Translate this page into:
Impact of rhizobacterium Bacillus sonorensis on propagation of Abelmoschus esculentus and its antimicrobial activity
⁎Corresponding authors. biosankaralingam@yahoo.co.in (Subbiah Sankaralingam), suribaskar@hotmail.com (Kathirvelu Baskar)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In order to evaluate the impact of Bacillus sonorensis on propagation of Abelmoschus esculentus and its antimicrobial activity was investigated. In the present study, A. esculentus was cultivated in B. sonorensis inoculated soil and also assessed the morphological as well as biochemical parameters of crops. The culture inoculum of B. sonorensis influenced growth and yield of treated plant. The root (39.5 cm), shoot length (20 cm), chlorophyll (1.06 mg), carotenoid (0.445 mg), protein and total soluble sugar content, NR & starch activity were higher in bioinoculated treated plant than control. The fresh biomass and dry weight were drastically increased in bioinoculated plants when compared to control. Gradual increase in composition of pivotal nutrients (N, P, K) and minor nutrients were observed in the bioinoculated plants. The culture filtrate possessed phytopathogenic activity against different phytopathogens. Among the three different phytopathogens, the maximum zone of inhibition (21 mm) was noticed in Aspergillus colletotrichum infected plants. The fermentation study was carried out in pilot scale fermentor and the synthesis of plant growth promoting substance was found to be 30.00 mg/l IAA.
Keywords
IAA
PGPR
Chlorophyll
Carotenoid
Starch
Soluble sugar
1 Introduction
Rapidly increasing human population and increasingly prevailing drought periods have led to deforestation and degradation of many ecosystems in the tropics, especially in India. The increasing population reflects on the environment, resulting in the destruction of biological productivity and biodiversity. Generally, the agriculture serves as a backbone for human population which fulfills the food supply (Pereira et al., 2020). Application of chemical fertilizer leads to soil pollution, contamination of ground water and threats to biodiversity because the gradual establishment of biomagnifications and eutrophication. The manufacture of quality food is due to maintain the supply of nutrients in sustainable compartment to make sure bio-safety. The innovative view of farm production attracts the growing demand of biological based organic fertilizers exclusive of an alternative to agrochemicals (Bhardwaj et al., 2014). In general, agriculture sector depends upon the fertility of soil, to enrich the supply of nutrients and to restore the nutrients in the field (Araujo et al., 2003).
Organic farming is one of the essential and important strategies to adopt food safety and also to maintain the biodiversity conservation (Megali et al., 2013). Biofertilizers from nature give a better yield, and beneficial to mankind by sustainable economic development to the farmers (Mishra and Dash, 2014). In this ground, we should advocate the naturally originated things in order to maintain the sustainable agriculture. PGPR is usually surrounds the superficial part of external surface of plant roots and it possesses positive effect on their host plant. Past few decades, PGPR has created a great interest due to its viable capacity with biofertilizer nature; it prevents diseases caused by various pests also to facilitate soil nutrition (Hu et al., 2018).
Directly or indirectly, PGPR has the ability to influence the plant growth and development. Production of phytohormones such as ethylene, gibberellins and auxin, iron chelating compounds, hydrogen cyanide and production of antibiotics are the essential traits of PGPR (Ahemad and Kibret, 2014). Plant rhizosphere samples are found to be sole biological niche with assorted microflora covered of bacteria, algae, fungi and protozoa; difference is based on the mechanism of PGPR; due to this organism supported nutritionally by a high input of organic materials derived from the rhizosphere (De Souza et al., 2015). Several earlier literatures have revealed the bacterial species viz., Bacillus, Pseudomonas, Azospirillum, Azotobacter, Klebsiella and Enterobacter, etc., have been exhibited a potentiality to induce the plant growth as well as secreting the industrially significant bioactive compounds like cellulase, exopolysaccharide etc. (Shankar et al., 2021; Maheswari et al., 2020; Sankaralingam et al., 2014).
In recent years we must create public awareness to promote eco-friendly organic products for sustainable agriculture in order to minimize the consumption of harmful energy intensive inputs. In this regard, we have selected flowering plant, A. esculentus, since it is prevalent cultivated crop in tropical areas. It is a good source of vitamins, minerals, calories and amino acid found in seeds. Also, it is chiefly cultivated as a garden crop on large wetlands in India. Most of the literatures have targeted the terrestrial as well as forest isolate on plant growth and development but only limited information is available on the impact of mangrove rhizobacterial strains on growth and yield of plant. With the above information, the present study is aimed to assess the effect of mangrove bacterium B. sonorensis on growth and yield of A. esculentus with their antibacterial activity.
