Translate this page into:
Impact of vitellogenin based dsRNA feeding on reproductive biology of red palm weevil, Rhynchophorus ferrugineus (Olivier), (Coleoptera: Dryophthoridae) under laboratory conditions
⁎Corresponding author at: Department of Plant Protection, College of Food and Agriculture Sciences, King Saud University, P.O. Box 2460, Riyadh 11451, Saudi Arabia. mbukhsh@ksu.edu.sa (Mureed Husain)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objective
Red palm weevil, Rhynchophorus ferrugineus is a crucial pest of date palm, in the Kingdom of Saudi Arabia including several other palm producing countries of the World. The vitellogenin gene play very important role for oocytes development in all oviparous animals. Recently, we have silenced the vitellogenin gene by injecting the RfVg-based dsRNA and proved that RNAi technology can be used to manage red palm weevil. The main challenge in RNAi-based plant protection methods is selecting a suitable tactic for successfully delivering the dsRNA.
Methods
In the present study, 9-10th instar red palm weevil larvae were provided with Vg-based dsRNA in diet and as drops to determine its effects on red palm weevil reproductive traits, such as pre-oviposition period, fecundity, oviposition period, post oviposition period, eggs size, and female and male life span.
Results
Results demonstrated that the RfVg gene function was successfully suppressed using Vg-based RNAi. When applied via drops, resulting in a significant decline in red palm weevil eggs hatchability and Vg expression measured by quantitative real time Polymerase chain reaction. However, when applied via diet, Vg-based dsRNA did not show any significant effect on fecundity, oviposition period, post oviposition period, eggs size, and female and male life span.
Conclusions
Based on present overall results and our previous findings, along with the documented information, we can conclude that Vg-based RNAi has a high potential for use as a target specific and eco-friendly technique for the sustainable control of this crucial pest.
Keywords
RNAi
Oviparous
Date palm
Vitellin
Gene function
Saudi Arabia
- Rf
-
Rhynchophorus ferrugineus
- Vg
-
Vitellogenin
- RNAi
-
RNA interference
- Vn
-
Vitellin
- ANOVA
-
one-way analysis of variance
- RPW
-
Red palm weevil
Abbreviations
1 Introduction
The red palm weevil (RPW), Rhynchophorus ferrugineus (Olivier), (Coleoptera: Dryophthoridae) is a highly damaging pest of date palm in the Kingdom of Saudi Arabia including several other palm producing countries (Gomez and Ferry 1998). It has been estimated that in the Kingdom of Saudi Arabia, approximately 80,000 date palms are severely infested by this pest and posing a threat to other neighboring orchards (Al-Sheaby 2010) Infestations have been reported in more than half of the world's date palm growing countries, extending to the entire Middle East (Faleiro 2006). On average, a female weevil lays about 48–139 eggs during its whole life period of 15–72 days (Aldawood et al., 2022).
In oviparous species, eggs production depends on the ability of Vg production and its accumulation in the oocytes. During reproductive phase, the female fat body produces a large amount the precursor Vg, which is subsequently reached to the oocytes through receptors (VgRs) through receptor-mediated endocytosis (Raikhel and Dhadialla 1992, Hagedorn et al., 1998, Sappington and Raikhel 1998, Snigirevskaya and Raikhel 2005, Tufail et al., 2005, Tufail and Takeda 2008, Tufail and Takeda 2009, Tufail and Takeda 2012, Tufail et al., 2014, Tufail and Takeda 2018). The biosynthesis of Vg in most, if not all, insect species is controlled at the transcriptional level.
Taken together the Vg gene is the key component of egg production, and might be an appropriate target for developing more effective pest control methods for the crucial pest insects. For example, if the Vg gene function is knocked down by the RNA interference (RNAi) technology, the egg production can be stopped. RNAi is a natural process which silences specific genes before being translated. Efficient methods for gene silencing as a means of controlling pests have been successfully demonstrated in the laboratory (Price and Gatehouse 2008). RNAi technology, both in the form of crop spray or transgenic plants have the prospective to efficiently silence the targeted genes (Baum et al., 2007, Mao et al., 2007, Zhao et al., 2008) and must be especially important for the pests showing resistance to the pesticide or those having the hidden nature in the host plant.
