Translate this page into:
Improved properties of keratin-based bioplastic film blended with microcrystalline cellulose: A comparative analysis
⁎Corresponding author. arungupta10@gmail.com (Arun Gupta)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
In the present study, bioplastic films were developed using the different ratio of keratin extracted from chicken feathers and cellulose. Firstly, bioplastic (K-60) was developed from the keratin, extracted from the chicken feathers using an alkaline agent (NaOH), and mixed with PVA/glycerol to synthesize protein-based bioplastic. Further, microcrystalline cellulose (2%) was used as an additive to K-60 bioplastic to develop an improved bioplastic (KC-60). The results of functional group analysis using FT-IR, showed the conformational arrangements of the keratin protein have mostly amides I–III and O—H groups in the bioplastic reinforced with microcrystalline cellulose and showed the substantial hydrogen bonding. The scanning electron microscopy analysis suggested the appropriate morphologies without edge, holes and cavities. The X-Ray diffraction analysis suggested the strong crystalline characteristics of synthesized bioplastic. Finally, the thermogravimetric analysis of K-60 and KC-60, showed the greater cross-linking efficiency between cellulose and keratin at higher temperature. Therefore, the results presented the development of keratin-based bioplastics with high structural strength and morphology good crystallinity which can be used in biomedical applications and manufacturing of food containers and others.
Keywords
Chicken feather
Keratin
Bioplastic
Microcrystalline cellulose
1 Introduction
Nowadays in the bioplastic, the plastic derivatives procured from the biomasses have gained worthy interest to develop food packaging materials and biomedical products (Shah et al. 2019; Nayak et al. 2019). However, due to expansive process of bioplastic synthesis created some barrier to develop it on large scale. However, the bioplastic production resolve the environmental- issues by means of the renewable and biodegradable resources of a commonly used material (Sharma et al., 2017; McLellan et al. 2019).
Globally, the chicken feathers are the foremost common waste material produced in poultry slaughterhouses and it consist of 90% of keratin which is used as a natural source for commercial applications (Sharma et al. 2019; Sharma and Kumar 2019).
The keratin protein has a major structure which provides the outer covering in most of mammals, birds, and reptiles in the form of hairs, wool, feathers horns and nails. It is an organic, biodegradable polymer where the biodegradation is due to strong covalent bonds and its prolonged cross-linking within its structure (Kumawat et al., 2018; Vasconcelos and Cavaco-Paulo, 2013; Sharma et al., 2017). The protein structure is mainly stabilized by hydrophobic interactions and a three-dimensional macromolecular network, reinforced by hydrogen and disulphide bonds. It also exhibits a high capacity in the thermosetting amendment due to the denaturation of the protein which is favorable for a wide variety materials (Sharma and Gupta, 2016). Therefore, the production of biodegradable materials using proteins could be a promising way for a variety of material based applications (Dou et al., 2015). Cellulose is a naturally abundant material, can reduce production costs and enhance the mechanical properties, biodegradability and thermal stability of the polymers (Herrera et al., 2015; Karande et al., 2014). Cellulose can be obtained from a wide variety of sources such as plants, algae, marine creatures and bacteria (Chen et al., 2011; Iwatake et al., 2008). DeMesquita et al (2010) reported that the manufacturing of cellulosic materials into micro and also nano dimensions which reinforce the low molecular weight, high crystallinity, biodegradability and renewability (de Mesquita et al., 2010). Microcrystalline cellulose is produced from natural cellulose through a combination of mechanical and chemical processing (Lu et al., 2008; Mathew et al., 2005; Kumar et al. 2015). The degree of crystallinity typically ranges from 55 to 80 %, depends on the source of the cellulose as well as processing variables such as the reaction temperature and duration, the drying (Chuayjuljit et al., 2010; Haafiz et al., 2013). Therefore, MCC has great potential for use as a biodegradable filler in polymer composites (Suvachittanont and Ratanapan, 2013; Arjmandi et al., 2015).