2 Materials and methods
2.1 Effect of PGB on A. esculentus
Earthen pots were filled with Garden soil, Red soil, Sandy soil in the ratio 1:1:1 and mixed with overnight cultures of the isolate. Seeds of A. esculentus were sown.
2.2 Morphological and phenological observations
Periodically, the plants were observed at various intervals of 30, 45 and 60 days. Then, the plants were removed, rinsed with tap water and distilled water without disturbing the root system. At that time the growth characters were also analyzed.
2.3 Root length and shoot length (cm)
Selected plants were examined to analyze the root and shoot length. The plants were uprooted from the soil without disturbing the plants other plants and washed with tap water. Then, the length of shoot and root systems were measured.
2.4 Fresh and dry weight of plants (g)
By using the tap water, the plants were meticulously cleansed in order to get rid of the soil particles. The fresh weight was measured using balance. For dry weight, the fresh plant from each treatment, control and PGPR treated plants were cut into pieces then dried at 80 °C for 24 hrs, then weighed.
2.5 Biochemical estimation in crop plant
During the study, random samples from each treated pot were harvested at the intervals of 30th, 45th and 60th day after treatment for biochemical evaluations. Proximal compositions of protein (Lowry et al., 1951), total sugars (Nelson, 1944), total phenol (Jagadish et al., 2009), carotenoids (Arnon, 1949), phosphorus (Simard et al., 1994) and NR activity (Kumar and Khan, 1982) were estimated.
2.6 Estimation of chlorophyll
At 150 mg of treated leaves were trimmed from the decayed leaves and were immersed in 50 mL of 80% acetone in a conical flask and incubated in dark at 24 hrs for the extraction of chlorophyll. Thereafter, the chlorophyll obtained was separated by using Whatman No.1 filter paper. Absorbance of the chlorophyll extract was measured at 645 nm and 663 nm using a colorimeter. The amount of chlorophyll (a, b) and total chlorophyll were calculated in mg/g fresh weight according to the following formula.
i) Chlorophyll –a (mg/g fresh weight of leaf)
= 12.7 × (OD-663) – 2.69 × (OD-645) V/ 1000 × W
ii) Chlorophyll-b (mg/g fresh weight of leaf)
= 22.9 × (OD-645) – 4.68 × (OD-663)V/1000 × W
iii) Total chlorophyll (mg/g fresh wt. of leaf)
= 20.2 × (OD-645) + 8.02 × (OD-663) V/ 1000 × W
where, V = Final volume of 80% acetone, W = Fresh weight (g) of corresponding number of fresh leaves used in the extraction
2.7 Estimation of starch
Anthrone method was used to quantify the amount of starch in leaves. The leaves (100 mg) were macerated with 80% ethanol and centrifuged at 5000 rpm for 10 min. The residue was repeatedly washed with 80% ethanol till the residue becomes colorless and dried. At 5 mL of water and 6.5 mL of 52% perchloric acid were added then centrifuged at 5000 rpm for 10 mins. The extraction was carried out with perchloric acid. Then, 0.2 mL of the supernatant was taken and made up to 5 mL (1 mL - water and 4 mL - anthrone reagent). The test tubes were kept in boiling water bath for ten minutes. The content was cooled and transmission density was read at 630 nm. The amount of starch was determined using starch as the standard.
2.8 Soil analysis
Analysis of soil was carried out at Soil Testing Laboratory, Department of Agriculture, Virudhunagar, Tamil Nadu, India. The major and minor nutrients like nitrogen, phosphorus, potassium, iron, manganese, zinc, copper, calcium carbonate, electrical conductivity and pH were analyzed.
2.9 Antimicrobial susceptibility - diffusion methods
2.9.1 Agar disk-diffusion method
Agar plates are inoculated with uniform inoculum of test microorganism. Well cutter was used for 6 mm well to evaluate test compound.