Although, several kinds of measures have been tried to manage the RPW such as, pheromone traps, chemical control, injection of entomopathogenic nematodes (Shamseldean and Abd-Elgawad 1994), and fungus (Sutanto et al., 2022), however, none of them is able to completely eliminate/control this weevil. One of the major reasons this insect evades pest control measures is concealed reproduction of this insect within the palm tree protecting it from several interventions applied in the past. Under such situation, the alternative control strategies, especially with a molecular approach need to be sorted out. As mentioned above, RNAi technology has become tremendously a good tactic for studying the gene functions and to explore the potential genes for pest-control. We believe this target-oriented and environment-friendly approach would be the best choice for RPW control.
This manuscript is the part of the research project in which we isolated the Vg gene from the to elucidate the reproduction mechanism of RPW and to exploit this molecular information to develop a system that can interfere the egg production using RNAi-technology as a target-oriented and environment-friendly pest control strategy for RPW in Kingdom of Saudi Arabia (see for detail our recently published manuscript in “Scientific Reports” (Rasool et al., 2021). Present study main objective was to testify the Vg-based dsRNA effectiveness through oral application against various biological parameters of RPW such as pre-oviposition period, fecundity, oviposition period, post oviposition period, eggs size, and female and male life span. We believe, RNAi-based strategy for control of this insect will be a great revolution in pest management system.
2 Materials and methods
2.1 Red palm weevil population
The red palm weevil (RPW), Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae), population (adult, larva, and pupa), primarily collected from infested date palm orchards in the Aldierab region, of Saudi Arabia (24.4164°N, 46.5765°E). The infested date palm trees were inspected visually and signs of infestation were observed. The major infestation symptoms are tunnels made by the grubs on the leaf base or trunk, oozing brownish fluid from the tunnels, chewed up plant tissues around the tunnel mixed with the fluid. After making sure that the inspected tree has RPW infestation; it was dissected and the RPW developmental stages were collected. These individuals were brought to the laboratory where they were reared on an artificial diet (Aldawood et al., 2022) to get the F1 progeny. The culture was established at a temperature of 25 ± 1 °C and 70 ± 5% relative humidity throughout the study. The adult weevil from the F1 progeny were allowed to copulate and lay eggs. To avoid the eggs from drying out, they were collected and kept in the petri dishes with wet filter paper at the bottom. These eggs were hatched into neonates which were provided the earlier described diet in order to grow and develop. These larvae were used for the RfVg based dsRNA feeding bioassay when they reached the 9th and 10th instars.
2.2 RfVg dsRNA feeding bioassy
In the present work the RfVg-based dsRNA feeding bioassay were performed on 9th and 10th instars old larval stage of RPW using the diet incorporation and direct drop feeding methods. The RfVg based dsRNA feeding bioassay was used in this study to assess their consequence on biological parameters and Vg expression. Briefly, the semi-synthetic diets, Diet-1 (artificial diet for RPW) and Diet-2 (diet used for Spodoptera spp. rearing), were cut into 2 cm3 pellets and 4 μg (100 μl) of RfVg dsRNA was incorporated into each pellet and every pellet was kept in a separate cup. Before bioassay, larvae were starved for 24 h and then fed on treated food pellets individually for 24 h. In another experiment, RfVg dsRNA 10 μl (4 μg) was delivered to larvae by oral feeding using drop method. The RfVg dsRNA drops were delivered directly into the mouth of 24-h starved larvae using a micropipette. However, in the control nuclease free water was incorporated into artificial synthetic food and provided to the 24-h starved larvae. There were 10 replications and each replication consist of an individual larva. Therefore, single larva was placed in each plastic cup and every plastic cup considered as a single replicate. After 24 h, the larvae were transferred to sugar cane in order to ensure cocoons (pupation), and followed daily till the adults eclosion. Experiments were maintained in the incubator at 25 °C ± 1 and 70 ± 5% relative humidity.
2.3 Biological studies to assess the impact of RfVg dsRNA
The newly emerged adults from the treated larvae were paired and placed in a separate plastic container (1 kg) containing a bit of cotton overloaded with a 10% sucrose solution. All the pairs from each treatment were kept separate, where they copulated and laid eggs until the females survived. The pre-oviposition period, oviposition period, eggs laid/day, total number of eggs laid, egg hatching percentage, post-oviposition period, and life span were observed.
2.4 Validation through qRT-PCR
To verify the effect of RfVg-based RNAi on Vg gene expression, a (real-time reverse transcription-PCR) qRT-PCR analysis was performed by using the RfVgRTF and RfVgRTR primers previously used in our study (Rasool et al., 2021). Four experimental units, including (RfVg dsRNA-based diet-I, RfVg dsRNA-based diet-II, RfVg dsRNA drop, and control); were subjected to real-time PCR. There were three replicates for each treatment, each replicate having one female, as well as three technical replicates. The reactions were prepared using SYBR® Green Supermix (BioRad) according to the standard protocol, and the real-time PCR was performed using the BioRad CFX-96 System.
