassin et al., 2020a).
2.3 Screening of antibacterial efficiency of different E. Cardamomum extracts
Disc diffusion assay was achieved to detect the susceptibility of the tested food poisoning strains to different seed extracts of E. cardamomum. Sterile filter paper discs (8 mm in diameter) were loaded with 10 mg of different extracts then the discs were placed over MHA plates which were previously seeded with the bacterial suspension as described above. Norfloxacin antibiotic disks (10 µg/disk) were used as positive controls while blank disks, loaded with 50 µl of solvents only without the extracts, were used as negative controls. The dishes were incubated for 24 h at 37° C, following which the results were determined by measuring the diameter of the inhibitory area using Vernier caliper (Yassin et al., 2022b).
2.4 Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MFC)
The ethanolic extract of E. cardamomum was tested for its MIC as this extract showed the highest antibacterial efficiency. MIC was determined for the ethanolic extract of E. cardamomum against S. aureus and E. coli strains exhibiting the highest susceptibility to the extract. 8 mm filter paper disks were loaded with concentrations of 0.125, 0.250, 0.50, 1.00, 2.00, and 4.00 mg/ml then placed over the seeded MHA plates which were previously prepared as described above. The dishes were incubated at 37 °C overnight, then the inhibitory zones were measured using a Vernier caliper, and the MIC was recorded as the lowest concentration showing clear zone formation. To measure MBC, streaks were obtained from suppressive zones of the MIC concentration and subsequent concentrations, inoculated in sterile MHA plates, and incubated overnight at 37 °C. The plates were then examined for bacterial growth, and MBC was defined as the lowest concentration that showed no microbial growth (Yassin et al., 2020b).
2.5 GC–MS analysis of E. Cardamomum ethanolic extract
Phytochemical active ingredients of E. cardamomum ethanolic extract were instrumentally detected using GC–MS analysis. The instrumental phytochemical analysis was performed using GC–MS Thermo Trace GC Ultra/TSQ Quantum GC equipped with TR5-MS capillary column. The operating analysis conditions were set as previously described by Yassin et al., 2020b. The effective compounds of the E. cardamomum ethanolic extract were identified by matching GC–MS results with spectral mass data and retention times of the NIST library.
2.6 Cytotoxicity assay
This cytotoxicity assay is based on the notion that tetrazolium is not reduced by dead cells or their products. The premise is that the mitochondrial enzyme succinate dehydrogenase cleaves the tetrazolium salt MTT into a blue-colored product (formazan). The amount of formazan produced by the cells was found to be related to the number of cells. The MCF7 breast cancer cells (ATCC HTB-22) were subcultured in minimal essential medium supplemented with gentamicin antibacterial agent of 0.1% concentration and 5% fetal calf serum. For cell adherence to the plate, MCF7 cells were injected in 96-well plates and incubated at 37 °C overnight in a 5% CO2 incubator. The crude seed extracts of E. cardamomum were dissolved in methanol (10 mg/mL) and serial dilutions were made accordingly. The extracts were added to the wells containing the adherent cells in doses ranging from 0.0065 to 1 mg/ml. The supernatants were removed after 48 h of treatment, and the developing solution (MTT) was applied at 5 mg/mL concentration to the wells for the aim of formazan crystals formation. The 96-well plates were further incubated at 37 °C for 4 hrs with the supernatants removed. Finally, 50 µl of dimethyl sulfoxide (DMSO) were injected to the wells to stabilize the formazan crystals that had formed. At a wavelength of 570 nm, the absorbance of the soluble formazan in plates was measured using a microplate reader (Yassin et al., 2020c). The absorbance corresponding to the concentration that inhibits cell viability by 50% (IC50) was estimated according to the following formula:
2.7 Statistical analysis
GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was used to analyze the data using one-way analysis of variance and Tukey's test. All experiments were done in triplicates, and the results were expressed as mean of triplicates ± standard error.
3 Results and discussion
3.1 Antibiotic susceptibility testing
Antibiotic sensitivity criteria of different tested antibiotics in the study were summarized as seen in Table 1. Methicillin resistant- Staphylococcus aureus (MRSA) strain exhibited resistance to all tested antibiotics except norfloxacin while E. coli strain showed susceptibility to all antibiotics used in the study. Furthermore, P. aeruginosa showed resistance to cefotaxime, Trimethoprim + Sulfamethoxazole, cefixime, ceftriaxone and cefaclor antibiotics while it was sensitive to norfloxacin antibiotic. Furthermore, S. aureus strain expressed resistance to cefatziodime, cefixime and ceftriaxone antibiotics while S. typhimurium strain showed resistance to ceftriaxone only. All the tested bacterial pathogens expressed sensitivity to norfloxacin antibiotic as shown in Table 2 and Fig. 1.
