In vitro antibacterial activity of crude extracts of 9 selected medicinal plants against UTI causing MDR bacteria
⁎Corresponding author. Tel.: +91 9437134982; fax: +91 6742432034. rnpadhy54@gmail.com (Rabindra N. Padhy)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Urinary tract infection (UTI) has become a more grievous problem today, due to multidrug resistance of infecting Gram-positive (GP) and Gram-negative (GN) bacteria, sometimes even with multiple infections. This study examines effectivity of 9 tropical flowering plants (Anogeissus acuminata, Azadirachta indica, Bauhinia variegata, Boerhaavia diffusa, Punica granatum, Soymida febrifuga, Terminalia chebula, Tinospora cordifolia and Tribulus terrestris) for possible use as source of antimicrobials for multidrug resistant (MDR) bacteria, along with main-stream antibiotics. Pathogenic bacteria were isolated from urine samples of patients attending and admitted in the hospital. Antibiograms of 11 isolated bacteria (GPs, Enterococcus faecalis and Staphylococcus aureus; and GNs, Acinetobacter baumannii, Citrobacter freundii, Enterobacter aerogenes, Escherichia coli, Klebsiella oxytoca, Klebsiella pneumoniae, Proteus mirabilis, Proteus vulgaris and Pseudomonas aeruginosa) were ascertained by the disc-diffusion method, and antibacterial effectivity of plant extracts was monitored by the agar-well diffusion method. Isolated bacteria were floridly MDR to most antibiotics of the day. Methanol extracts of 9 plants were used, and extracts of 3 plants, A. acuminata, P. granatum and S. febrifuga at least caused 25–29 mm as the maximum size of zone of inhibition on bacterial lawns. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) values of methanol extracts of 9 plants were recorded. The methanol extract of A. acuminata had 0.29 mg/ml as the lowest MIC value and 0.67 mg/ml as the lowest MBC value, against MDR S. aureus, signifying effectivity; but, it had the highest MIC value of 3.41 mg/ml. and the highest MBC value of 4.27 mg/ml for most other MDR bacteria including E. coli. Qualitative phytochemical analysis was done for these 9 plants and information on leading phytochemicals was presented retrieved from PubChem database. Thus, three effective-most plants in controlling MDR-UTI bacteria in vitro were A. acuminata, P. granatum and S. febrifuga, which can be promoted as complementary medicine.
Keywords
Tropical plants
Urinary tract infection
Complementary medicine
Multidrug resistant bacteria
In vitro antibacterial activity
1 Introduction
Urinary tract infection (UTI) was mostly caused by Gram negative (GN) bacteria, predominately by Escherichia coli and by mixed infections of (Gram positive, GP) Staphylococcus aureus, Enterococcus faecalis, including other GNs, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, Enterobacter aerogenes, Proteus mirabilis, Citrobacter freundii, Proteus vulgaris and Klebsiella oxytoca in the decreasing order of prevalence, when monitored in the Institute of Medical Sciences and Sum Hospital, for example (Mishra et al., 2013). The infecting bacteria normally constitute the faecal flora, and the UTI episode is initiated, when the urine flow in an individual is obstructed by one of several reasons such as, strictures, calculi, tumours, prostatic hypertrophy, vesicourethral reflux, diabetes, anal disease, pregnancy, catheterization, some surgical procedure at the urinogenital region and cystoscopy (Saint et al., 2002). The infecting bacteria invade urethra and bladder with a compromised body defense mechanism and decreased urine flow. In these conditions, the bacteria ascend via urethra move to the bladder mucosa, colonize, multiply and cause inflammation; this causes intolerable pain, burning, frequency and urgency of urination, nocturia, foul smelling, cloudy urine and haematuria. Indeed, at the onset of the problem, the patient reports to the physician and an empiric therapy is started before the culture report of the urine sample is obtained.
If any infection in a patient is not controlled, infecting microbes get resistance to the applied antibiotics intrinsically and a drug resistant cell survives and predominates with concomitant bacterial genetic exchanges mechanisms (McMurry and Levy, 2011). In short, there are several factors of antibiotic resistance in pathogenic bacteria and this situation has become a clinical consternation. A physician often prescribes some higher generation antibiotics in empiric therapy to avoid the debacle from treatment failure arising from the possible presence of MDR bacteria. And the UTI being a graver problem than imagined because of frequent attacks in females mainly, some complementary/adjuvant/synergistic therapeutic strategy is warranted.
Furthermore, information from ethnobotany/traditional medicine has been seen useful for several health complications other than infectious diseases. Nine flowering plants (Anogeissus acuminata, Azadirachta indica, Bauhinia variegata, Boerhaavia diffusa, Punica granatum, Soymida febrifuga, Terminalia chebula, Tinospora cordifolia and Tribulus terrestris) (Fig. 1) were used. These plants are in use traditionally by local ethnic tribes against infectious diseases; and these were examined in a systematic screening with UTI causing bacteria for use as source of non-microbial antimicrobials, so that these could serve as complementary medicine, along with mainstream drugs, the antibiotics, as it takes 3–4 days of time of arrival of the culture report of the urine during which period, infecting bacteria cause further problems. UTI episodes need to be controlled with an iron hand. And to avoid the use of any antibiotic of higher generation during empiric therapy, complementary or synergistic therapy using phyto-drugs with any ongoing antibiotic could be prudently used against UTI.

- Photographs of plants: a, Anogeissus acuminata; b, Azadirachta indica; c, Bauhinia variegata; d, Boerhaavia diffusa; e, Punica granatum; f, Soymida febrifuga; g, Terminalia chebula; h, Tinospora cordifolia; i, Tribulus terrestris.
In continuation to previous work on scientific verification of ethnomedicinal information of a group of plants from Kalahandi (Odisha) forest (Mishra and Padhy, 2013; Rath and Padhy, 2014), the cited 9 plants were selected. These plants were too recorded in Indian pharmacopeia (Anonymous, 2014), as plants frequently used against general infectious diseases: A. acuminata is in use for UTI and skin diseases; whole plant of A. indica is used as an antiseptic; B. variegata is used against diarrhoea and throat infections; B. diffusa is used for UTI and dysentery; P. granatum is used for treating diarrhoea, dysentery and throat problems; S. febrifuga is used against diarrhoea, dysentery and UTI; T. chebula is used for diarrhoea and dysentery; T. cordifolia has anti-tubercular activity; and T. terrestris is used against UTI. Several other plants, Terminalia alata, Lantana camara, Combretum albidum and Woodfordia fruticosa had comparable antibacterial activities, which had been used for further pharmacognostical studies (Rath and Padhy, 2012; Rath and Padhy, 2013; Dubey et al., 2014; Sahu et al., 2014), but these 9 cited plants were not used for the isolation of individual compounds against any bacteria. Detailed antibacterial work with pathogenic bacteria isolated from clinical samples of patients in the hospital, with 9 plants is described.
2 Materials and methods
2.1 Collection of plants and extract preparation
Plants reported were collected from the Kandha tribe at hills of Eastern range of mountains of India, in the district Kalahandi, Odisha in January 2014. About 50 respondents of 25 hamlets were interviewed in a forest patch and the recorded information was documented (Table 1, Fig. 1), with the snowball method of survey and sampling. Methanol extracts of dried leaf samples dissolved in 10% v/v dimethyl sulfoxide (DMSO) were used, as detailed (Mishra and Padhy, 2013).