2.5 Statistical analysis
To examine the differences between the four test groups (RfVg dsRNA-based diet-I, RfVg dsRNA-based diet-II, RfVg dsRNA drop, and control) for biological studies, one-way analysis of variance (ANOVA) was used followed by multiple-comparison testing with the least significant difference (LSD) test (α = 0.05) by using SAS program ver. 9.2 (SAS 2008). Moreover, the qRT-PCR quantification results were analyzed and mean cycle threshold (CT) values were calculated to see the effect of dsRNA on the vitellogenin expression when fed to the RPW at larval stages.
3 Results
3.1 Effects of RfVg-dsRNA feeding on RPW biological parameters
The Vg gene knockdown impact in adult RPW females as a result of feeding RfVg-dsRNA to 9th and 10th instars larvae in diet and drops was determined in terms of pre-oviposition, oviposition, and post-oviposition. The RfVg-dsRNA application significantly influenced some biological parameters in treated females. Results revealed that larvae fed with RfVg-dsRNA by drops having significantly longer pre-oviposition period as compared to larvae that were provided with RfVg-dsRNA in diets and control (df = 3, F = 6.59, P < 0.0041) (Fig. 1). There was no significant difference in the oviposition period between larvae fed RfVg-dsRNA and controls (df = 3, F = 0.15, P < 0.9291) (Fig. 2). Similarly, no significant difference (df = 3, F = 0.60, P < 0.6268) was observed for post-oviposition period between larvae feed on RfVg-dsRNA and control (Fig. 3).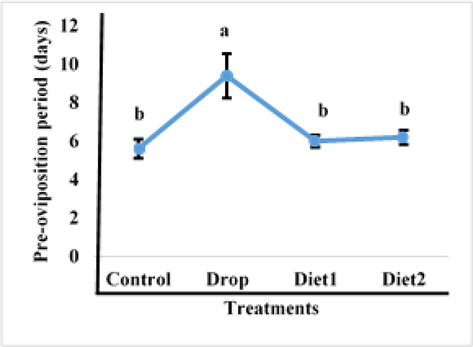
Effects of RFVgdsRAN feeeding on pre-oviposition period.
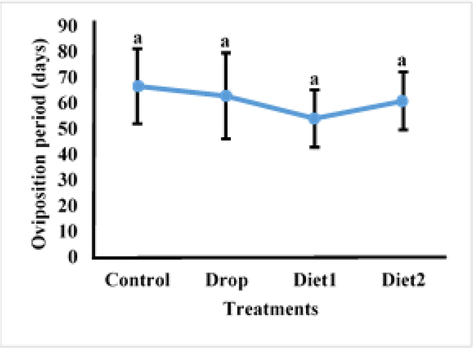
Effects of RFVgdsRAN feeeding on oviposition period.
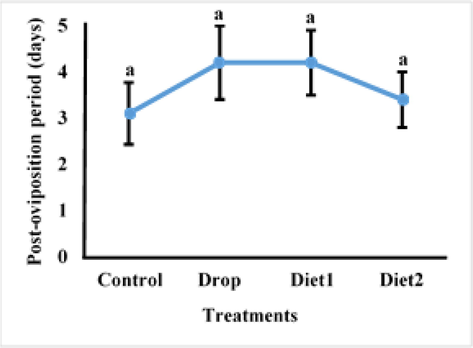
Effects of RFVgdsRAN feeeding on post-oviposition period.