The interaction between fibre particles and polymer matrix could improve the properties of the biocomposite (Lubis et al., 2016). Therefore, cellulose filler was added to examine the improvement of the produced bioplastic properties. Enhancing cellulose filler has assured to be the most favorable materials to use as a major component (Husain et al. 2018; Siagian and Tarigan, 2016). The interactions between keratin and MCC film matrix supported the main role in enhancing the result of keratin bioplastic. Thus, this interaction mainly attributed to the strong hydrogen bonding between the keratin and MCC (Lubis et al., 2016; Wang et al., 2006).
This study aimed to develop a bioplastic using a different proportion of chicken feather extracted keratin and cellulose. Firstly, bioplastic (K-60) was developed from the keratin, extracted from the chicken feather using an alkaline agent (NaOH), and mixed with PVA/glycerol to synthesize protein-based bioplastic. Further, microcrystalline cellulose (2%) was used as an additive to K-60 bioplastic to develop an improved bioplastic (KC-60) The bioplastic developed characterize using different techniques to saw the differences among the both.
2 Materials and methods
2.1 Materials
For the extraction of keratin, chicken feather was procured from (Balok Poultry Farm Sdn. Bhd. Kuantan, Malaysia). Sodium hydroxide, hydrochloric acid, Glycerol, 99.5%, Polyvinyl Alcohol and microcrystalline cellulose were purchased from (Sigma-Aldrich, Kuala Lumpur, Malaysia).
2.2 Keratin extraction from chicken feather
Keratin protein solution was prepared in the chemical engineering lab at University Malaysia Pahang by cleaning feathers according to previously studied methods (Sharma et al., 2017a). The 50 g of cleaned, dried and blended chicken feathers were added in 1 L of sodium hydroxide (1 N) solution in a conical flask. The solution was incubated at 50 °C under continuous shaking for five hours, After that, the solution was filtered by stainless steel filter and centrifuged at 10,000 rpm for 10 min (Bao et al., 2011; Sharma et al., 2017a). The keratin solution was carefully collected and stored for the synthesis of bioplastic.
2.3 Synthesis of keratin-based bioplastic
PVA powder (15 g) was dissolved in 100 ml of milli Q water and stirred at 80 °C for one hour. The first sample, the keratin (K-60) was mixed with 10% of glycerol and 30% of PVA solution at 60 °C. The second sample, cellulose (2%) was added to the total weight of the bioplastic (K-60) and stirred at 60 °C. Then, the both mixtures were poured in glass petri plates having a diameter of 20 cm and desiccated in oven at 60 °C for 24 h. Later, both films; (K-60) and (KC-60) were separated from glass petri plates and stored for the further analysis.
2.4 Characterization
2.4.1 Fourier transform infrared spectroscopy (FTIR)
The K-60 and KC-60 films were investigated using the program FTIR Spectrum Software. The FTIR spectra of Perkin-Elmer Model 1000 series equipment was used and supplied with an Attenuated Total Reflectance accessory (ATR). Powdered-form samples weighing about 1–2 mg were placed on the FTIR spectroscopy test area. The spectrum for each sample was recorded with 40 scans in the frequency range from 4000 to 500 cm−1 with a resolution of 4 cm−1.
2.4.2 Scanning electron microscopy (SEM)
The scanning of the surface morphologies of K-60 and KC-60 films were studied at 30 s of acquisition time and accelerating voltage of 15.0 kV. The identification of the molecular structure of the samples was analyzed in a Hitachi's Tabletop Electron microscope TM3030 Plus.
2.4.3 X-ray diffraction (XRD)
The crystallinity of K-60 and KC-60 films were determined using Rigaku Miniflex-II X-ray Diffractometer system (XPERT-3) with copper K-α radiation (X = 1.54060 Å) and generator settings at 45 kV and 40 mA. At the rate 0.0217°/min, the data within the scattering angles range of 5° to 50° were recorded. The essential spacing resulted from the position of the peak in the XRD diagram has resulted according to the Bragg's equation: Where: n is an integer, λ is the wavelength of the electrons, d is the spacing of the crystals planes and θ is the scattering angle.