Antibiotic agent
Disk potency (µg/disc)
Inhibition zone diameter (mm)
Resistant
Intermediate
Sensitive
Cefaclor (CFC)
30
≤14
15–17
≥18
Cefatziodime (CAZ)
30
≤14
15–17
≥18
Cefixime (CFM)
5
≤15
16–18
≥19
Cefotaxime (CTX)
30
≤14
15–22
≥23
Ceftriaxone (CRO)
30
≤24
25–26
≥27
Norfloxacin (NOR)
10
≤12
13–16
≥17
Trimethoprim + Sulfamethoxazole (TS)
25
≤15
16–18
≥19
Antibiotics (µg/disk)
Inhibition zone diameter (mm)
MRSA
P. aeruginosa
S. typhimurium
S. aureus
E. coli
Cefaclor (CFC)
0.00 ± 0.00 (R)
0.00 ± 0.00 (R)
18.76 ± 0.31 (S)
18.75 ± 0.26(S)
20.55 ± 0.22 (S)
Cefatziodime (CAZ)
0.00 ± 0.00 (R)
16.12 ± 0.21 (I)
17.97 ± 0.38 (I)
0.00 ± 0.00 (R)
26.06 ± 0.23 (S)
Cefixime (CFM)
0.00 ± 0.00 (R)
0.00 ± 0.00 (R)
18.91 ± 0.14 (I)
0.00 ± 0.00 (R)
19.55 ± 0.46(S)
Cefotaxime (CTX)
0.00 ± 0.00 (R)
0.00 ± 0.00 (R)
22.45 ± 0.43 (I)
21.17 ± 0.28 (I)
31.61 ± 0.42(S)
Ceftriaxone (CRO)
0.00 ± 0.00 (R)
12.05 ± 0.14 (R)
23.25 ± 0.17 (R)
20.05 ± 0.31(R)
28.17 ± 0.34 (S)
Norfloxacin (NOR)
18.95 ± 0.35 (S)
21.26 ± 0.18 (S)
21.82 ± 0.24 (S)
19.71 ± 0.37 (S)
29.98 ± 0.25(S)
Trimethoprim + Sulfamethoxazole (TS)
15.65 ± 0.26 (I)
0.00 ± 0.00 (R)
18.93 ± 0.45 (I)
18.75 ± 0.29 (I)
28.45 ± 0.42 (S)
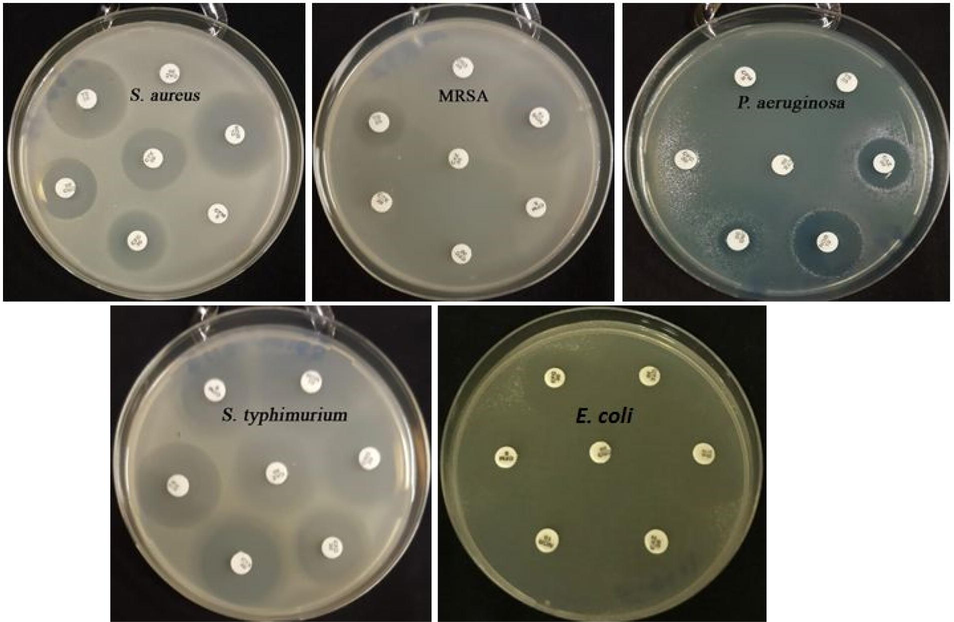
Antibacterial resistance profile of different food poisoning strains to the concerned standard antibiotics.
3.2 Extracts yield
The aqueous extract demonstrated the highest productivity yield followed by ethanol, n-Hexane and ethyl acetate solvents recording yield productivity of 9.56%, 7.29%, 6.84% and 4.13% respectively.