| Sl. No | Plant name | Family | English name; Local name | Parts used | Ethnomedicinal uses |
|---|---|---|---|---|---|
| 1 | Anogeissus acuminata (Roxb.ex.DC) wall.ex Guill. and perr.) | Combretaceae | Button tree; Phasi | Leaf/Bark | Its leaf has wound healing activity; used for inflammation, urinary tract infection (UTI) and skin diseases. Its bark is used to treat diabetes |
| 2 | Azadirachta indica L. Adelb | Meliaceae | Neem; Neemba | Leaf | It is used as an antiseptic and for antiviral action (chicken pox). It is used for the treatment of acne |
| 3 | Bauhinia variegata L. | Fabaceae | Mountain ebony; Kanchan | Leaf/Root | Its leaf is used for burning sensation during urination. The roots are used for digestive problems, diarrhoea and throat infections |
| 4 | Boerhaavia diffusa L. nom. cons. | Nyctaginaceae | Hog weed; Atika podi | Leaf | It is used to improve eyesight, to treat UTI, dysentery and diabetes |
| 5 | Punica granatum L. | Lythraceae | Pomegranate; Dalimba | Leaf/Bark/Fruits | It is used for diarrhoea, dysentery, intestinal parasites, kidney problems, heart and throat problems; it is used to stop nose bleeds and gum bleeds and as an eye drop to slow the development of cataracts |
| 6 | Soymida febrifuga Roxb. | Meliaceae | Indian redwood; Rohini | Leaf/Bark | It is used in the treatment of diarrhoea, dysentery, UTI, fever, vaginal infections, rheumatism swellings |
| 7 | Terminalia chebula Retz. | Combretaceae | Chebulic myrobala; Harida | Leaf/Fruits | Its leaves are used in skin disorders, anaemia, narcosis, piles, fever, diarrhoea, dysentery, cough and UTI; fruits are used for constipation and anorexia |
| 8 | Tinospora cordifolia (Thunb.) Miers | Menispermaceae | Heart-leaved moonseed; Guluchi | Leaf/Bark | It has hepato-protective activity; commonly it is used for diabetes and also to treat tuberculosis |
| 9 | Tribulus terrestris L. | Zygophyllaceae | Puncture vine; Gokhura | Leaf | It is used to treat kidney, bladder, UTI and sexual problems |
2.2 Isolation, identification of bacterial strains and antibiotic sensitivity test
Two GPs, E. faecalis and S. aureus including 9 GNs, A. baumannii, C. freundii, E. aerogenes, E. coli, K. oxytoca, K. pneumoniae, P. mirabilis, P. vulgaris and P. aeruginosa were used in this study. All these bacteria were directly isolated from urine samples of UTI patients attending and other patients admitted in the hospital (see, Mishra et al., 2013). Identification of pathogenic bacterial strains was done depending upon gross colony morphology and biochemical tests of isolated pure bacterial cultures, along with Microbial Type Culture Collection (MTCC), Chandigarh, reference strains (Mishra and Padhy, 2013). All bacterial strains were subjected to antibiotic sensitivity tests by the Kirby-Bauer’s disc diffusion method, using a 4 mm thick Mueller–Hinton (MH) agar (HiMedia, Mumbai) medium, following the standard antibiotic susceptibility test chart of Clinical Laboratory Standard Institute guidelines (CLSI, 2011). The use of urine samples does warrant ethical approval of the institute.
2.3 Antibacterial test of plant extracts
One strain from each bacterial species having resistance to a maximum number of presently used antibiotics was further used for monitoring antibacterial potentiality of methanol leaf extracts with gentamicin 30 μg/ml as the reference standard, by the agar-well diffusion method, as previously detailed (Mishra and Padhy, 2013; Rath and Padhy, 2014). Antibacterial activities were evaluated as before (Mishra and Padhy, 2013) and results of the third repetition are presented.
2.4 Determinations of MIC and MBC of plant extracts
Original stock solutions of leaf extracts were prepared with methanol, at 44.44 mg plant extract/ml 10% DMSO solution, with distilled water. Each stock solution was diluted to obtain final concentrations of 0.29, 0.67, 1.51, 3.41, 4.27, 9.63, 21.67 and 44.44 mg/ml with the DMSO solution. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the methanol leaf extracts were determined in a well on a 96-welled (12 × 8) micro-titre plate (Fig. 2), as described elsewhere (Mishra and Padhy, 2013; Rath and Padhy, 2014).
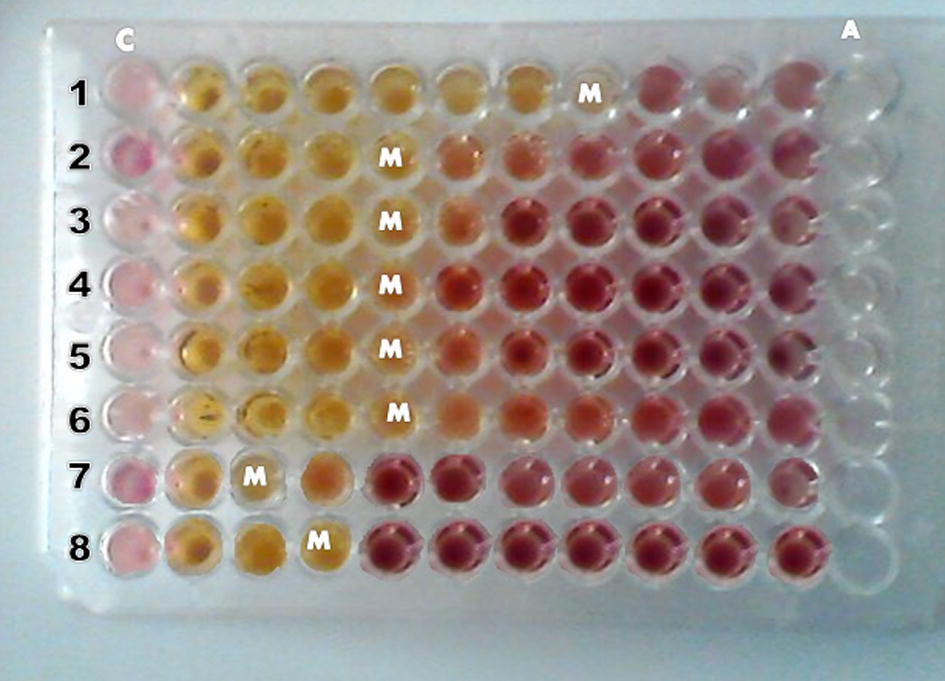
- Determination of minimum inhibitory concentrations (MICs), in a 96-well microtiter plate, of 44.44 mg/ml of methanol leaf extract of P. granatum against 8 MDR UTI causing pathogenic bacteria (1 = S. aureus, 2 = E. faecalis, 3 = A. baumannii, 4 = E. aerogenes, 5 = K. pneumoniae, 6 = K. oxytoca, 7 = P. mirabilis, 8 = P. vulgaris). M = MIC at numbers that signifies the lowest concentration of leaf extract. C = control without plant leaf extract; A = Gentamicin (30 μg/ml) as control without any plant leaf extract.
2.5 Qualitative phytochemical analyses
The following tests were performed for selected 9 medicinal plants for the presence of alkaloids, carbohydrates, saponins, flavonoids, steroids/terpenes, tannins, resins, glycosides, and anthraquinones, as detailed previously (Dubey and Padhy, 2013).
2.6 Statistical analysis
Kruskal–Wallis H test for data of zone of inhibition as antibacterial activities in agar-cup method of 3 comparable plants against 11 bacteria was done using the Statistical Package for Medical Science version 17.0 (SPSS Inc., IL, USA).
3 Results
3.1 Collection of plants
Ethnomedicinal information on 9 selected medicinal plants are documented along with the details of modalities on crude extracts as medicine for many ailments used by local ethnic aborigine groups (Table 1). Most of these plants are used for infectious diseases and were found edible as medicines by the aborigine society.