2.4.4 Thermo gravimetric analysis (TGA)
TGA analysis of the samples were conducted utilizing a Mettler Toledo Thermal Analyzer instrument air and nitrogen atmosphere. From room temperature, the samples were gradually heated to 800 °C at the rate of 10 °C /min and flow rate of 20 ml/min. The mass loss within the differential thermal analysis profile was recorded as a function of temperature
3 Results and discussion
3.1 FTIR
FTIR spectra of K-60 and KC-60 shown in Fig. 1. Both films showed a decline in the spectrum intensity of C-O (1000–1260 cm−1) and the stretching bands C—H (2800–2950 cm−1) were found. Besides this, KC-60 showed O—H (3300–3400 cm−1) stretching vibration bands broader than the K-60. The conformational changes in the protein were examined by amides I–III (Ramakrishnan et al., 2018; Sharma et al., 2017b; Sharma et al., 2017c). The absorption bands corresponds to amide I i.e. carbonyl stretching vibration at 1600–1700 cm−1, amide II for N—H bending and C—N stretching at 1400–1580 cm−1, and amide III for C⚌O, C—N stretching and N—H bending at 1257 cm−1 were studied in the samples (Ramakrishnan et al., 2018; Yue et al., 2012; Sharma et al., 2016). The absorption bands at 1095 cm−1 and 1033 cm−1 were attributed to the symmetric and asymmetric S⚌O stretching vibration respectively. The sharp peak at 1034 cm−1 indicated an elevated content of cysteine-S-sulfonated residues in the samples (Shavandi et al., 2017). However, the peak at 2940 cm−1 is similar in both the samples, due to hydroxyl groups which can form more hydrogen bonds, and add to the total hydrogen bonded peptides groups. The other alteration in characteristic absorption bands frequency and intensity possibly indicated the role of cellulose as a strengthening filler in the bioplastic networks (Lubis et al., 2016).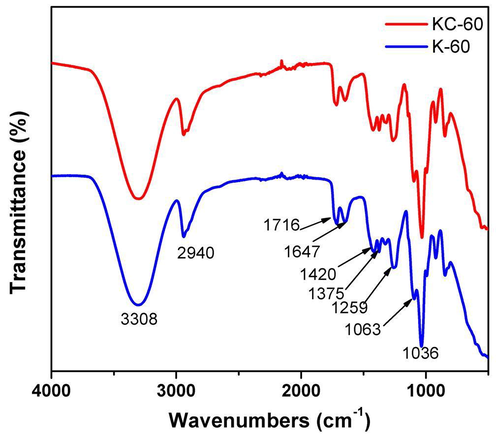
FT-IR spectrum of synthesised bioplastics.