3.3 Antimicrobial screening of different Elettaria cardamomum solvent extracts against the concerned strains
The ethanolic extract of E. cardamomum showed the highest antibacterial proficiency against S. aureus, MRSA, E. coli and P. aeruginosa recording inhibition zone diameters of 26.81 ± 0.24, 24.34 ± 0.12, 22.48 ± 0.36 and 11.91 ± 0.17 mm respectively while E. cardamomum ethyl acetate extract recorded the highest bioactivity against S. typhimurium strain with suppressive zone diameter of 12.44 ± 0.19 mm as seen in Fig. 2. On the other hand, the aqueous extract of E. cardamomum showed no antimicrobial action against P. aeruginosa strain.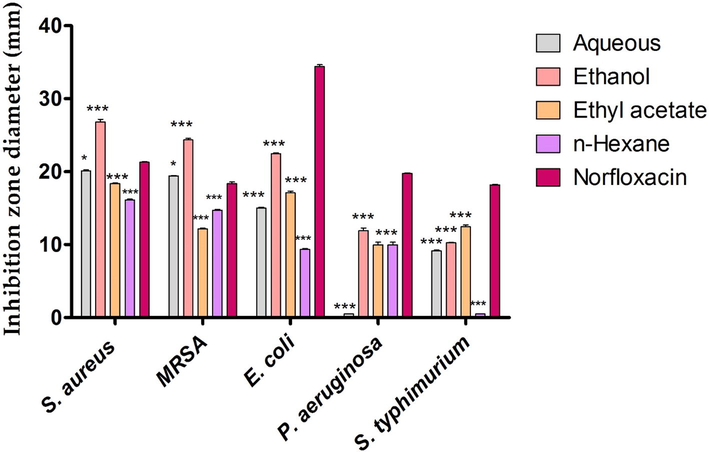
Antibacterial efficiency of Elettaria cardamomum solvent extracts against food poisoning bacterial strains.* Asterisks indicated that values were significantly different compared to control (*** P < 0.001), (*P ≤ 0.05).
3.4 Determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The ethanolic extract of E. cardamomum showed the highest antibacterial potency against the tested strains so its MIC was evaluated. S. aureus strain exhibited the highest susceptibility to E. cardamomum ethanolic extract with MIC value of 0.250 mg/disk demonstrating inhibition zone diameter of 16.83 ± 0.14 mm as seen in Fig. 3. On the other hand, MIC value of E. cardamomum ethanolic extract was 0.500 mg/disk with suppressive zone diameter of 12.34 ± 0.18 mm as seen in Table 3. The minimum bactericidal concentrations (MBC) of E. cardamomum ethanolic extract against S. aureus and E. coli were 0.500 and 1.000 mg/disk respectively.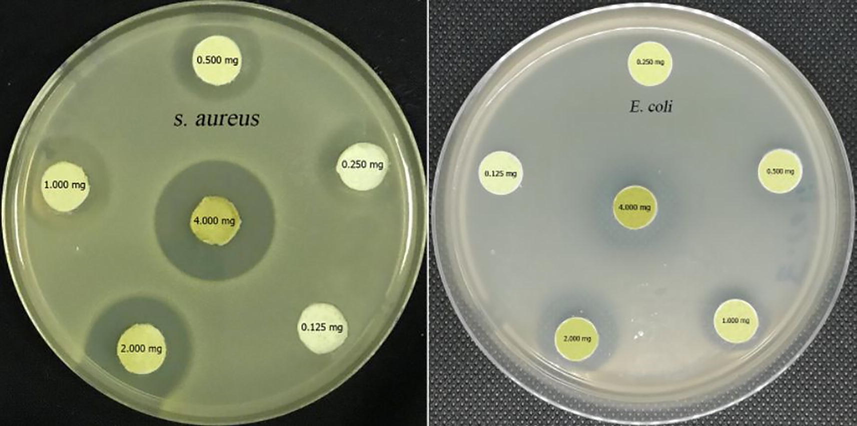
Minimum inhibitory concentrations of Elettaria cardamomum ethanolic extract against S. aureus and E. coli strains.
Concentration (mg/disc)
Inhibition zone diameter
S. aureus
E. coli
0.125
0.00 ± 0.00
0.00 ± 0.00
0.250
16.83 ± 0.14
0.00 ± 0.00
0.500
17.39 ± 0.35
12.34 ± 0.18
1.000
18.15 ± 0.27
14.89 ± 0.41
2.000
19.24 ± 0.51
18.45 ± 0.23
4.000
20.82 ± 0.46
19.32 ± 0.16
3.5 Phytochemical characterization of Elettaria cardamomum ethanolic extract
GC–MS analysis of the ethanolic extract of E. cardamomum was performed as this extract exerted the highest antibacterial efficacy against the tested strains. α-Terpinyl acetate (41.24%) was the major active ingredient of E. cardamomum ethanolic extract followed by 1,8-cineole (28.14%), β‑pinene (5.98%), p-linalool (4.52%), sabinene (4.24%), α-terpineol (3.24%), linalyl acetate (2.78%), myrcene (2.57%), n-hexadecanoic acid (1.87%), a-farnesene (1.46%), p-cymene (1.21%), p-cresol (1.12%), nerol (0.89%) and geranyl acetate (0.65%) as seen in Table 4. The chemical structures of the bioactive components of E. cardamomum ethanolic extract exhibiting the highest antibacterial efficiency were shown in Fig. 4.