3.2 Antibiotic sensitivity test of bacteria
The antibiotic profile of each pathogenic bacterium was determined using specified antibiotic discs (Table 2). GP isolates, E. faecalis were resistant to 17 and S. aureus were resistant to 13 of 18 antibiotics used. Among the nine GN isolates, A. baumannii, E. aerogenes, and E. coli were resistant to 11, C. freundii, K. pneumoniae, K. oxytoca and P. aeruginosa were resistant to 12, P. mirabilis and P. vulgaris were resistant to 10 antibiotics of the total 14 antibiotics used. The details of individual antibiotics resistant profiles of individual bacteria are presented (Table 2). Thus, all isolated bacterial strains were MDR.
| Bacterium | Susceptibility to prescribed antibiotics | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aminoglycosides | β-lactams | Cephalosporin | Fluoroquinolones | Glyco-peptides | Lincosa-mide | Sulfonamide | Stand alones | |||||||||||
| Ac | Ge | Ak | Am | Ox | Pt | Ce | Cf | Of | Le | Nx | Gt | Tei | Va | Cd | Cot | Ch | Lz | |
| E. faecalis∗ | R | R | R | R | R | R | R | R | R | R | R | R | R | S | R | R | R | R |
| S. aureus∗ | R | R | R | R | MS | R | R | R | R | R | R | R | MS | MS | MS | R | R | S |
| A. baumannii | R | R | R | R | ND | R | R | R | R | R | MS | S | ND | ND | ND | R | R | S |
| C. freundii | R | R | R | R | ND | R | R | R | R | R | R | MS | ND | ND | ND | R | R | S |
| E. aerogenes | R | R | R | R | ND | R | R | R | R | R | R | MS | ND | ND | ND | R | MS | S |
| E. coli | R | R | R | R | ND | S | R | R | R | R | R | R | ND | ND | ND | S | R | S |
| K. oxytoca | R | R | R | R | ND | R | R | R | R | R | R | MS | ND | ND | ND | S | R | R |
| K. pneumoniae | R | R | R | R | ND | R | R | R | R | R | R | S | ND | ND | ND | R | R | S |
| P. mirabilis | R | R | R | R | ND | S | R | R | S | R | S | MS | ND | ND | ND | R | R | R |
| P. vulgaris | R | R | R | S | ND | R | R | S | S | R | R | S | ND | ND | ND | R | R | R |
| P. aeruginosa | R | R | R | R | ND | R | R | R | R | R | R | MS | ND | ND | ND | R | R | S |
Note: ‘∗’ marked bacteria are Gram-positives and the rest are Gram-negatives. R: Resistant; S: Sensitive; MS: moderately sensitive; ND: not done. Antibiotics (μg/disc), Ac: amikacin 30; Ak: amoxyclav 30; Am: ampicillin 10; Cd: clindamycin 2; Cf: cefpodoxime 10; Ch: chloramphenicol 30; Cot: co-trimoxazole 25; Ce: ceftriaxone 30; Ge: gentamicin 10; Gt: gatifloxacin 5; Nx: norfloxacin 10; Le: levofloxacin 5; Lz: linezolid 30; Of: ofloxacin 5; Ox: oxacillin 1; Pt: piperacillin/tazobactam 100/10; Tei: teicoplanin 5; Va: vancomycin 30.
3.3 Antibacterial test of plant extracts
Methanol extracts of medicinal plants when tested against MDR strains of 11 bacteria, 3 plants, A. acuminata, P. granatum and S. febrifuga were seen most effective, with at least causing 25 to 29 mm diameter-sizes of zone of inhibition against any bacterium (Table 3). The A. acuminata leaf extract registered the highest value of inhibition zone of 27 mm, against S. aureus and the lowest value of 20 mm against P. mirabilis was recorded; but, inhibition zone values due to A. acuminata were recorded for other given bacteria (mm): E. faecalis (24), A. baumannii (23), C. freundii (22), E. aerogenes (21), E. coli (22), K. oxytoca (23), K. pneumoniae (24), P. vulgaris (21) and P. aeruginosa (25). Thus, A. acuminata extract was effectively capable of controlling all the 11 MDR bacteria. Methanol leaf extract of A. indica had shown the highest inhibition-zone size of 22 mm against E. aerogenes, while the lowest value was 12 mm against P. mirabilis. Methanol leaf extract of P. granatum had the highest value of inhibition-zone size, 26 mm against S. aureus and the lowest value of 17 mm was against P. mirabilis; the extract was effectively capable of controlling all the 11 MDR pathogens by registering values of inhibition zones ranging from 18 to 25 mm. The highest value of inhibition-zone size of 25 mm against S. aureus and the lowest value of 17 mm against E. aerogenes had been noted due to the methanol extract of S. febrifuga; and 20, 23, 21, 19, 22, 21, 19, 20 and 28 mm values of size of zone of inhibition were recorded against the pathogenic bacteria, E. faecalis, A. baumannii, C. freundii, E. coli, K. oxytoca, K. pneumoniae, P. mirabilis, P. vulgaris and P. aeruginosa, respectively. The methanol leaf extract of S. febrifuga had an effective controlling capacity over all the pathogens. Methanol leaf extracts of the rest 6 plants had moderate control capacity on all bacterial strains (Table 3). The total size of zone of inhibition of all used plants is arranged according to the decreasing order, A. acuminata > P. granatum > S. febrifuga > A. indica > B. variegata > T. terrestris > T. cordifolia > T. chebula > B. diffusa. Moreover, Kruskal–Wallis H test for data of zone of inhibition of the 3 best plants, A. acuminata, P. granatum and S. febrifuga yielded the H-value of 0.83, which was statistically not significant; so, these 3 plants were inferred as equally effective for antibacterial activity.
| Bacteria | Size of zone of inhibition by plants (Nos. 1 to 9) methanol extracts (mm) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A. acuminata | A. indica | B. variegata | B. diffusa | P. granatum | S. febrifuga | T. chebula | T. cordifolia | T. terrestris | Ge 30 μg/ml | |
| E. faecalis | 24 | 18 | 17 | 15 | 23 | 20 | 18 | 17 | 19 | 25 |
| S. aureus | 27 | 20 | 21 | 17 | 26 | 25 | 23 | 19 | 21 | 28 |
| A. baumannii | 23 | 17 | 14 | 13 | 22 | 23 | 15 | – | 15 | 20 |
| C. freundii | 22 | 21 | 13 | 12 | 24 | 21 | – | 13 | 13 | 21 |
| E. aerogenes | 21 | 22 | 15 | 14 | 20 | 17 | – | 14 | – | 23 |
| E. coli | 22 | 19 | 19 | 10 | 25 | 19 | 14 | 16 | 17 | 26 |
| K. oxytoca | 23 | 15 | 15 | 13 | 21 | 22 | 15 | 15 | 16 | 22 |
| K. pneumoniae | 24 | 17 | 17 | 15 | 18 | 21 | 13 | 13 | 15 | 20 |
| P. mirabilis | 20 | 12 | 12 | – | 17 | 19 | 11 | 11 | 18 | 22 |
| P. vulgaris | 21 | 13 | 14 | – | 19 | 20 | – | – | 17 | 23 |
| P. aeruginosa | 25 | 15 | 20 | 15 | 24 | 18 | 16 | 18 | 20 | 26 |
| Total zone size | 252 | 189 | 177 | 124 | 239 | 225 | 125 | 136 | 171 | |
Note: Numbers 1 to 9 are serial numbers of plants given in Table 1; Ge, gentamicin. Values are measurements of zone of inhibition due to methanol-extracts. “–” sign denotes no activity. Kruskal–Wallis H test for data of zone of inhibition of 3 plants, A. acuminata, P. granatum and S. febrifuga yielded the H-value of 0.83, which was statistically not significant; so, these 3 plants were equally effective for antimicrobial activity. The rest other 6 plants were clearly lesser in antimicrobial activity in comparison to cited 3 plants. It was confirmed that 10% DMSO had no inhibitory effect on any bacterium.