2.9.2 Phytopathogenic and biocontrol activity
The isolates obtained from PDA plates were patched on Muller Hinton Agar to observe antimicrobial activity against A. niger, A. colletotrichum and Pseudomonas fluorescens. The subculture of B. sonorensis was added to all the wells. The bacterial plates were observed after incubation at 36 ± 1 °C and fungal plates were incubated 48 hrs at 27 ± 1 °C. After the incubation, the antimicrobial activity of B. sonorensis against A. niger, A. colletotrichum and P. fluorescens were evaluated by zone of inhibition (Talapatra et al., 2017). Biocontrol activity was observed in A. esculentus (Control, Fungus treated and Bioinoculant + A. niger) by A. niger strain was procured from the Department of Microbiology, Bharathidasan University, Tiruchirappalli.
3 Results
3.1 Growth and development
Effect of PSB on growth and development of A. esculentus was conducted in potted plant at Botanical garden of Saraswathi Narayanan College (Figs. 1a–c).
Root length of Abelmoschus esculentus after treatment of Bacillus sonorensis. b. Growth of Abelmoschus esculentuswas observed at 60 days after treatment of Bacillus sonorensis. c. Biocontrol activity of Abelmoschus esculentus against Aspergillus niger.
3.2 Root length
In the present study, the root length was studied after 30th, 45th and 60th days inoculation of candidate bacterial strains B. sonorensis in treated A. esculentus and control plants. The root length was higher in B. sonorensis treated plants as 39.5 cm of 60th days than the control, without the addition of inoculums (Table 1a & Fig. 1a).
Treatment
Preflowering (Cm)
Flowering (Cm)
Post Flowering (Cm)
(30 days)
(45 days)
(60 days)
T1
34.0 ± 0.02
35.5 ± 0.02
36.5 ± 0.02
T2
36.0 ± 0.02
39.0 ± 0.02
39.5 ± 0.02
3.3 Shoot length
Effect of bioinoculated and uninoculated on A. esculentus were carried out after 30th, 45th and 60th days. The shoot length was higher (20 cm) in bioinoculated i.e., B. sonorensis when compared to the control plants (Table 1b & Fig. 1b).
Treatment
Preflowering (cm)
Flowering (cm)
Post Flowering (cm)
(30 days)
(45 days)
(60 days)
T1
12.7 ± 0.02
14 ± 0.02
17 ± 0.02
T2
16 ± 0.02
19.2 ± 0.02
20 ± 0.02
3.4 Estimation of chlorophyll content
The chlorophyll content of A. esculentus was carried out by inoculation of B. sonorensis and control plant. In our study, chlorophyll (1.06 mg) was predominantly enhanced in plant supplemented with B. sonorensis then control after 60 days treatment (Table 1c).
Treatment
Preflowering (mg)
Flowering (mg)
Post Flowering (mg)
(30 days)
(45 days)
(60 days)
T1
1.02 ± 0.04
1.02 ± 0.04
1.02 ± 0.04
T2
1.03 ± 0.04
1.04 ± 0.04
1.06 ± 0.04
3.5 Carotenoid content
The carotenoid content of A. esculentus was determined for bioinoculated and control plants. In the present investigation, amount of carotenoid (0.445 mg) was predominantly high in bioinoculated plants than control plant (Table 1d).
Treatment
Preflowerig (mg) (30 days)
Flowering (mg) (45 days)
Post Flowering (mg) (60 days)
T1
0.587 ± 0.05
0.570 ± 0.05
0.343 ± 0.05
T2
0.678 ± 0.05
0.595 ± 0.05
0.445 ± 0.05
3.6 Estimation of protein
The protein content of A. esculentus was determined for bioinoculated and control plant. In the present study, protein content was predominantly higher (20.24 mg) in B. sonorensis inoculated plants than control after 60 days of treatment (Table 2a).
Treatment
Preflowerig (mg) (30 days)
Flowering (mg) (45 days)
Post Flowering (mg) (60 days)
T1
14.13 ± 0.05
19.81 ± 0.05
16.12 ± 0.05
T2
18.13 ± 0.05
23.76 ± 0.05
20.24 ± 0.05
3.7 Estimation of sugar
The total soluble sugar of A. esculentus was determined by the inoculation of candidate bacterial strain B. sonorensis and control plant. In our study, total soluble sugar was higher (10.53 mg) in bioinoculated plant than the control plants (Table 2b).