Phytochemical constituents
Chemical formula
Mol. weight
RT(min.)
% of Total
β‑Pinene
C10H16
136.23
4.874
5.98
Sabinene
C10H16
136.23
5.382
4.24
Myrcene
C10H16
136.23
6.257
2.57
1,8-Cineole
C10H18O
154.25
7.895
28.14
p-Cymene
C10H14
134.21
8.754
1.21
p-Linalool
C10H18O
154.25
10.357
4.52
α-Terpineol
C10H18O
154.25
11.218
3.24
Nerol
C10H18O
154.25
13.385
0.89
Linalyl acetate
C12H20O2
196.29
15.571
2.87
α-Terpinyl acetate
C12H20O2
196.29
16.173
41.24
Geranyl acetate
C12H20O2
196.29
17.945
0.65
a-Farnesene
C15H24
204.36
19.214
1.46
p-Cresol
C7H8O
108.14
21.785
1.12
n-Hexadecanoic acid
C16H32O2
256.42
25.564
1.87
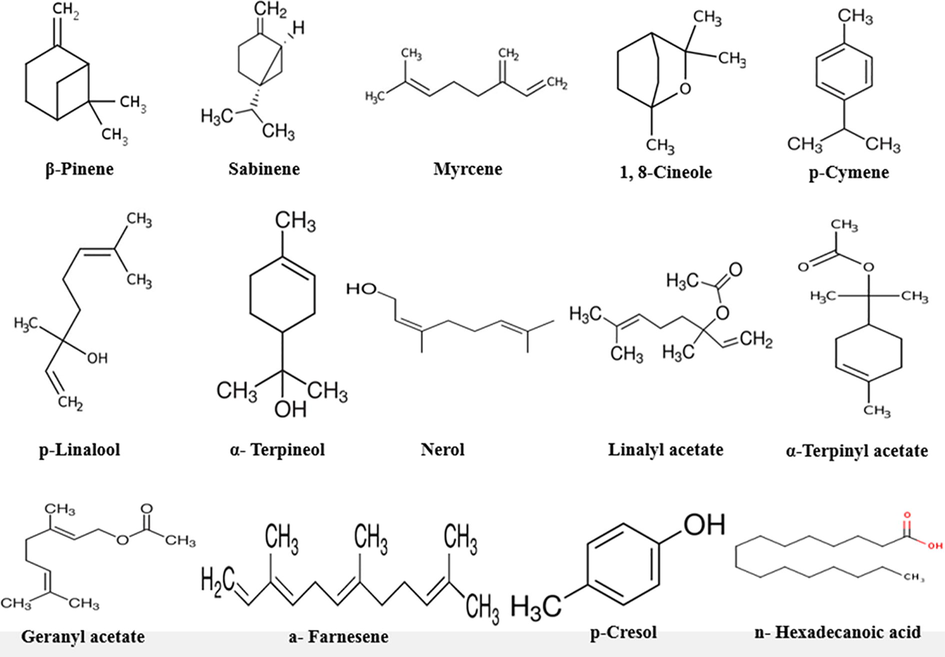
Phytochemical active components of Elettaria cardamomum ethanolic extract.
3.6 Cytotoxicity assay
The ethyl acetate extract exhibited the highest cytotoxicity against MCF7 cell line while the aqueous extract showed the lowest cytotoxicity with IC50 of 114.8 and 187.9 µg/ml respectively. The ethanolic and hexanic extracts of E. cardamomum exhibited moderate toxicities with IC50 of 156.9 and 128.2 µg/ml respectively as seen in Fig. 5.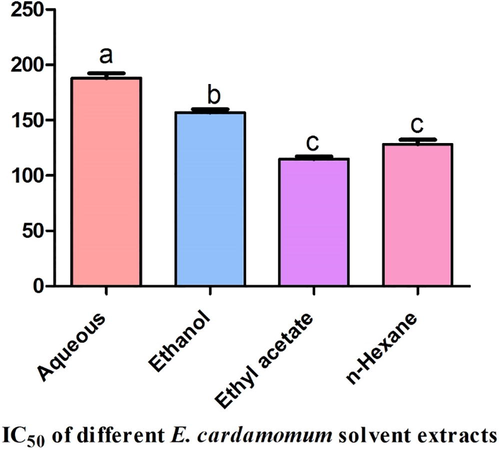
IC50 of different solvent extracts of Elettaria cardamomum against MCF7 cell line.* Different letters indicated that values were significantly different (P ≤ 0.05).