3.4 MIC and MBC of plant extracts
The methanol leaf extract of A. acuminata had the lowest MIC value, 0.29 mg/ml and the lowest MBC value 0.67 mg/ml against S. aureus; MIC value of 0.67 mg/ml and MBC value of 1.51 mg/ml against E. faecalis, K. pneumoniae and P. aeruginosa, while MIC value of 1.51 mg/ml and MBC value of 3.41 mg/ml against A. baumannii and K. oxytoca were recorded by A. acuminata. On the other hand, the highest MIC value of 3.41 mg/ml, and the highest MBC value of 4.27 mg/ml due to A. acuminata extract were noted for C. freundii, E. aerogenes, E. coli, P. mirabilis and P. vulgaris. The methanol leaf extract of P. granatum showed the lowest MIC value of 0.29 mg/ml and the lowest MBC value of 0.67 mg/ml against S. aureus; MIC value of 0.67 mg/ml and MBC value of 1.51 mg/ml was noted against C. freundii, E. coli and P. aeruginosa; MIC value of 1.51 mg/ml and MBC value of 3.41 mg/ml was recorded against E. faecalis; MIC value of 3.41 mg/ml and MBC value of 4.27 mg/ml were recorded against A. baumannii, E. aerogenes, K. oxytoca and K. pneumoniae. The highest MIC value of 4.27 mg/ml and MBC value of 9.63 mg/ml by the extract of P. granatum were noted against P. mirabilis and P. vulgaris. The methanol leaf extract of S. febrifuga showed the lowest MIC value of 0.67 mg/ml and the lowest MBC value of 1.51 mg/ml against S. aureus. MIC value of 1.51 mg/ml and MBC value of 3.41 mg/ml were recorded against A. baumannii. By the extract of S. febrifuga, MIC value 3.41 mg/ml and MBC value 4.27 mg/ml were noted against E. faecalis, C. freundii, K. oxytoca, K. pneumoniae and P. vulgaris and the highest MIC value of 4.27 mg/ml and MBC value of 9.63 mg/ml were recorded against E. aerogenes, E. coli, P. mirabilis and P. aeruginosa. The MIC and MBC values of leaf extracts of rest other plants were recorded (Table 4). A lower MIC/MBC value signifies that a minimum amount of plant extract is used, whereas, a higher value signifies the use of comparatively more amount of plant extract for the control of any bacterium.
| Bacterium | MIC and MBC values by methanol extracts of 9 plants (mg/ml) | Gentamicin μg/ml | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | |
| E. faecalis | 0.67 | 1.51 | 3.41 | 4.27 | 4.27 | 9.63 | 9.63 | 21.67 | 1.51 | 3.41 | 3.41 | 4.27 | 4.27 | 9.63 | 4.27 | 9.63 | 4.27 | 9.63 | 0.93 | 1.87 |
| S. aureus | 0.29 | 0.67 | 3.41 | 4.27 | 3.41 | 4.27 | 4.27 | 9.63 | 0.29 | 0.67 | 0.67 | 1.51 | 1.51 | 3.41 | 4.27 | 9.63 | 3.41 | 4.27 | 0.46 | 0.93 |
| A. baumannii | 1.51 | 3.41 | 4.27 | 9.63 | 9.63 | 21.67 | 9.63 | 21.67 | 3.41 | 4.27 | 1.51 | 3.41 | 9.63 | 21.67 | – | – | 9.63 | 21.67 | 3.75 | 7.50 |
| C. freundii | 3.41 | 4.27 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 0.67 | 1.51 | 3.41 | 4.27 | – | – | 9.63 | 21.67 | 9.63 | 21.67 | 1.87 | 3.75 |
| E. aerogenes | 3.41 | 4.27 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 3.41 | 4.27 | 4.27 | 9.63 | – | – | 9.63 | 21.67 | – | – | 0.93 | 1.87 |
| E. coli | 3.41 | 4.27 | 4.27 | 9.63 | 4.27 | 9.63 | – | – | 0.67 | 1.51 | 4.27 | 9.63 | 9.63 | 21.67 | 4.27 | 9.63 | 4.27 | 9.63 | 1.87 | 3.75 |
| K. oxytoca | 1.51 | 3.41 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 3.41 | 4.27 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 4.27 | 9.63 | 0.93 | 1.87 |
| K. pneumoniae | 0.67 | 1.51 | 4.27 | 9.63 | 4.27 | 9.63 | 9.63 | 21.67 | 3.41 | 4.27 | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | 9.63 | 21.67 | 1.87 | 3.75 |
| P. mirabilis | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | – | – | 4.27 | 9.63 | 4.27 | 9.63 | 9.63 | 21.67 | 9.63 | 21.67 | 4.27 | 9.63 | 3.75 | 7.50 |
| P. vulgaris | 3.41 | 4.27 | 9.63 | 21.67 | 9.63 | 21.67 | – | – | 4.27 | 9.63 | 3.41 | 4.27 | – | – | – | – | 4.27 | 9.63 | 1.87 | 3.75 |
| P. aeruginosa | 0.67 | 1.51 | 9.63 | 21.67 | 3.41 | 4.27 | 9.63 | 21.67 | 0.67 | 1.51 | 4.27 | 9.63 | 9.63 | 21.67 | 4.27 | 9.63 | 3.41 | 4.27 | 0.46 | 0.93 |
Note: Numbers 1 to 9 are serial numbers of plants given in Table 1. Values are measurements of MIC and MBC due to methanol extracts. “–” sign denotes no activity; Gentamicin was used as dilutions from 30 μg/ml.
3.5 Qualitative phytochemical analyses
Qualitative phytochemical analyses were done for methanol leaf extracts. Phytochemicals, alkaloids, flavonoids, carbohydrates, terpenoids, steroids, tannins, resins, saponins and anthraquinones, which could be attributed to the recorded significant antibacterial activities in most extracts (Table 5). From PubChem database, structure and related information of one leading antimicrobial compound of each plant was presented (Table 6). From previous studies, the antibacterial nature of these cited 9 compounds were ascertained (Table 6). The Molsoft tool (http://molsoft.com/mprop/) was used to find out the drug likeness scores of each compound, according to their structure canonical ‘simplified molecular-input line-entry system’ (SMILES). Thus theoretically, based on the available data on drug likeness scores, phytochemicals could be arranged in the decreasing order of drug-likeness scores mentioned against: quercetin (0.93) > berberin (0.91) > luteolin-7-O-glugoside (0.86) > kaempferol (0.77) > ursolic acid (0.65) > argungenin (0.61) and mahmoodin (0.61) > conocarpan (0.13) and dihydrodedrodehydrodiconiferylalcohol (0.13) > anolignan B (−0.78). It could be inferred here that S. febrifuga among the 3 best plants is the most leading plant with luteolin-7-O-glugoside, which has a good score of drug-likeness (Table 6).
| Sl.No. | Plants | Alkaloids | Resins | Glycosides | Terpenoids | Carbohydrates | Saponins | Tannins | Flavonoids | Steroids | Anthraquinones |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A. acuminata | + | − | + | + | + | + | + | + | + | + |
| 2 | A. indica | − | + | + | + | + | + | − | − | + | − |
| 3 | B. variegata | + | + | + | − | + | + | + | − | + | + |
| 4 | B. diffusa | + | − | − | − | − | − | + | + | + | + |
| 5 | P. granatum | + | − | + | + | − | + | + | + | + | + |
| 6 | S. febrifuga | + | + | + | + | + | − | + | + | + | + |
| 7 | T. chebula | + | + | + | + | + | − | + | + | − | + |
| 8 | T. cordifolia | + | − | + | − | + | + | − | − | + | − |
| 9 | T. terrestris | − | − | − | + | − | − | − | − | + | − |
Note: “+” sign denotes presence, and “− “sign denotes absence of the compound in a plant.