3.2 Effects of RfVg-dsRNA feeding on biological parameters of adult female RPW
The effects of Vg gene knockdown in adult females as a result of feeding RfVg-dsRNA to 9th and 10th instars larvae in diet and drops was also assessed in terms of fecundity, eggs hatching percentage, and adult female life span. The larvae fed on RfVg-dsRNA showed no significant difference (df = 3, F = 0.18, P < 0.9096) for no. of eggs laid between the control and RfVg-dsRNA treatments as drops and in diets (Fig. 4). But larvae feed on RfVg-dsRNA irrespective of feeding methods revealed significantly lower eggs hatchability percentage (df = 3, F = 3.22, P < 0.0509) as compared to control (Fig. 5). Results did not show any significant difference for adult female life span (df = 3, F = 0.13, P < 0.9386) between larvae feed on RfVg-dsRNA and control (Fig. 6). Moreover, results also not showed any significant difference for eggs length (df = 3, F = 5021, P < 0.0031) as well as width (df = 3, F = 1.56, P < 0.2081) between control and RfVg-dsRNA feeding through different methods (Fig. 7).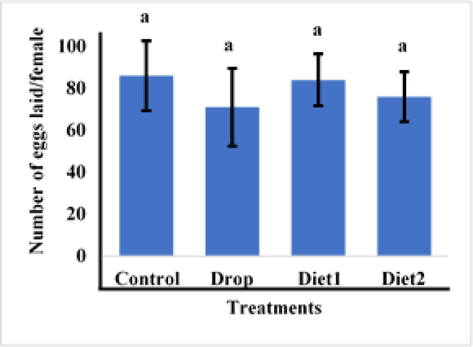
Effects of RFVgdsRAN Feeeding feeding on mean number of eggs laid pre female.
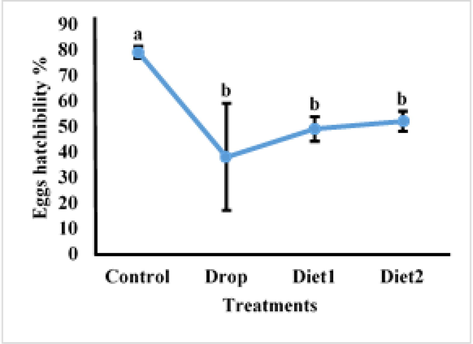
Effects of RfVg-dsRNA feeding on eggs hatchability %.
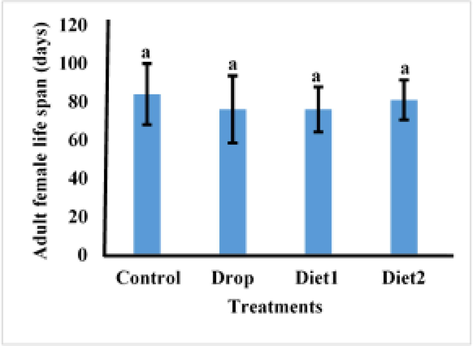
Effects of RfVg-dsRNA feeing on adult female life span.
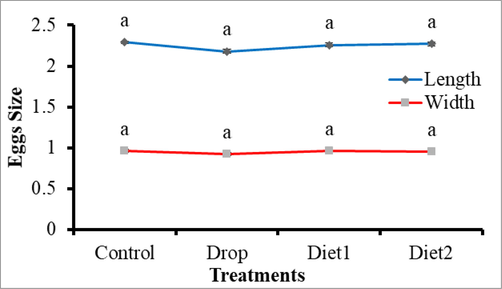
Effects of RfVg-dsRNA feeding on egg size.
3.3 Validation of RfVg gene expression
The RNAi effect on Vg gene expression was validated through qRT-PCR. The dsRNA targeting a unique region (locus 3538–3938) showing minor similarity with different insect Vgs fed in diets and as drops to 9-10th instars larvae. Real time PCR results indicated that the expression level of Vg gene reduced significantly in all treated groups especially where dsRNA was administered as drops in comparison with control group. The cycle threshold (CT) average was recorded as 24, 19, 32, and 17 for RfVg dsRNA-based diet-I, RfVg dsRNA-based diet-II, RfVg dsRNA drop, and control 15-days post-application, respectively (Fig. 8).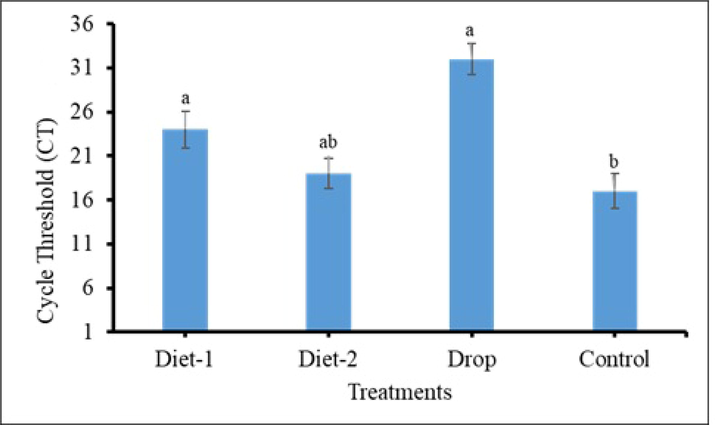
The cycle threshold (CT) average recorded for different treatments after 15-days of dsRNA application.