3.2 SEM
SEM micrographs of the (Fig. 2) K-60 and KC-60 showed a fluctuating and continuous structure, with better compatible morphology with no cavities, edges, and holes. Thus, confirmed the superior bonding among components, which was attributed to the existence of chemical interactions between keratin and cellulose. The cellulose assisted to make a homogenous mixture. It showed no separation and single-phase morphology which was observed in related reports (Lubis et al., 2016; Yue et al., 2012; Sharma et al., 2018). SEM analysis on the structure of the specimen showed the mildness at the unified surface of the bioplastic indicates the cross-linked structures. This is evident from their smooth surface morphology with cracks, indicating its resilient nature. The dark color showed carbon background onto which sample was mounted and light color corresponds to the samples of bioplastic made. However, KC-60 have small cracks than the K-60, which is possibly due to the formation of large number of intermolecular bondings (Aluigi et al., 2008).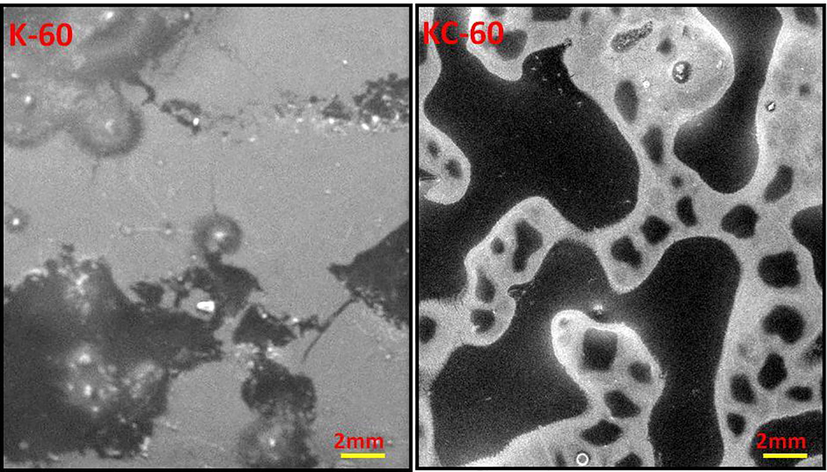
SEM images of synthesised bioplastics.
3.3 XRD
The XRD patterns of K-60 and KC-60 are shown in Fig. 3. K-60 showed primary peaks at 2θ = 19°, 23°, 41° and KC-60 displays 2θ = 19°, 32°, 41°, 45°, 66°, which coincide with earlier reported (Lubis et al., 2016; Priyaah et al., 2017). The change to upper angle specified a decrease in the subsequent interlayer spacing, which implies that the mixed constituent had an arranged structure. The K-60 has the higher intensity of shifted peaks. The KC-60 composite showed the typical peaks which are ascribed to that the cellulose filler has hydrogen bond interaction with the residual functional groups. The inclusion of the cellulose leads to the decline in the intensity. However, the strong crystalline characteristic was showed and the d-spacing of both bioplastic film indicated that the blends have a well-arranged structure (Harkins et al., 2014). Fig. 3 indicated that bioplastic K-60 is amorphous in nature while the crystallinity increased after the addition of MCC as evident from the sharp peaks appeared in the KC-60.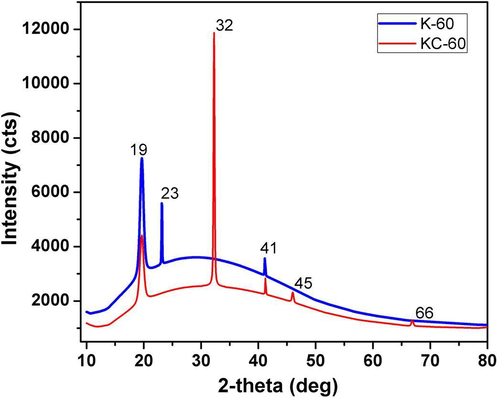
X-ray diffractogram of synthesised bioplastics.
3.4 TGA
The TGA curves in Fig. 4 showed three stages of weight loss for both samples. In the first stage, the mass loss (less than 10 wt%) occurs between room temperature and 100 °C, due to evaporation of absorbed moisture (Sharma et al., 2017a). In the second stage, the samples decomposed rapidly at 160 to 230 °C, with a mass loss of 20 to 40 wt% due to the decomposition of glycerol and PVA, as confirmed by the previous study (Ullah et al., 2011). At the last stage, decomposition of the keratin protein occurs at temperatures higher than 260 °C. At all temperature ranges, less mass loss was observed in the KC-60 as compared with K-60, which showed an improvement of thermal stability for the cross-linked film. The enhanced thermal stability is probably attributed to the formation of strong amine (–CH = N–) bonds formed which indicate that the interactions between KC-60 with increasing temperature are greater than these of K-60, probably due to the higher cross-linking efficiency of cellulose upon heating (Siqueira et al., 2010).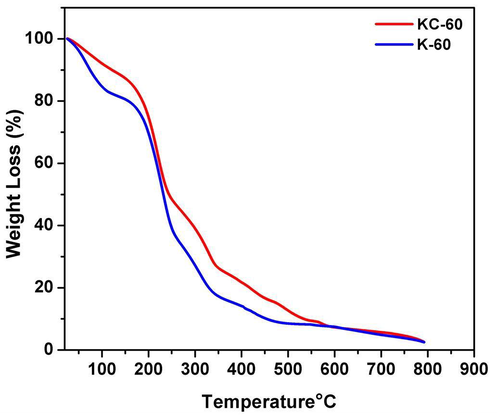
TGA graph of synthesised bioplastics.