| Plant name | Leading antimicrobial phytochemical structure | Information and properties of leading phytochemical | Drug-likeness score | References |
|---|---|---|---|---|
| A. acuminata |
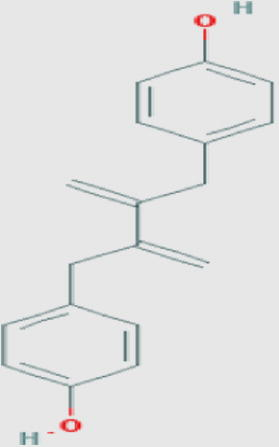
|
Anolignan B (oc) Molecular weight: 266.33432 [g/mol] Molecular formula: C18H18O2 XLogP3-AA: 5.5 H-Bond donor: 2 H-Bond acceptor: 2 |
−0.78 | Rimando et al. (1994a,b), Eldeen et al. (2006) |
|
Conocarpan (oc) Compound ID: 6474521 Molecular Weight: 266.33432 [g/mol] Molecular Formula: C18H18O2 XLogP3-AA: 4.4 H-Bond donor: 1 H-Bond acceptor: 2 |
0.13 | ||
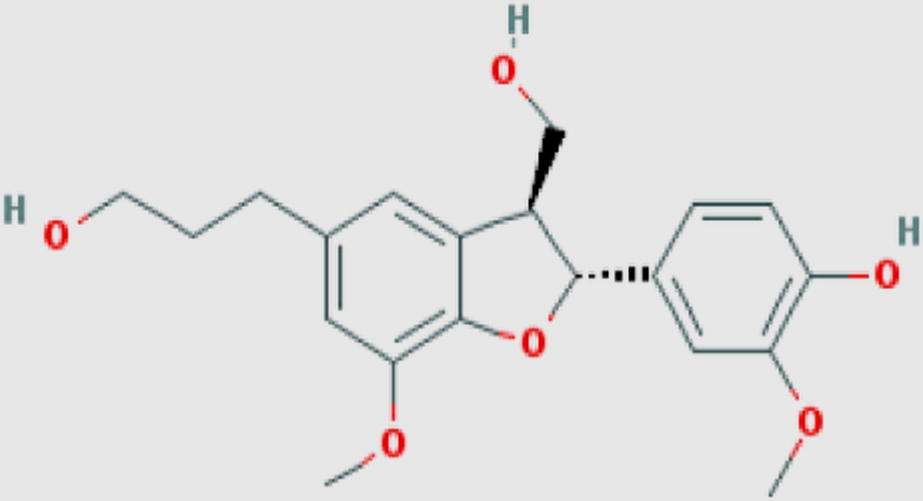
|
Dihydrodehydrodiconiferylalcohol (oc) Compound ID: 5274623 Molecular weight: 360.40096 [g/mol] Molecular formula: C20H24O6 XLogP3-AA: 2.1 H-Bond donor: 3 H-Bond acceptor: 6 |
0.13 | ||
| A. indica |

|
Mahmoodin (l) Compound ID: 126566 Molecular weight: 526.61792 [g/mol] Molecular formula: C30H38O8 XLogP3-AA: 3.7 H-Bond donor: 1 H-Bond acceptor: 8 |
0.61 | Siddiqui et al. (1992) |
| B. variegata |
|
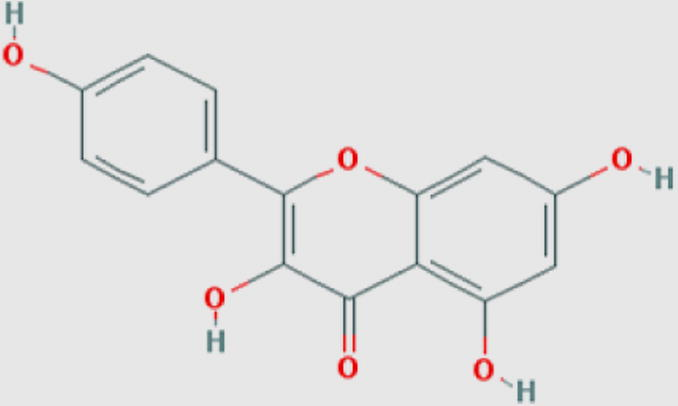
Kaempferol (f) Compound ID: 5280863 Molecular weight: 286.2363 [g/mol] Molecular formula: C15H10O6 XLogP3: 1.9 H-Bond donor: 4 H-Bond acceptor: 6 |
0.77 | Holler et al. (2012) |
| B. diffusa |
|
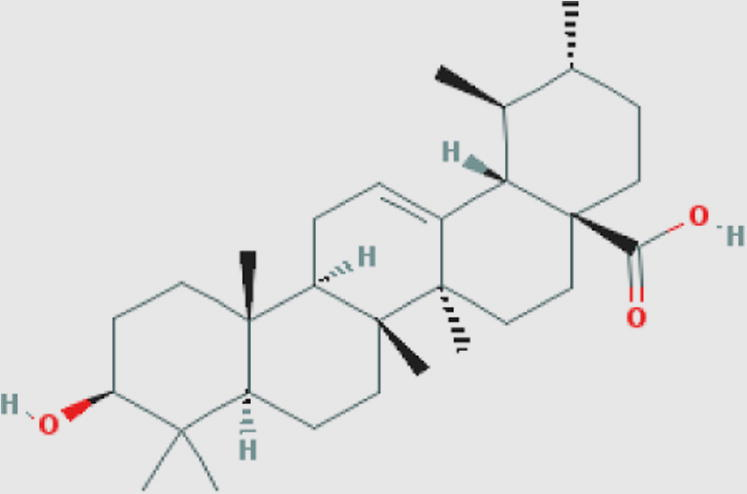
Ursolic acid (t) Compound ID: 64945 Molecular weight: 456.70032 [g/mol] Molecular formula: C30H48O3 XLogP3-AA: 7.3 H-Bond donor: 2 H-Bond acceptor: 3 |
0.65 | Jiménez-Arellanes et al. (2013) |
| P. granatum |
|
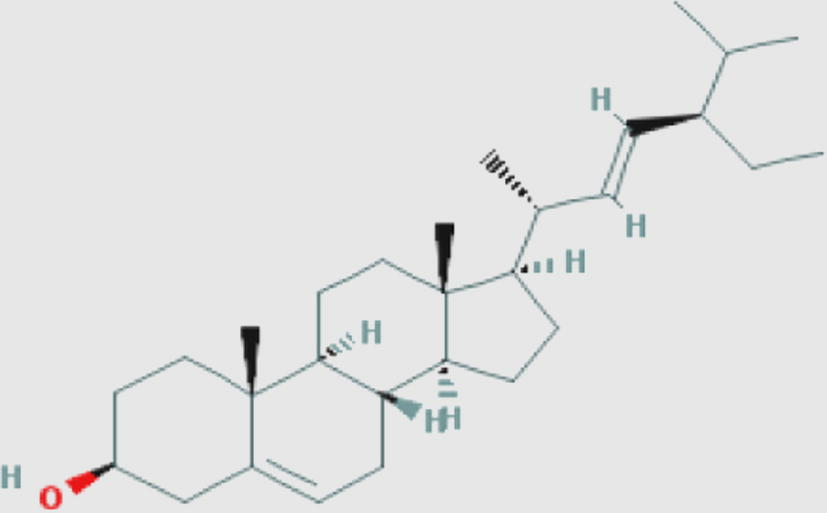
Stigmasterol (s) Compound ID: 5280794 Molecular weight: 412.69082 [g/mol] Molecular formula: C29H48O XLogP3-AA: 8.6 H-Bond donor: 1 H-Bond acceptor: 1 |
0.73 | Awouafack et al. (2013) |
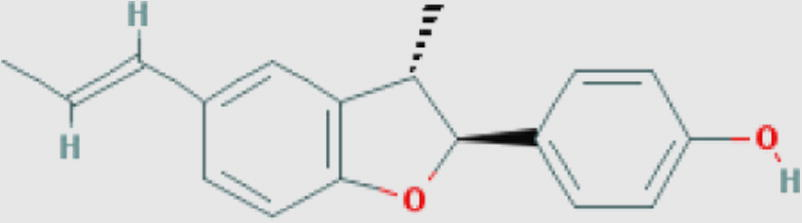
| S. febrifuga |
|
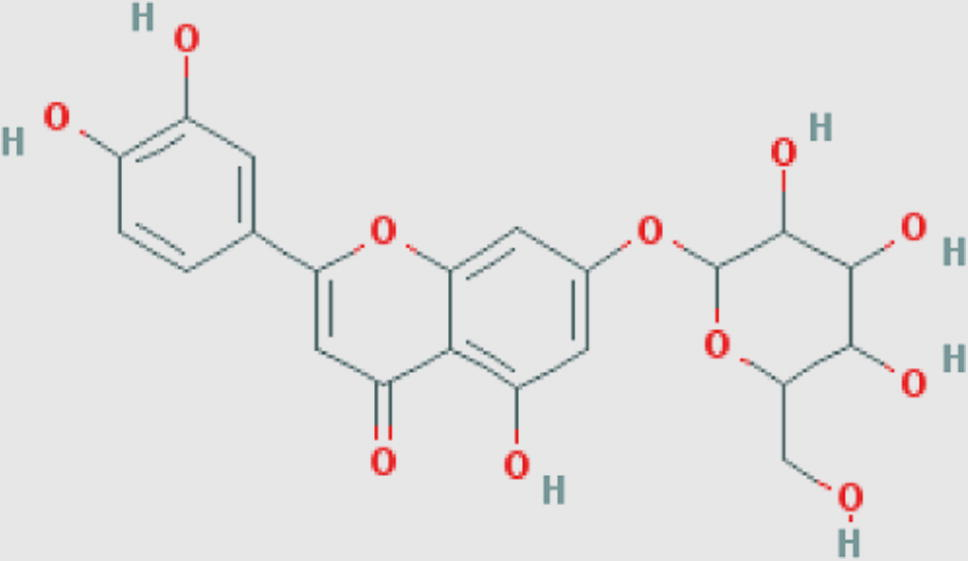
Luteolin-7-O-glucoside (f) Compound ID: 5291488 Molecular weight: 448.3769 [g/mol] Molecular formula: C21H20O11 XLogP3-AA: 0.5 H-Bond donor: 7 H-Bond acceptor: 11 |
0.86 | Khatkar et al. (2014) |
| T. chebula |
|
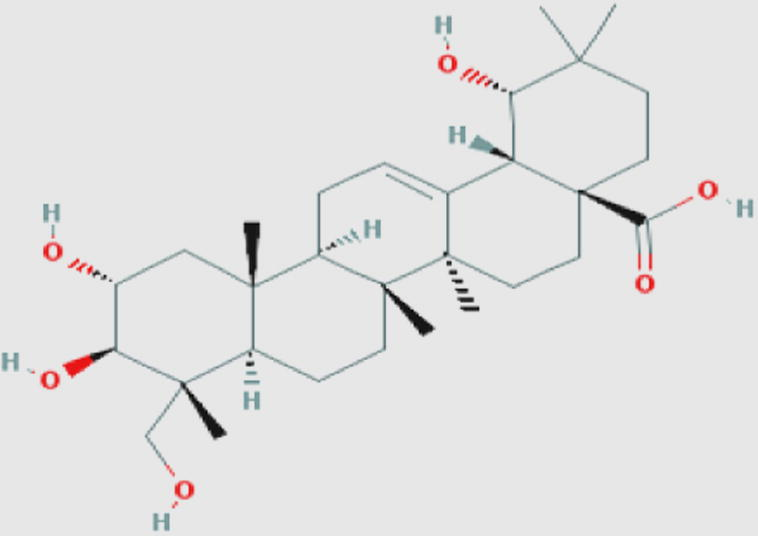
Arjungenin (t) Compound ID: 12444386 Molecular weight: 504.69852 [g/mol] Molecular formula: C30H48O6 XLogP3-AA: 4.5 H-Bond donor: 5 H-Bond acceptor: 6 |
0.61 | Manosroi et al. (2013) |
| T. cordifolia |
|
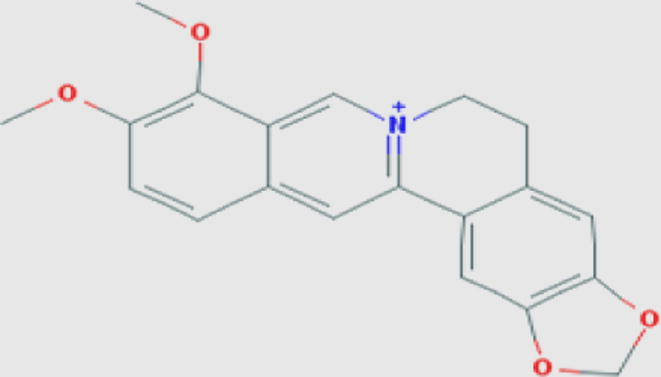
Berberin (a) Compound ID: 2353 Molecular weight: 336.36122 [g/mol] Molecular formula: C20H18NO4+ XLogP3-AA: 3.6 H-Bond donor: 0 H-Bond acceptor: 4 |
0.91 | Choudhary et al. (2013) |
| T. terrestris |
|
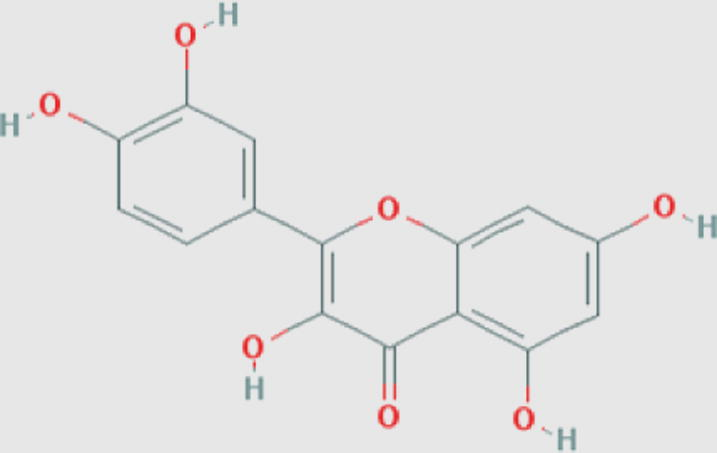
Quercetin (f) Compound ID: 5280343 Molecular weight: 302.2357 [g/mol] Molecular formula: C15H10O7 XLogP3: 1.5 H-Bond donor: 5 H-Bond acceptor: 7 |
0.93 | Rashed and Butnariu (2014) |
Note: a, alkaloid; f, flavonoid; l, limonoid; oc, organic compound; s, sterol; t, terpene.
4 Discussion
All of these 9 plants were used by an ethnic tribe, the Kandha tribe of Kalahandi district, since time immemorial for primary healthcare needs specifically for infectious diseases. It was seen that, 11 bacteria isolated from urine samples were resistant to the following: aminoglycosides, β-lactams (amoxyclav and ampicillin), two cephalosporins (ceftriaxone and cefpodoxime) as well as, chloramphenicol, signifying most bacterial strains as resistant to most antibiotics. Additionally, plants, A. acuminata, A. indica, B. variegata, P. granatum and S. febrifuga had control capacity on all the 11 strains of MDR bacteria. Moreover, among these 3 best plants in the control of MDR bacteria in vitro were A. acuminata, P. granatum and S. febrifuga. P. granatum has stigmasterol, a sterol with the drug-likeness score, 0.73 while, S. febrifuga has luteolin-7-O-glucoside, a flavonoid with the drug-likeness score, 0.86. Further work on antibacterial bioactive compounds of A. acuminata is in progress, as it had significant microbial activity. However, the plant, T. terrestris has the famous antimicrobial agent, quercetin, which has an effective drug likeness score of 0.93; but this plant did not have the best antibacterial activity, in this study or with these bacteria.