Treatment
Preflowering (mg) (30 days)
Flowering (mg) (45 days)
Post Flowering (mg) (60 days)
T1
9.846 ± 0.02
7.645 ± 0.04
8.500 ± 0.03
T2
14.345 ± 0.02
9.888 ± 0.04
10.530 ± 0.03
3.8 Assay for nitrate reductase
The nitrate reductase activity of A. esculentus was analyzed by the inoculation of candidate bacterial strain B. sonorensis and control plants. In our study, we observed that the nitrate reductase activity was predominantly higher in bioinoculated plant after 60 days when comparatively higher than plants which were grown without the addition of culture 8.14 µm NO2 g−1 (Table 3c).
Treatment
Pre-flowering (µm NO2 g−1) (30 days)
Flowering (µm NO2 g−1) (45 days)
Post Flowering (µm NO2 g−1) (60 days)
T1
7.34 ± 0.04
7.36 ± 0.04
7.38 ± 0.04
T2
7.49 ± 0.04
8.11 ± 0.04
8.14 ± 0.04
3.9 Estimation of starch and phosphate
The amount of starch in A. esculentus was carried out by the inoculation of candidate bacterial strain B. sonorensis and control plants. In our study, amount of starch activity was 1.232 mg in bioinoculated plants than the normal plants at 60th days after treatment (Table 3d).
Treatment
Preflowering (mg) (30 days)
Flowering (mg) (45 days)
Post Flowering (mg) (60 days)
T1
1.198 ± 0.04
1.212 ± 0.04
1.219 ± 0.04
T2
1.226 ± 0.04
1.229 ± 0.04
1.232 ± 0.04
The amount of phosphate in A. esculentus was carried out by the inoculation of candidate bacterial strain B. sonorensis and control plants. In our study, amount of starch content was predominantly higher (0.33 mg) in bioinoculated plants than the control plants (Table 3a).
Treatment
Preflowering (mg) (30 days)
Flowering (mg) (45 days)
Post Flowering (mg) (60 days)
Control
0.14 ± 0.04
0.17 ± 0.04
0.19 ± 0.04
Treated
0.24 ± 0.04
0.27 ± 0.04
0.33 ± 0.04
3.10 Fresh and dry weight of fruit sample
The fresh and dry weight of fruit was determined for both control and treatment. In our investigation, fresh biomass slightly increased in bioinoculated plants when compared to control at 60th day observation. The influence of the bacterial innoculum got reflected in the increase of weight of the fruit (Table 3b). The dry weight was determined in A. esculentus (control and treated plants) and the values were recorded after 60 days. In our present study, the dry weight was predominantly increased in A. esculentus after 60 days in bioinoculated plants than the control.
Treatment
Fresh weight of plant (kg)
Dry weight of plant (kg)
Fresh weight of fruit (gm)
Dry Weight of fruit (gm)
T1
0.445
0.210
13.75
6.75
T2
0.450
0.215
12.5
7.5
3.11 Soil analysis