4 Discussion
Staphylococcus aureus strain has the ability to induce antibiotic resistance owing to its capability to gain antimicrobial resistance as the methicillin resistance via number of mechanisms (Craft et al., 2019). In our current study, the methicillin-resistant Staphylococcus aureus (MRSA) exhibited resistance to β- lactam antibiotics as cefaclor which belongs to the second generation cephalosporins. In this sense, β-lactam resistance of MRSA strains represents a serious burden for eradication of S. aureus infections (Lee et al., 2018). Cefaclor resistance was found in 91.2% of clinical MRSA isolates, while cefaclor resistance was detected in 25% of strains isolated from healthy individuals (Alharbi 2020). On the other hand, MRSA strain exhibited resistance to third generation cephalosporins used in the current study as ceftriaxone, cefatziodime, cefixime and cefotaxime antibiotics. Our results were in accordance with that of Naimi et al., 2017 who reported that the clinical isolates of MRSA were less susceptible to cefixim, azithromycin, ceftriaxone, and penicillin antibiotics (Naimi et al., 2017). Furthermore, Gashe et al., 2018 reported resistance to third-generation cephalosporins such as cefriaxone and cefazidime in S. aureus isolates obtained from Jimma University Specialized Teaching Hospital (JUSH), with resistance percentages of 23.4% and 34% of the total number of isolates, respectively. The expression of the β-lactamase enzyme, which is capable of inactivating antibiotics by hydrolyzing the β-lactam ring, is one of the key mechanisms of β-lactam resistance in Staphylococci (Kim et al., 2020). The other main mechanism of β-lactam resistance is the acquirement of penicillin-binding protein (PBP 2a) gene which has the potential to remain active in the presence of β-lactam antibiotics so that enables the bacterial growth in presence of β-lactam antibiotics (Foster 2019). Similarly, P. aeruginosa strain showed resistance to the third generation cephalosporins tested in the current study while exhibited an intermediate susceptibility to cefatziodime antibiotic. Pseudomonas aeruginosa, on the other hand, exhibited resistance to trimethoprim + sulfamethoxazole (co-trimoxazole) antibiotics. These findings were inconsistent with that of Maclean et al., 2022 who reported the sensitivity of all P. aeruginosa strains isolated from Sodwana Bay at South Africa to Bactrim (trimethoprim–sulfamethoxazole) antibiotic. The overexpression of multidrug efflux system enhance the intrinsic resistance of P. aeruginosa strain to trimethoprim–sulfamethoxazole antibiotics (Behzadi et al., 2021). Salmonella typhimurium exhibited resistance to ceftriaxone antibiotic while showed an intermediate susceptibility to cefatziodime, cefixime, cefotaxime and trimethoprim + sulfamethoxazole antibiotics.
The microbiological resistance of the tested food poisoning bacterial strains, such as S. aureus, MRSA, P. aeruginosa, and S. typhimurium, to the antibiotics utilized in this study necessitates the development of novel antimicrobial drugs to address the high rate of antibacterial resistance (Manso et al., 2022). Furthermore, chemical agents employed in food preservation have been linked to a high occurrence of multidrug resistant strains, in addition to the negative effects of these agents on humans and animals, necessitating the development of novel antimicrobial agents (Miethke et al., 2021). To achieve this goal, the antibacterial efficiency of different E. cardamomum extracts against food poisoning strains was evaluated. E. cardamomum oil is well-known for its distinctive aroma and is frequently utilized as a flavoring and fragrance agent in the food and cosmetic industries (Anwar et al., 2016).
On the other hand, the Gram positive bacterial strain, S. aureus, has the capability of releasing staphylococcal enterotoxins in food which are characterized by their high thermostability (Mahros et al., 2021). Moreover, the staphylococcal food poisoning disease is considered one of the main culprits of foodborne outbreaks results in causing severe gastroenteritis (Mourenza et al., 2021). The antibacterial efficacy of the ethanolic extract of E. cardamomum against S. aureus and the multidrug resistant strain (MRSA) was significantly higher than that of the control (Norfloxacin).
Moreover, E. coli is a commensal bacterium that lives in the human colon and frequently causes severe diarrhea (Boxall et al., 2020). P. aeruginosa, on the other hand, causes diarrhea in immune-compromised patients and is difficult to relief due to its intrinsic resistance to various medications, whereas P. aeruginosa can cause severe gastrointestinal illnesses if it is spread through the food chain (Langendonk et al., 2021; Fakhkhari et al., 2022). Salmonella typhimurium is another Gram negative bacterial strain that causes enteric fever disease resulting in gastroenteritis and septicemia (Gal-Mor et al., 2014). The ethanolic extract of E. cardamomum was highly efficient against Gram negative microbes, with suppressive zone widths of 11.91 ± 0.17 and 22.48 ± 0.36 mm for P. aeruginosa and E. coli, respectively. In contrast, Salmonella typhimurium expressed the highest sensitivity to E. cardamomum ethyl acetate extract at the concentration of 10 mg/disk recording zone diameter of 12.44 ± 0.19 mm. The remarkable efficacy of E. cardamomum extracts against several etiological agents of food poisoning illness demonstrates the potential for employing these extracts in the bioformulation of safe and potentially efficient food preservatives.