Antibiotic sensitive pathogens have a limited capacity of virulence as the employed antibiotic controls them in vivo. At a particular density, the host defense system too helps control pathogens, when the later are in a limiting number. As known, for the internal protection, antibiotic producing organisms harbour antibiotic resistant genes in plasmids and chromosomes, as well as the transfer mechanisms remain active (Mamelli et al., 2009). Therefore, such genes and/or transposon are taken up, horizontally by the susceptible group of bacteria, through bacterial transformation and/or conjugation (Pages et al., 2008; Warnes et al., 2012).
Moreover, bacteria having simple genomes undergo intrinsic (mutations) or acquired genetic (conjugations and transformation) changes in the presence of an antibiotic, as a stress factor (Groisman and Ochman, 1996). As a result, accrual antibiotic resistance mechanisms are the clinical determinants of the pathogenesis. It had been known that in areas, where a particular group of antibiotics are used bacteria resistance to same antibiotics were in higher numbers (Shrestha et al., 2002). Indeed, the horizontal transfer of genetic materials from one organism to another appears faster than mutational changes, a phenomenon popularly called as ‘evolution of quantum leaps’ (Groisman and Ochman, 1996). Progressively, the use of more antibiotics even of higher generations for the control of infectious diseases have led to multiple resistances, i.e., too many antibiotics are ineffective to progressively increasing resistant strains of pathogens, as if growth and momentum gained by a descending snow-ball, during the passage of time by mutation and acquisition of genes from related/unrelated bacteria, ending in shockingly repellant multidrug bacterial resistance. Older antibiotics slowly become moribund, even the resistant mechanism against those are found in certain bacteria for which, those antibiotics were never applied. Drug resistant bacteria gain the capability of surviving and multiplying under antibiotic-stress conditions, confirming the biological rule, ‘any limiting condition for the majority would be an excellent opportunity for the minority’. In the presence of a drug in a body in vivo, the progeny of a drug sensitive strain is eliminated and the resistant strain survives, multiplies as if, developing from a doppelgänger, and predominates ultimately in causing a characteristic pathogenesis. It is because a suitable emulating agent for the control is absent, and if plant-based antimicrobial would be present in parallel along with the employed antibiotic, there would be the coveted blithesome result, since no bacterium how much genetically well-equipped be it may as in a cohort of MDR bacteria, can never over-ride complexities of phytochemicals for survival. This fact is repeatedly seen in vitro with several plant extracts (Dubey and Padhy, 2013; Mishra and Padhy, 2013; Rath and Padhy, 2013; Sahu et al., 2015). Thus, those in a coalesced manner, as in a crude extract, have a combined controlling effect.