In A. esculentus, there was a gradual increase in composition of major nutrients (N, P, K) in the bioinoculated soil than control. The influence of minor nutrients was also well established in the bionoculated plants grown soil when compared to control (Table 4).
CONTROL
TREATED
Soil texture
Clayey sandy soil
Clayey sandy soil
Calcium Carbonate
high
High
Electrical conductivity (dSm−1)
0.4
0.7
pH
8.4
8.5
Major Nutrients
Nitrogen
63.0
68
Phosphorus
23.1
25.8
Potassium
216.1
224.7
Micro Nutrients
Iron
2.8
3.0
Manganese
3.9
3.9
Zinc
0.2
0.4
Copper
0.9
1.2
3.12 Antifungal activity
In the present study, the screened candidate isolate B. sonorensis was evaluated for phytopathogenic activity against different phytopathogens. Measurement of inhibition of zone (expressed in mm) was observed in Muller Hinton Agar by well diffusion assay method (Figs. 2a–c). Among the three different phytopathogens, maximum zone of incubation (21 mm) was noticed in A. colletotrichum. A good biocontrol activity was noticed when compared to control and the plant which was treated alone with A. niger. We could clearly notice that the plants treated with the combined inoculum along with A. niger exhibited good biocontrol activity (Table 5).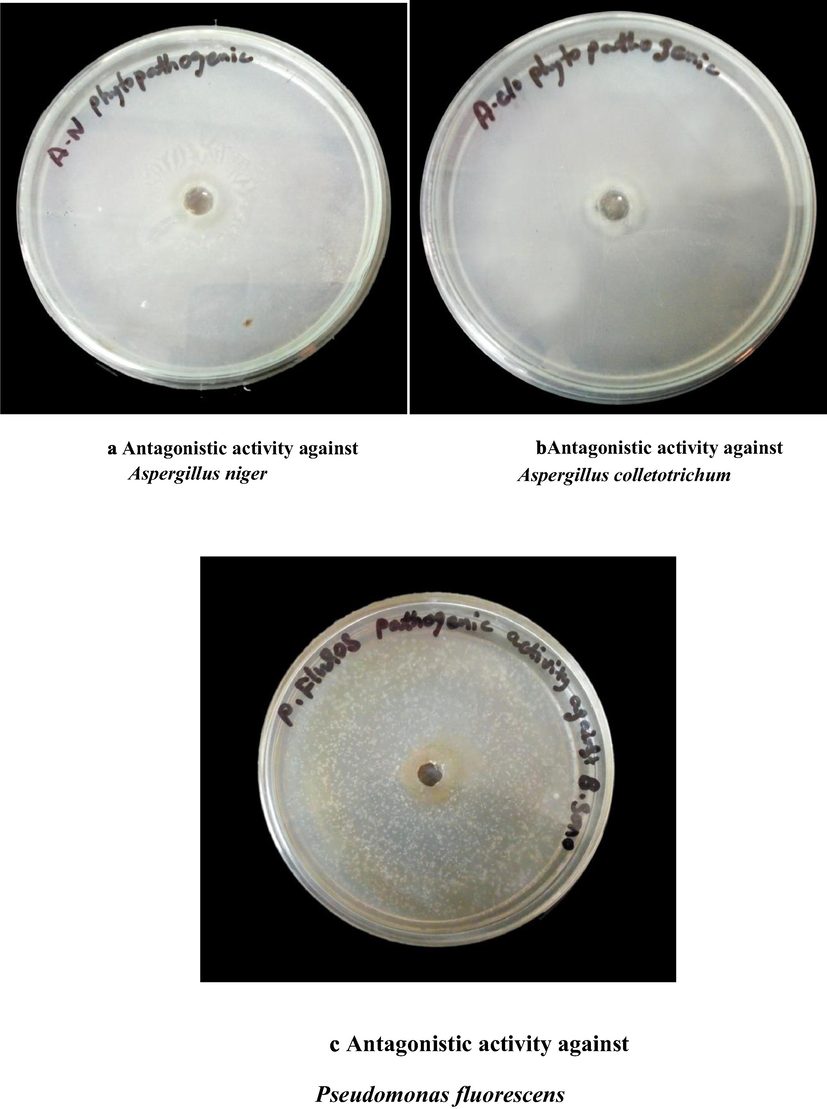
Antagonistic activity against Aspergillus niger. b. Antagonistic activity against Aspergillus clorotrichum. c. Antagonistic activity against Pseudomonas fluorescens.
SPECIES
Zone of inhibition (mm)
Aspergillus niger
18
Aspergillus colletotrichum
21
Pseudomonas fluorescens
13
3.13 Mass production of IAA
Based on the large scale production by pilot scale fermentor, 30 mg/L of IAA was produced by B. sonorensis.
4 Discussion
Previous findings suggest that the presence of PGPR, P. fluorescens (PF) colonized in roots of sparingly induces systemic stress tolerance in plants, responsible for the metabolic processes (Saakre et al., 2017; Prabhu et al., 2013). Application of biofertlizer will stimulate the nutrient content especially major nutrients (N, P, K) by the process of mineralization which favors microbial activity which in turn increases the quantity of protein. The activity of nitrate reductive enzymes also increases which helped in synthesis of certain amino acids and proteins (Sankaralingam et al., 2014; Singh et al., 2008). Earlier researchers like Singh et al. (2015) have reported the addition of vermicompost and biofertilizer on strawberry; application of vermicompost + Azotobacter + PSB + Arbuscular mycorrhiza produced maximum plant elevation, number of leaves, width and its yield. The earlier report is in strong correlation with our results, we got better yield in plant height, number of leaves & fruits, fresh & dry weight of plant.