Accordingly, the high efficiency of E. cardamomum ethanolic extract against majority of the tested food poisoning strains was recorded so its MIC was evaluated. Minimum inhibitory concentrations of E. cardamomum ethanolic extract were 0.250 and 0.500 mg/disk against S. aureus and E. coli strains recording clear zone diameters of 16.83 ± 0.14 and 12.34 ± 0.18 mm respectively. These findings were contrasted with that of Asghar et al., 2017 who reported that MIC value of E. cardamomum oil against S. typhimurium and S. aureus was 10 mg/ml. Another study evaluated MIC of E. cardamomum oil against E. coli and P. aeruginosa strains recording values of 1.0 and 0.5 mg/ml respectively (Alam et al., 2021). Accordingly, the substantial bioactivity of E. cardamomum extracts in the current investigation, when compared to previous findings, ensures the extraction procedure's efficiency. A prior study confirmed the antibacterial efficiency of both cardamom seed and fruit extracts against Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, Prevotella intermedia and Porphyromonas gingivalis recording MIC of 0.25% [v/v], 0.5%, 0.125% and 0.062% respectively while MBC were found to be 0.25% [v/v], 1.0%, 0.25% and 0.062% respectively (Souissi et al., 2020). Chemical analysis of the active ingredients of E. cardamomum ethanolic extract indicated the presence of three main oxygenated monoterpenes compounds, namely, α-terpinyl acetate, 1,8-cineole and β‑pinene, recording relative percentages of 41.24, 28.14 and 5.98%, respectively. Our results were coincident with that of Alam et al. (2021b) who reported that α- terpinyl acetate and 1,8-cineole were the main phytochemical compounds of E. cardamomum oil (Alam et al., 2021a). Another previous study detected the chemical composition of cardamom essential oil using GC–MS and showed the presence of 26 phytoactive compounds with α-terpinyl acetate (38.4%), 1,8-cineole (28.71%), linalool acetate (8.42%), sabinene (5.21%), and linalool (3.97%) as major phytochemical active constituents (Asghar et al., 2017). These monoterpene compounds were reported to possess antimicrobial effectiveness contributing to the potential bioactivity of E. cardamomum extracts (Nazzaro et al., 2013). The antibacterial activity of oxygenated monoterpenes was previously reported to be mediated by their penetration into the fatty acid acyl chains of membrane lipid bilayers, which disrupted cell membrane permeability and fluidity (Tsuchiya 2015). Cytotoxicity assay revealed that the E. cardamomum ethyl acetate extract exhibited the highest antiproliferative efficiency while the aqueous extract showed the highest cytotoxic activity against MCF7 cell line recording IC50 of 114.8 and 187.9 µg/ml respectively. These findings were in accordance with that of Manjunath and Mahurkar 2021 who demonstrated that cardamom oil exhibited a significant anticarcinogenic activity against skin cancer cell line recording IC50 of 166.63 µg/ml (Manjunath and Mahurkar 2021). The major monoterpene component in E. cardamomum extract is terpinyl acetate, which has been shown to have anticancer properties (Chowdhury and Kumar 2020).
5 Conclusion
Antibiotic resistance profile of some etiological agents of food poisoning illness revealed the high resistance of MRSA and P. aeruginosa strains while E. coli showed susceptibility to all antibiotics used in the current study. The ethanolic extract of E. cardamomum exhibited a remarkable antibacterial potency against the tested strains highlighting the potentiality of utilizing these extracts in biofacbrication of natural and effective food preservatives. Moreover, the ethyl acetate of E. cardamomum extract showed the highest antiproliferative efficiency against MCF7 cell line indicating the bioeffieicny of these extracts in formulation of safe and natural antiproliferative agents. Collectively, Cardamom extracts could be utilized for production of natural, eco-friendly, safe and effective antimicrobial and anticarcinogenic agents owing to their high content of oxygenated monoterpene compounds as demonstrated by GC–MS analysis.
Acknowledgements
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/362), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abebe, E., Gugsa, G., Ahmed, M. 2020. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med.
- Antimicrobial resistance, mechanisms and its clinical significance. Dis Mon.. 2020;66(6):100971
- [Google Scholar]
- In vitro antioxidant and anti-inflammatory activities of green cardamom essential oil and in silico molecular docking of its major bioactives. J. Taibah Univ. Sci.. 2021;15(1):757-768.
- [Google Scholar]
- Effects of essential oils of Elettaria cardamomum grown in India and Guatemala on gram-negative bacteria and gastrointestinal disorders. Molecules.. 2021;26(9):2546.
- [Google Scholar]
- Methicillin-Resistant Staphylococcus aureus (MRSA): one health perspective approach to the bacterium epidemiology, virulence factors, antibiotic-resistance, and zoonotic impact. Infect Drug Resist. 2020;13:3255.