It has been demonstrated with Salmonella enterica serotype typhimurium (Alekshun and Levy, 1999). Moreover, MDR Neisseria gonorrheae had been known to acquire ‘MTR and SAP A MDR’ systems of genes, from S. enterica serotype typhimurium (Hagman and Shaferm, 1995). Discovery and development of antibiotics in the last century have not only saved countless human lives, but have provided assurances in clinical management all over. But, concomitant development of antibiotic-resistance mainly in bacteria has dismayed both preventive and therapeutic potencies of antibiotics today. In the odyssey of drug development, antibiotics are introduced continually and a few of them are modified suiting to the need to overcome bacterial resistance. Eventually, today there are a large number of antibiotics in use. The demand for newer antibiotics for MDR bacteria in colossal scale has arisen, which has become difficult to meet, as these small molecules are extremely complex in functionality linked to chemical structure. Secondly, an antibiotic ensconced for a typical set of infections cannot ordinarily be abandoned as an obsolete drug, due to reports of dogmatic/realistic resistance in a geographical zone; rather, along with the same antibiotic the introduction of complementary or adjuvant drug could be aimed, when considered with contemplation the problem of morbidity/mortality from infections due to MDR bacteria (Davies and Davies, 2010).
Applied antibiotics, being of microbial origin, are readily won over by pathogenic microbes in vivo. The cell producing an antibiotic has the characters of the self-protective mechanism as characters/genes, which direct the modes of resistance such as, alteration in the cell membrane by efflux mechanism or production of external enzymes like, β-Lactamases are intrinsically transmitted to similar bacteria, as discussed (Rout et al., 2014). In short, suitable antibiotics are required in colossal scale globally, which would give a way to the chance of development of resistant strain(s) of pathogenic bacteria in a Darwinian way, further. Indeed, the present methodology of methanol-extraction of phytochemicals is a unique approach, as this solvent helps extraction of most polar to non-polar phytocompounds (Rezaie et al., 2015). The β-Lactam group of antibiotics consisting of penicillins, cephalosporins, monobactams, glycopeptides and penems target the peptidoglycan biosynthesis of bacteria. Tetracyclines, aminoglycosides, macrolides, lincosamides, streptogramins, oxazolidinone (linezolid) and phenicols cause a thwart to the translation process in the parasitic cell; quinolones inhibit the DNA replication. Pyrimidines and sulphonamides alter carbon metabolism during inhibition of parasitic growth; and the most recent ones such as, daptomycin and colistin inhibit cell membrane functions (see, Davies and Davies, 2010). However, the first effective antimicrobials were the sulphonamides, which have been amply lent themselves for further uses in the drug development process as antibacterials in the last several decades. Thus, the concept of complementary use of phytocompounds along with main stream drugs, the antibiotics has immersed when the myriad mechanisms of drug resistance is considered in the face of success of phytocompounds as non-microbial antimicrobials (Sahu et al., 2015).
Our school has screened out about 250 plants in the last 4 years (Rath and Padhy, 2012; Rath et al., 2012; Dubey and Padhy, 2012; Mishra and Padhy, 2013; Rath and Padhy, 2014; Sahu et al., 2015), using mainly ethanol and water as extracting solvents. Among them for 47 plants methanol was used as the solvent (Mishra and Padhy, 2013; Rath and Padhy, 2014). A comparative account of MIC values of the best plants among 47 against MDR strains of 8 gruesome bacteria is considered, along with the present 3 best plants (Table 7). It is discernible that methanol extracts of most plants had MIC values as 3.41 or more, except those of Cinnamomum tamala, A. acuminata, P. granatum and S. febrifuga, which had comparatively lower MIC values, as 1.51 or 0.67 or 0.29 mg/ml.
| Bacteria | Ef | Sa | Ab | Cf | Ea | Ec | Kp | Pa | References |
|---|---|---|---|---|---|---|---|---|---|
| Plants | |||||||||
| Allium sativum | NE | 9.63 | 9.63 | 4.27 | 3.41 | NE | 9.63 | NE | Rath and Padhy (2014) |
| Amomum aromaticum | 3.41 | 4.27 | NE | NE | 9.63 | NE | NE | 4.27 | Rath and Padhy (2014) |
| Artocarpus heterophyllus | 9.63 | 9.63 | 9.63 | 9.63 | 9.63 | NE | 9.63 | 9.63 | Mishra and Padhy (2013) |
| Cinnamomum tamala | 3.41 | 1.51 | NE | NE | 3.41 | NE | 9.63 | 4.27 | Rath and Padhy (2014) |
| Dalbergia latifolia | 9.63 | 3.41 | 9.63 | 9.63 | 9.63 | 9.63 | NE | 4.27 | Mishra and Padhy (2013) |
| Gmelina arborea | 9.63 | 3.41 | 9.63 | 9.63 | NE | 9.63 | 9.63 | 9.63 | Mishra and Padhy (2013) |
| Melia azedarach | 9.63 | 3.41 | 9.63 | NE | NE | NE | NE | 9.63 | Mishra and Padhy (2013) |
| Mentha spicata | NE | 9.63 | 9.63 | NE | 1.51 | NE | 3.41 | 4.27 | Rath and Padhy (2014) |
| Mimusops elengi | 4.27 | 4.27 | 9.63 | NE | 9.63 | 9.63 | 9.63 | 9.63 | Mishra and Padhy (2013) |
| Myristica fragrans | 3.41 | NE | NE | 4.27 | 3.41 | NE | 9.63 | 9.63 | Rath and Padhy (2014) |
| Pongamia pinnata | 9.63 | 4.27 | NE | NE | 9.63 | NE | NE | 9.63 | Mishra and Padhy (2013) |
| Pterocarpus marsupium | 4.27 | 4.27 | 9.63 | 9.63 | 9.63 | 9.63 | NE | 9.63 | Mishra and Padhy (2013) |
| Shorea robusta | 9.63 | 4.27 | 9.63 | 9.63 | NE | NE | 9.63 | 3.41 | Mishra and Padhy (2013) |
| Anogeissus acuminata | 0.67 | 0.29 | 1.51 | 3.41 | 3.41 | 3.41 | 0.67 | 0.67 | Present work |
| Punica granatum | 1.51 | 0.29 | 3.41 | 0.67 | 3.41 | 0.67 | 3.41 | 0.67 | Present work |
| Soymida febrifuga | 3.41 | 0.67 | 1.51 | 3.41 | 4.27 | 4.27 | 3.41 | 4.27 | Present work |
Note: Ef, E. faecalis; Sa, S. aureus; Ab, A. baumannii; Cf, C. freundii; Ea, E. aerogenes; Ec, E. coli; Kp, K. pneumoniae; Pa, P. aeruginosa, ND, not done; NE, no effect.