Yadav et al. (2011) stated that the supreme tallness of the plant, fruit length and fruit width after collective application of vermicompost and Azotobacter with 100% recommended NPK in papaya. Our report is similar with earlier finding which suggests that increase in fruit length. Maximum shoot length and plant height was observed in the combination of inoculation of Azospirillum and phosphate solubilizing bacteria. The similar finding was observed in chick pea plants by Khan et al., (2020). Inoculation of soil isolate Bacillus sp. has influenced the shoot and dry weight of chick pea plants due to possessing the PGPR activity. In our investigation found that amount of carotenoid (0.445 ± 0.05 mg) present in A. esculentus after 60th days treatment. The carotenoid content was elevated by the supplementation of bioinoculant when compared to control (Harinathan et al., 2015)
The nutrient present in the fertilizer has drastically increased the starch and carbohydrates might have resulted in the increase of bulb diameter and shoot thickness. The results of the present investigation in terms of bulb diameter and shoot thickness are in association with the findings reported by Shinde et al. (2013). This previous finding is in accordance with our result which suggests that the usage of bioinoculant has resulted in the growth of the plant and yielded a higher accumulation of starch.
Kaur et al. (2015) observed the physico-chemical and biological characteristics of soil are improved by the supplementations with vermicompost. Soil fertility depends upon growth of plants and enzyme production. Research has revealed that vermicompost has an active role in refining growth and yield of diverse field crops, including vegetables, ornamental plants, cereals and fruit crops. This is in collaboration with our findings which reveals that the physio-chemical characteristics of soil are induced by the addition of bacterial culture as a suitable biofertilizer. Fagwalawa and Yahaya (2016) observed number of fruits and weight were increased by more than 100% when treated with poultry manure. He also reported that manure has increased the yield. This report is in close association with our finding, supplementation of PSB has a great influence of fruit weight in okra plants. In our study, prolific increase in fresh, dry and fruit weight when compared to control.
Sankaralingam et al. (2013) who have stated that the PSB augment N, P, K and protein content in fruit by the nutrient availability in the root zone from the soil. This bacterium enriches the accessibility of phosphorus to plants and facilitates the supply of nutrients by the crop which promotes root development. N and P might have resulted in superior content and uptake in okra. The nutrient content is higher due to the activity of microbes in the rhizosphere under inoculation of PSB (Singaravel et al., 2008). In our findings, there is a dynamic increase in major nutrients and uptake of nitrogen significantly when compared to control plants. The above finding by previous workers corroborates with our finding. Doifode and Nandkar (2014) reported that total number of functional leaves per plant was observed from 30 to 150 DAP. The maximum number of leaves at 120 DAP (552.38) was found in 100% RDF of NPK and AZT + PSB (463.25), than the control. The treatments had more mean number of leaves respectively per plant over the control. It is in conformity with our investigation total number of leaves got increased by the supplementation of bioinoculant.
Chlorophyll is highly useful biomolecule because it is very much essential one in photosynthesis. It makes chlorophyll content a significant experimental parameter in agronomy and plant biology research (Lamb et al., 2015). Sevik et al. (2012) reported that the quantity of chlorophyll displays alteration depending on many edaphic, climatic factors depending upon the time of vegetation period. The above result investigated by previous researchers has a strong correlation with our result.
Developed accumulation of osmolytes like total protein, sugar and free amino acids increase in the solute concentration, in drought exposed plants assisting the absorption of water from dry soil (Fahad et al., 2017). This is in same line with our report that the PGPR treated plants produced better result than control plants. Results demonstrated that B. sonorensis has enhanced the nitrate reductase activity in A. esculentus. Our findings correlate with the earlier work of many researchers who have confirmed the bacterial inoculation had influence the growth of crops (Santos and Esposito, 2014). Our results revealed the production of IAA was 30.0 mg/L. Our findings are bit coherent with the previous study of Jatav et al. (2017) who reported that the production of IAA in two Bacillus sp.