- [Google Scholar]
- Screening of antibiotic-resistant staphylococci in the nasal cavity of patients and healthy individuals. Saudi J. Biol. Sci.. 2020;27(1):100-105.
- [Google Scholar]
- Epidemiology of multidrug-resistant Pseudomonas aeruginosa in the Middle East and North Africa Region. Msphere.. 2021;6(3):e00202. e00221
- [Google Scholar]
- Cardamom (Elettaria cardamomum Maton) Oils Essential oils in food preservation, flavor and safety. Elsevier; 2016. p. :295-301.
- Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J. Food Sci. Technol.. 2017;54(8):2306-2315.
- [Google Scholar]
- Botany, traditional uses, phytochemistry and biological activities of cardamom [Elettaria cardamomum (L.) Maton]–A critical review. J. Ethnopharmacol.. 2020;246:112244
- [Google Scholar]
- It’s not easy being green: a narrative review on the microbiology, virulence and therapeutic prospects of multidrug-resistant Pseudomonas aeruginosa. Antibiotics.. 2021;10(1):42.
- [Google Scholar]
- Carriage of Staphylococcus aureus among food handlers: An ongoing challenge in public health. Food Control.. 2021;130:108362
- [Google Scholar]
- Bacterial Infections of the Small and Large Intestine. Textbook of Pediatric Gastroenterology, Hepatology and Nutrition. Springer; 2022. p. :203-218.
- Antimicrobial resistance profiles of diarrhoeagenic Escherichia coli isolated from travellers returning to the UK, 2015–2017. J. Med. Microbiol.. 2020;69(7):932-943.
- [Google Scholar]
- Impact of the Escherichia coli heat-stable enterotoxin b (STb) on gut health and function. Toxins.. 2020;12(12):760.
- [Google Scholar]
- Antimicrobial, aflatoxin B1 inhibitory and lipid oxidation suppressing potential of anethole-based chitosan nanoemulsion as novel preservative for protection of stored maize. Food Bioproc Tech. 2020;13(8):1462-1477.
- [Google Scholar]
- Alpha-terpinyl acetate: A natural monoterpenoid from Elettaria cardamomum as multi-target directed ligand in Alzheimer’s disease. J. Funct. Foods.. 2020;68:103892
- [Google Scholar]
- Methicillin-resistant Staphylococcus aureus (MRSA): antibiotic-resistance and the biofilm phenotype. MedChemComm.. 2019;10(8):1231-1241.
- [Google Scholar]
- Gut Microbiota Is an Important Source of Bacteriocins and Their In Situ Expression Can Be Explored for Treatment of Bacterial Infections. Probiotics Antimicrob. Proteins.. 2021;13(6):1759-1765.
- [Google Scholar]
- Emergence of Resistance to Ceftazidime-Avibactam in a Pseudomonas aeruginosa Isolate Producing Derepressed bla PDC in a Hollow-Fiber Infection Model. Antimicrob. Agents Chemother.. 2021;65(6):e00124-e00221.
- [Google Scholar]
- Methicillin-resistant Staphylococcus aureus: livestock-associated, antimicrobial, and heavy metal resistance. Infect Drug Resist.. 2018;11:2497.
- [Google Scholar]
- Involvement of Pseudomonas aeruginosa in the occurrence of community and hospital acquired diarrhea, and its virulence diversity among the stool and the environmental samples. Int. J. Environ. Health Res.. 2022;32(1):61-71.
- [Google Scholar]
- Can β-lactam antibiotics be resurrected to combat MRSA? Trends Microbiol.. 2019;27(1):26-38.
- [Google Scholar]
- Same species, different diseases: how and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol.. 2014;5:391.
- [Google Scholar]
- Antimicrobial Resistance Profile of Different Clinical Isolates against Third-Generation Cephalosporins. J. Pharm.. 2018;2018
- [Google Scholar]
- Research on the drug resistance mechanism of foodborne pathogens. Microb. Pathog.. 2022;162:105306
- [Google Scholar]
- Foodborne pathogens Food safety engineering. Springer; 2020. p. :25-49.
- A comprehensive evaluation of heavy metal contamination in foodstuff and associated human health risk: a global perspective Contemporary environmental issues and challenges in era of climate change. Springer; 2020. p. :33-63.
- Pathogenesis of gram-negative bacteremia. Clin. Microbiol. Rev.. 2021;34(2):e00234. e00220
- [Google Scholar]
- Zoonotic Significance and Antimicrobial Resistance in Salmonella in Poultry in Bangladesh for the Period of 2011–2021. Zoonotic Dis.. 2021;1(1):3-24.
- [Google Scholar]
- Biodegradable green packaging with antimicrobial functions based on the bioactive compounds from tropical plants and their by-products. Trends Food Sci Technol.. 2020;100:262-277.
- [Google Scholar]
- The importance of porins and β-lactamase in outer membrane vesicles on the hydrolysis of β-lactam antibiotics. Int. J. Mol. Sci.. 2020;21(8):2822.