5 Conclusion
Antibiograms of 11 isolated pathogenic bacteria with 17 antibiotics of the day ascertained that all were amply MDR. The work on individual 9 plants in controlling all MDR strains of bacteria was evident, mostly with lower MIC and MBC values. All these used plants have ethnomedicinal uses and 3 best plants could be promoted as complementary medicine. The recorded data of 3 best plants, A. acuminata, P. granatum and S. febrifuga, are anticipated to trigger work on the isolation of pure compounds for further scientific use in the crusade of the control of MDR bacteria. Phytocompounds, stigmasterol and luteolin-7-O-glucoside already isolated from the second and the third best antibacterial plant, respectively have significant drug-likeness scores. Thus, the presently used three best plants could be regarded as the most effective plants studied for further consideration for complementary medicine as sources of non-microbial anti-microbials against most MDR UTI causing bacteria.
Acknowledgements
MPM and SR contributed equally to the work, which was supported by a major research project on ‘Development of standardized herbal extracts against urinary tract bacterial infection’ (grant no. BT/PR8214/PBD/17/863/2013) from Department of Biotechnology, Govt. of India, New Delhi. We are grateful to Prof. Dr. SC Si, Dean, SPS, S’O’A University, Bhubaneswar for encouragements, and Prof. Dr. MR Nayak, President, S’OA University, Bhubaneswar, for extended facilities.
References
- Anonymous, 2014. Indian Pharmacopoeia. Vol. 1, seventh ed. <http://pharmapdfdownloads-blogspot.in/2014/05/indian-pharmacopoeia-download.html>.
- The mar regulon: multiple resistances to antibiotics and other toxic chemicals. Trends Microbiol.. 1999;7:410-413.
- [Google Scholar]
- Antimicrobial activity and cytotoxicity of the ethanol extract, fractions and eight compounds isolated from Eriosema robustum (Fabaceae) BMC Complement. Alternat. Med.. 2013;13:289.
- [Google Scholar]
- Tinospora cordifolia: ethnobotany, phytopharmacology and phytochemistry aspects. Int. J. Pharma. Sci. Res.. 2013;4:891-899.
- [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance standard for antimicrobial susceptibility testing: twenty-first informational supplement (third ed.). Wayne, PA, USA: Clinical and Laboratory Standards Institute; 2011. (Document M200–S21)
- Origins and evolution of antibiotic resistance. Microbiol. Molec. Boil. Rev.. 2010;74:417-433.
- [Google Scholar]
- Surveillance of multidrug resistance of two Gram-positive pathogenic bacteria in a teaching hospital and in vitro efficacy of 30 ethno-medicinal plants used by an aborigine of India. Asian Pac. J. Trop. Dis.. 2012;2:273-281.
- [Google Scholar]
- Antibacterial activity of Lantana camara L. against multidrug resistant pathogens from ICU patients of a teaching hospital. J. Herb. Med.. 2013;3:65-75.
- [Google Scholar]
- In vitro antibacterial activity, GC-MS analysis of Woodfordia fruticosa Kurz. leaf extract and host toxicity testing with in vitro grown lymphocytes from human umbilical cord blood. Osong Publ. Health Res. Persp.. 2014;5:298-312.
- [Google Scholar]
- Anolignan B: a bioactive compound from the roots of Terminalia sericea. J. Ethnopharmacol.. 2006;103:135-138.
- [Google Scholar]
- Pathogenicity islands: bacterial evolution in quantum leaps. Cell. 1996;87:791-794.
- [Google Scholar]
- Transcriptional control of the mtr efflux system of Neisseria gonorrhoeae. J. Bacteriol.. 1995;177:4162-4165.
- [Google Scholar]
- Novel inhibitory activity of the Staphylococcus aureus norA efflux pump by a kaempferol rhamnoside isolated from Persea lingue Nees. J. Antimicrob. Chemother.. 2012;67:1138-1144.
- [Google Scholar]
- Ursolic and oleanolic acids as antimicrobial and immunomodulatory compounds for tuberculosis treatment. Complement. Alternat. Med.. 2013;13:258.
- [Google Scholar]
- Synthesis, antimicrobial evaluation and QSAR studies of gallic acid derivatives. Arab. J. Chem. 2014
- [CrossRef] [Google Scholar]
- New antibiotic molecules: bypassing the membrane barrier of Gram-negative bacteria increases the activity of peptide deformylase inhibitors. PLoS ONE. 2009;4:6443.
- [Google Scholar]
- Biological activities of phenolic compounds and triterpenoids from the galls of Terminalia chebula. Chem. Biodivers.. 2013;10:1448-1463.
- [Google Scholar]
- The periplasmic protein mppA is not involved in regulation of marA in Escherichia coli. Antimicrob. Agen. Chemother.. 2011;55:4939-4942.
- [Google Scholar]
- Surveillance of multidrug resistant uropathogenic bacteria in hospitalized patients – an Indian study. Asian Pac. J. Trop. Biomed.. 2013;3:315-324.
- [Google Scholar]
- In vitro antibacterial efficacy of 21 Indian timber-yielding plants against multidrug resistant bacteria causing urinary tract infection. Osong Public Health Res. Persp.. 2013;4:347-357.
- [Google Scholar]
- The porin and the permeating antibiotic: a selective diffusion barrier in Gram-negative bacteria. Nat. Rev. Microbiol.. 2008;6:893-903.
- [Google Scholar]
- Isolation and antimicrobial and antioxidant evaluation of bio-active compounds from Eriobotrya japonica stems. Adv. Pharm. Bull.. 2014;4:75-81.
- [Google Scholar]
- Surveillance of multidrug resistance of 6 uropathogens in a teaching hospital and in vitro control by 25 ethno- medicinal plants used by an aborigine of India. Asian Pac. J. Trop. Biomed.. 2012;2:S818-S829.
- [Google Scholar]
- Surveillance of multidrug resistance of 10 enteropathogens in a teaching hospital and in vitro efficacy of 25 ethnomedicinal plants used by an Indian aborigine. Asian Pac. J. Trop. Dis.. 2012;2:S336-S346.
- [Google Scholar]
- Monitoring in vitro antibacterial efficacy of Terminalia alata Heyne ex. Roth, against multidrug resistant enteropathogenic bacteria. J. Acute Med.. 2013;3:93-102.
- [Google Scholar]
- Monitoring in vitro antibacterial efficacy of 26 Indian spices against multidrug resistant urinary tract infecting bacteria. Integr. Med. Res.. 2014;3:133-141.
- [Google Scholar]
- Surveillance of extended-spectrum β-lactamase producing bacteria in an Indian teaching hospital. J. Taibah Univ. Med. Sci.. 2014;9:274-281.
- [Google Scholar]
- Ultrasonic-assisted extraction of antioxidative compounds from Bene (Pistacia atlantica subsp. mutica) hull using various solvents of different physicochemical properties. Food Chem.. 2015;173:577-583.
- [Google Scholar]
- New lignans from Anogeissus acuminata with HIV-1 reverse transcriptase inhibitory activity. J. Nat. Prod.. 1994;57:896-904.
- [Google Scholar]
- Revision of the NMR assignments of pterostilbene and of dihydrodehydrodiconieferyl alcohol: cytotoxic constituents from Anogeissus acuminata. Nat. Prod. Lett.. 1994;4:267-272.
- [Google Scholar]
- Monograph. In vitro efficacy of 30 ethnomedicinal plants used by an Indian aborigine against 6 multidrug resistant Gram- positive pathogenic bacteria. Asian Pac. J. Trop. Dis.. 2015;5:136-150.
- [Google Scholar]
- In vitro combination-efficacy of ceftriaxone and leaf extract of Combretum albidum against multidrug resistant Pseudomonas aeruginosa and host-toxicity testing with human lymphocytes. J. Acute Med.. 2014;4:26-34.
- [Google Scholar]
- Indwelling urinary catheters: a one-point restraint? Ann. Intern. Med.. 2002;137:125-127.
- [Google Scholar]
- Multidrug resistant Shigella species in Nepal, a retrospective study conducted at national public health laboratory (NPHL), 1999 to 2002. J. Nepal Health Res. Commun.. 2002;4:51-59.
- [Google Scholar]
- Constituents of Azadirachta indica: isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin, and a new protolimonoid, naheedin. J. Nat. Prod.. 1992;55:303-310.
- [Google Scholar]
- Horizontal transfer of antibiotic resist- ance genes on abiotic touch surfaces: implications for public health. mBio. 2012;3:e00489-12.
- [Google Scholar]