4 Discussion
The red palm weevil is a major pest of palm trees all over the world. It causes tremendous economic losses to the palm farming community, especially in Middle East RPW proved a very deleterious pest of date palm trees. Despite numerous attempts to control RPW, no effective alternative method for eliminating this destructive pest has approved. Recent molecular studies revealed that RNAi can be used to silence any specific genes before being translated. RNAi has been successfully used as target specific and eco-friendly technique for controlling several insect pests. Gene silencing using RNAi for controlling insect pests has been successfully proven in the laboratory (Price and Gatehouse 2008). The use of dsRNA as a spray or in transgenic plants has a high potential for effectively silencing the targeted genes (Baum et al., 2007, Mao et al., 2007, Zhao et al., 2008) and work more efficiently against insect showing pesticide resistance. Recently, we have silenced RfVg gene by injecting the Vg-based dsRNA and demonstrated that RNAi could be used to manage RPW, the ruined pest of date palm trees (Rasool et al., 2021). Similarly, in the present study RfVg-based RNAi significantly impaired the reproductive performance in terms of pre-oviposition period, oviposition period, eggs laying, and eggs hatching in all treatments as compared to control.
In current study, pre-oviposition period was prolonged in females treated with dsRNA especially where dsRNA was offered through oral drops as compared to dsRNA provided in diets and control. Similar results were reported in our previous studies where dsRNA was delivered through dorsal inject (Rasool et al., 2021). Effects of Vg silencing on pre-oviposition period have also been reported in several insect pests (Coelho et al., 2016, Moriyama et al., 2016). Our previous findings along with those reported by others have shown that silencing of Vg gene expression resulted in the deformed eggs due to failure of Vg protein expression (Coelho et al., 2016, Moriyama et al., 2016, Rasool et al., 2021). The present study has also revealed similar effects on egg size of RPW.
In RfVg-dsRNA treated females eggs hatchability% was zero as compared to control (Rasool et al., 2021). Though in present study eggs hatchability was not up to zero but reduced significantly in RfVg-dsRNA-treated groups. Some studies reported a significant decline in fecundity of RfVg-dsRNA-treated female (Tokar et al., 2014, Moriyama et al., 2016) but in present study we did not record any significant decrease in number of eggs laying in RfVg-dsRNA-treated groups.
We also tested the effect of RfVg-based RNAi on Vg gene expression, validated through qRT-PCR. The results of qRT-PCR indicated that the expression level of Vg gene reduced significantly in all treated groups especially where dsRNA was administered as drops in comparison with control group. In another study VgmRNA suppressed to 95.3% after 15-days of dsRNA application while suppression was further increased to 96.6% and 99.4% when tested after 20 and 25 days of dsRNA application through injection, respectively (Rasool et al., 2021). Successful suppression of Vg mRNA in RPW, in response to dsRNA application is a positive sign aimed at choosing Vg-based RNAi for RPW management. Literature reveals that coleopteran insects are more vulnerable to RNAi as compared to other insect species such as Diabrotica virgifera (Baum et al., 2007), Tribolium castaneum (Whyard et al., 2009), Leptinotarsa decemlineata (Zhu et al., 2011), and Anthonomus grandis (Coelho et al., 2016). Based on the present promising results and literature reviewed we can suggest that Vg-based RNAi has a great potential to be used as a target specific and eco-friendly technique for the sustainable control of RPW and several other major insect pests. Future research should focus on developing a most suitable technique to deliver RfVg-dsRNA into palm/ date palm trees where it should be readily available to attack RPW.
5 Conclusions
The present study demonstrated that Vg-based RNAi effectively suppressed the function of RfVg gene, resulting into a significant decline in the hatchability % of RPW eggs and Vg expression as measured by qRT-PCR. Results did not show any significant effect of Vg-based dsRNA on fecundity, oviposition period, post oviposition period, eggs size, and female and male life span, when applied through diet. Our present results along with those of previous findings very clearly demonstrate that Vg-based RNAi has a great potential to be used as a target specific and eco-friendly technique for the sustainable control of RPW.
Acknowledgments
This project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia (Award Number 13-BIO 1407-02).