- [Google Scholar]
- Antibiotic usage in poultry production and antimicrobial-resistant Salmonella in poultry Food Safety in Poultry Meat Production. Springer; 2019. p. :47-66.
- The building blocks of antimicrobial resistance in Pseudomonas aeruginosa: implications for current resistance-breaking therapies. Front. Cell. Infect. Microbiol.. 2021;11:307.
- [Google Scholar]
- Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Primers.. 2018;4(1):1-23.
- [Google Scholar]
- Antimicrobial Susceptibility Profiles among Pseudomonas aeruginosa Isolated from Professional SCUBA Divers with Otitis Externa, Swimming Pools and the Ocean at a Diving Operation in South Africa. Pathogens.. 2022;11(1):91.
- [Google Scholar]
- Multidrug-, methicillin-, and vancomycin-resistant Staphylococcus aureus isolated from ready-to-eat meat sandwiches: An ongoing food and public health concern. Int. J. Food Microbiol.. 2021;346:109165
- [Google Scholar]
- In vitro cytotoxicity of cardamom oil, lemon oil, and jasmine oil on human skin, gastric, and brain cancer cell line. J. Cancer Res. Ther.. 2021;17(1):62.
- [Google Scholar]
- Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics.. 2022;11(1):46.
- [Google Scholar]
- Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem.. 2021;5(10):726-749.
- [Google Scholar]
- Novel Treatments and Preventative Strategies Against Food-Poisoning Caused by Staphylococcal Species. Pathogens.. 2021;10(2):91.
- [Google Scholar]
- Determination of antimicrobial susceptibility patterns in Staphylococcus aureus strains recovered from patients at two main health facilities in Kabul. Afghanistan. BMC Infect. Dis.. 2017;17(1):1-7.
- [Google Scholar]
- Effect of essential oils on pathogenic bacteria. Pharmaceuticals.. 2013;6(12):1451-1474.
- [Google Scholar]
- Introduction to nutraceuticals, medicinal foods, and herbs Aromatic Herbs in Food. Elsevier; 2021. p. :1-34.
- Rodrigues, G. L., Panzenhagen, P., Ferrari, R. G., Dos Santos, A., Paschoalin, V. M. F., Conte-Junior, C. A. 2020. Frequency of antimicrobial resistance genes in Salmonella From Brazil by in silico whole-genome sequencing analysis: An overview of the last four decades. Front. Microbiol. 1864.
- Biovolume and spatial distribution of foodborne Gram-negative and Gram-positive pathogenic bacteria in mono-and dual-species biofilms. Food Microbiol.. 2021;94:103616
- [Google Scholar]
- Dietary polyphenol supplementation in food producing animals: Effects on the quality of derived products. Animals.. 2021;11(2):401.
- [Google Scholar]
- Effect of household water treatment with chlorine on diarrhea among children under the age of five years in rural areas of Dire Dawa, eastern Ethiopia: a cluster randomized controlled trial. Infect. Dis. Poverty.. 2020;9(1):1-13.
- [Google Scholar]
- Antibacterial and anti-inflammatory activities of cardamom (Elettaria cardamomum) extracts: Potential therapeutic benefits for periodontal infections. Anaerobe.. 2020;61:102089
- [Google Scholar]
- Molecular epidemiology of methicillin-resistant Staphylococcus aureus from dairy farms in North-eastern Italy. Int. J. Food Microbiol.. 2020;332:108817
- [Google Scholar]
- Membrane interactions of phytochemicals as their molecular mechanism applicable to the discovery of drug leads from plants. Molecules.. 2015;20(10):18923-18966.
- [Google Scholar]
- In vitro anticandidal potency of Syzygium aromaticum (clove) extracts against vaginal candidiasis. BMC complement. med. ther.. 2020;20(1):1-9.
- [Google Scholar]
- Anticandidal efficiency of Cinnamomum zeylanicum extracts against vulvovaginal candidiasis. Curr. Sci.. 2020;118(5):796.
- [Google Scholar]
- Anticandidal and anti-carcinogenic activities of Mentha longifolia (Wild Mint) extracts in vitro. J. King Saud Univ. Sci.. 2020;32(3):2046-2052.
- [Google Scholar]
- In Vitro Evaluation of Biological Activities and Phytochemical Analysis of Different Solvent Extracts of Punica granatum L. (Pomegranate) Peels. Plants. 2021;10(12):2742.
- [Google Scholar]
- In vitro antimicrobial activity of Thymus vulgaris extracts against some nosocomial and food poisoning bacterial strains. Process Biochem. 2022;115:152-159.
- [Google Scholar]
- Facile green synthesis of silver nanoparticles using aqueous leaf extract of Origanum majorana with potential bioactivity against multidrug resistant bacterial strains. Crystals. 2022;12(5):603.
- [Google Scholar]