4 Conclusions
In summary, the synthetic natural-based bioplastic was successfully developed using the extracted keratin from chicken feathers and microcrystalline cellulose. Although the conducted analysis showed excellent characteristics of bioplastic (K-60), the desired improvement was noticed in the presence of cellulose. FTIR spectra showed that the absorbent peaks appeared in the films indicating that they sustain the macromolecular structure of the chicken feather keratin, extracted and there are possible hydrogen bonds between keratin protein and cellulose. Moreover, the good surface morphology, high crystallinity, and thermal properties were proven with SEM, XRD and TGA analysis. Conclusively, the developed keratin-based bioplastic has a promising future in various industrial applications.
References
- Composite biomaterials from fibre wastes: Characterization of wool–cellulose acetate blends. Compos. Part A Appl. Sci. Manuf.. 2008;39:126-132.
- [Google Scholar]
- Effect of microcrystalline cellulose on biodegradability, tensile and morphological properties of montmorillonite reinforced polylactic acid nanocomposites. Fibers Polym.. 2015;16:2284-2293.
- [Google Scholar]
- Synthesis and swelling behaviors of sodium carboxymethyl cellulose-g-poly (AA-co-AM-co-AMPS)/MMT superabsorbent hydrogel. Carbohydr. Polym.. 2011;84:76-82.
- [Google Scholar]
- Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym.. 2011;83:1804-1811.
- [Google Scholar]
- Poly (vinyl chloride) film filled with microcrystalline cellulose prepared from cotton fabric waste: properties and biodegradability study. Waste Manag. Res.. 2010;28:109-117.
- [Google Scholar]
- Biobased nanocomposites from layer-by-layer assembly of cellulose nanowhiskers with chitosan. Biomacromolecules. 2010;11:473-480.
- [Google Scholar]
- Keratin/polyvinyl alcohol blend films cross-linked by dialdehyde starch and their potential application for drug release. Polymers (Basel).. 2015;7:580-591.
- [Google Scholar]
- Properties of polylactic acid composites reinforced with oil palm biomass microcrystalline cellulose. Carbohydr. Polym.. 2013;98:139-145.
- [Google Scholar]
- Chitosan–cellulose composite for wound dressing material. Part 2. Antimicrobial activity, blood absorption ability, and biocompatibility. J. Biomed. Mater. Res. Part B Appl. Biomater.. 2014;102:1199-1206.
- [Google Scholar]
- Plasticized polylactic acid/cellulose nanocomposites prepared using melt-extrusion and liquid feeding: mechanical, thermal and optical properties. Compos. Sci. Technol.. 2015;106:149-155.
- [Google Scholar]
- Synthesis of PVA/PVP based hydrogel for biomedical applications: a review. Energy Sources, Part A: Recovery, Utilization, Environm. Effects. 2018;40(20):2388-2393.
- [Google Scholar]
- Cellulose nanofiber-reinforced polylactic acid. Compos. Sci. Technol.. 2008;68:2103-2106.
- [Google Scholar]
- Cotton linter nano-fibers as the potential reinforcing agent for guar gum. Iran. Polym. J.. 2014;23:869-879.