Disclosure of Funding
The authors declare that they don’t have any particular funding for this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Semi-artificial diet developed for the successful rearing of red palm weevil: Rhynchophorus ferrugineus (Coleoptera: Dryophthoridae) in the laboratory. J. King Saud Univ.-Sci.. 2022;34(7):102272
- [Google Scholar]
- Al-Sheaby, F., 2010. SABIC launches Red Palm Weevil workshop as part of its commitment to global corporate social responsibility. Online at: http://www.sabic.com/corporate/en/newsandmediarelations/news/20100331-2.aspx.
- Control of coleopteran insect pests through RNA interference. Nat. Biotechnol.. 2007;25(11):1322-1326.
- [Google Scholar]
- Vitellogenin knockdown strongly affects cotton boll weevil egg viability but not the number of eggs laid by females. Meta Gene.. 2016;9:173-180.
- [Google Scholar]
- A review of the issues and management of the red palm weevil Rhynchophorus ferrugineus (Coleoptera: Rhynchophoridae) in coconut and date palm during the last one hundred years. Int. J. Trop. Insect Sci.. 2006;26(3):135-154.
- [Google Scholar]
- Gomez, V., Ferry, M., 1998. Attempts at biological control of date palm pests recently found in Spain. Proceedings of the first regional symposium for applied biological control in mediterranean countries, Cairo.
- The evolution of vitellogenins, cyclorrhaphan yolk proteins and related molecules. Adv. Insect Physiol.. 1998;27:335-384.
- [Google Scholar]
- Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat. Biotechnol.. 2007;25(11):1307-1313.
- [Google Scholar]
- Suppression of bedbug’s reproduction by RNA interference of vitellogenin. PLoS One. 2016;11(4):e0153984.
- [Google Scholar]
- RNAi-mediated crop protection against insects. Trends Biotechnol.. 2008;26(7):393-400.
- [Google Scholar]
- Accumulation of yolk proteins in insect oocytes. Annu. Rev. Entomol.. 1992;37(1):217-251.
- [Google Scholar]
- Silencing of vitellogenin gene contributes to the promise of controlling red palm weevil, Rhynchophorus ferrugineus (Olivier) Sci. Rep.. 2021;11(1):1-12.
- [Google Scholar]
- Molecular characteristics of insect vitellogenins and vitellogenin receptors. Insect Biochem. Mol. Biol.. 1998;28(5–6):277-300.
- [Google Scholar]
- SAS, 2008. SAS/STAT 9.2. user guide. SAS Institute, Carry, NC. USA.
- Laboratory evaluation of six Egyptian isolates of heterorhabditid nematodes for control of the red palm weevil. Egyptian J. Appl. Sci.. 1994;9(3):670-679.
- [Google Scholar]
- Receptor-mediated endocytosis of yolk proteins in insect oocytes. Reproductive Biol. Invertebrates. 2005;12(Part B):215-244.
- [Google Scholar]
- Persistency of indigenous and exotic entomopathogenic fungi isolates under Ultraviolet B (UV-B) irradiation to enhance field application efficacy and obtain sustainable control of the red palm weevil. Insects. 2022;13(1):103.
- [Google Scholar]
- Vitellogenin RNAi halts ovarian growth and diverts reproductive proteins and lipids in young grasshoppers. Am. Zool.. 2014;54(5):931-941.
- [Google Scholar]
- Tufail, M., Takeda, M., 2018. Vitellogenesis and yolk proteins in insects.
- Biosynthesis and processing of insect vitellogenins. Progress in vitellogenesis. Reproductive Biol. Invertebrates. 2005;12(part B):1-32.
- [Google Scholar]
- Molecular characteristics of insect vitellogenins. J. Insect Physiol.. 2008;54(12):1447-1458.
- [Google Scholar]
- Insect vitellogenin/lipophorin receptors: molecular structures, role in oogenesis, and regulatory mechanisms. J. Insect Physiol.. 2009;55(2):88-104.
- [Google Scholar]
- Hemolymph lipoproteins: role in insect reproduction. Hemolymph proteins and functional peptides: Recent advances in insects and other arthropods.. 2012;1:3-19.
- [Google Scholar]
- Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem. Mol. Biol.. 2009;39(11):824-832.
- [Google Scholar]
- Phyllotreta striolata (Coleoptera: Chrysomelidae): Arginine kinase cloning and RNAi-based pest control. Eur. J. Entomol.. 2008;105(5):815.
- [Google Scholar]
- Ingested RNA interference for managing the populations of the Colorado potato beetle. Leptinotarsa decemlineata. Pest Manage. Sci.. 2011;67(2):175-182.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102701.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