- [Google Scholar]
- Cellulose binding domain assisted immobilization of lipase (GSlip–CBD) onto cellulosic nanogel: characterization and application in organic medium. Colloids Surf. B Biointerfaces. 2015;136:1042-1050.
- [Google Scholar]
- Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials. Compos. Part A Appl. Sci. Manuf.. 2008;39:738-746.
- [Google Scholar]
- Effect of Microcrystalline Cellulose (MCC) from Sugar palm fibres and glycerol addition on mechanical properties of bioplastic from avocado. Seed Starch (Persea Americana Mill) 2016
- [Google Scholar]
- Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC) J. Appl. Polym. Sci.. 2005;97:2014-2025.
- [Google Scholar]
- Keratin-based biofilms, hydrogels, and biofibers. In: Keratin as a Protein Biopolymer. Springer; 2019. p. :187-200.
- [Google Scholar]
- Keratin-based biotechnological applications. In: Keratin as a Protein Biopolymer. Springer; 2019. p. :201-224.
- [Google Scholar]
- Synthesis of wound-healing keratin hydrogels using chicken feathers proteins and its properties. Int. J. Pharm. Pharm. Sci.. 2017;9:171-178.
- [Google Scholar]
- Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018
- [Google Scholar]
- Keratin production and its applications: current and future perspective. In: Keratin as a Protein Biopolymer. Springer; 2019. p. :19-34.
- [Google Scholar]
- Sustainable management of keratin waste biomass: applications and future perspectives. Brazilian Arch. Biol. Technol. 2016:59.
- [Google Scholar]
- Dissolution and characterization of biofunctional keratin particles extracted from chicken feathers. In: IOP Conference Series: Materials Science and Engineering. IOP Publishing; 2017. p. :12013.
- [Google Scholar]
- Characterization of keratin microparticles from feather biomass with potent antioxidant and anticancer activities. Int. J. Biol. Macromol.. 2017;104:189-196.
- [Google Scholar]
- Study of different treatment methods on chicken feather biomass. IIUM Eng. J.. 2017;18:47-55.
- [Google Scholar]
- An efficient conversion of waste feather keratin into ecofriendly bioplastic film. Clean Technol. Environ. Policy 2018:1-11.
- [Google Scholar]
- Extraction and characterization of keratin from chicken feather waste biomass: a study. In: Proceedings of the national conference for postgraduate research (NCON-PGR 2016). Pekan: Universiti Malaysia Pahang (UMP); 2016. p. :693-699.
- [Google Scholar]
- Keratin: an introduction. In: Keratin as a Protein Biopolymer. Springer; 2019. p. :1-18.
- [Google Scholar]
- Keratin as a Protein Biopolymer. Springer; 2019.
- Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J. Bioact. Compat. Polym.. 2017;32:163-177.
- [Google Scholar]
- Production of starch based bioplastic from cassava peel reinforced with microcrystalline celllulose avicel PH101 using sorbitol as plasticizer. J. Physics: Conf. Ser. IOP Publishing 2016:12012.
- [Google Scholar]
- Cellulosic bionanocomposites: a review of preparation, properties and applications. Polymers (Basel).. 2010;2:728-765.
- [Google Scholar]
- Optimization of micro crystalline cellulose production from corn cob for pharmaceutical industry investment. J. Chem. Chem. Eng.. 2013;7:1136.
- [Google Scholar]
- Ullah, A., Vasanthan, T., Bressler, D., Elias, A.L., Wu, J., 2011. Bioplastics from Feather Quill.
- The use of keratin in biomedical applications. Curr. Drug Targets. 2013;14:612-619.
- [Google Scholar]
- Effects of cellulose whiskers on properties of soy protein thermoplastics. Macromol. Biosci.. 2006;6:524-531.
- [Google Scholar]
- Preparation and characterisation of bioplastics made from cottonseed protein. Green Chem.. 2012;14:2009-2016.
- [Google Scholar]