Antibiogram provides qualitative results by categorizing bacteria as susceptible, intermediate or resistant (Jorgensen and Ferraro, 2009). Phenotype can be identified to treat diseases with the help of antibiotics (Caron, 2012). Many species of microbes are used for plant protection to understand the bactericidal and bacteriostatic effect (Abanda-Nkpwatt et al., 2006). Rice pathogens are inhibited by some strains like Xanthomonas oryzae. The naturally occurring Bacillus isolate was found to be good biocontrol agents against X. oryzae (Niño-Liu et al., 2006). These results closely associate with our findings, because which exhibit maximum sensitivity in X. oryzae. In general, the plant growth is induced by auxins. IAA is produced by using tryptophan as a precursor in the medium. On the basis of the microorganism employed the production differs by its growth and suitable culture conditions, growth stage and availability of substrates (Swarnalakshmi et al., 2020). Our finding has a strong agreement in which influence of oxygen had increased for the production of IAA in B. sonorensis. Our study is in line with the previous researchers who reported that biocontrol activity was observed in potato plants to control the fungal disease (Garbelotto et al., 2019). The present study is suggested that the candidate bacterial strain Bacillus sonorensis possessed good PGPR activity based on the pot culture studies. The bioactive potential along with PGPR traits was determined for the development of biofertilizers in sustainable agriculture/organic farming.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group NO (RGP-271) and also authors thanks to University Grants Commission F.No. - 43- 109/2014(SR), Govt. of India, New Delhi.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Dual antagonism of aldehydes and epiphytic bacteria from strawberry leaf surfaces against the pathogenic fungus Botrytis cinereain vitro. BioControl. 2006;51:279-291.
- [CrossRef] [Google Scholar]
- Mechanisms and applications of plant growth promoting rhizobacteria: current perspective. J. King Saud Univ-Sci.. 2014;26(1):1-20.
- [CrossRef] [Google Scholar]
- Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere. 2003;52:799-804.
- [CrossRef] [Google Scholar]
- Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol.. 1949;24(1):1-15.
- [CrossRef] [Google Scholar]
- Biofertilizers function as key player in sustainable agriculture by improving soil fertility, plant tolerance and crop productivity. Microb. Cell Fact.. 2014;13:66.
- [CrossRef] [Google Scholar]
- Antimicrobial susceptibility testing: a four facets tool for the clinician. Journal des Anti-infectieux. 2012;14(4):168-174.
- [CrossRef] [Google Scholar]
- Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol Biol.. 2015;38(4):401-419.
- [CrossRef] [Google Scholar]
- Influence of Biofertilizers on the growth, yield and quality of brinjal crop. Int. J. Life Sci.. 2014;A2:17-20.
- [Google Scholar]
- Effect organic manure on the growth and yield of okra. Imperial Journal of Interdisciplinary Research. 2016;2(3):130-133.
- [Google Scholar]
- Evidence for inhibition of a fungal biocontrol agent by a plant microbiome. J. Plant Pathol.. 2019;101(3):457-466.
- [CrossRef] [Google Scholar]
- Efficiency of Plant Growth Promoting Rhizobacteria for the Enhancement of Sesbania grandiflora and Moringa oleifera Growth. Advances in Biological Research. 2015;9(4):230-235.
- [CrossRef] [Google Scholar]
- Effects of long-term fertilization on phoD-harboring bacterial community in Karst soils. Sci. Total Environ.. 2018;628–629:53-63.
- [CrossRef] [Google Scholar]
- Jagadish, L.K., Krishnan, V.V., Shenbhagaraman, R., Kaviyarasan, V., 2009. Comparative study on the antioxidant, anticancer and antimicrobial property of Agaricus bisporusim (J. E. Lange) Imbach before and after boiling. Afr. J. Biotechnol. 8(4), 654-661 http://www.academicjournals.org/AJB
- Production of plant growth hormones indole-3-acetic acid (iaa) using Bacillus by batch fermentation. Global J. Biosci. Technol.. 2017;6(4):612-616.
- [Google Scholar]
- Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clinical Infectious Diseases. 2009;49(11):1749-1755.
- [CrossRef] [Google Scholar]
- Effect of guar gum and xanthan gum on pasting and noodle-making properties of potato, corn and mung bean starches. J. Food Sci. Technol.. 2015;52(12):8113-8121.
- [CrossRef] [Google Scholar]
- Peroxidase and polyphenol oxidase in excised ragi (Eleusine coracana cv. PR 202) leaves during senescence. Ind. J. Exp. Botany. 1982;20(5):412-416.
- [Google Scholar]
- A practical solution for 77 K fluorescence measurements based on LED excitation and CCD array detector. PLOS ONE. 2015;10:e0132258
- [CrossRef] [Google Scholar]
- Protein measurement with the Folin phenol reagent. J. Biol. Chem.. 1951;193:265-275.
- [Google Scholar]
- Maheswari, P., Kumar, K.A., Sankaralingam S., Natesan, S., 2020. Optimization and characterization of exopolysaccharide from marine soil bacteria. Research Journal of Pharmacy and Technology. 13, (6) 2540 -2544
- Fertilization with beneficial microorganisms decreases tomato defenses against insect plants Agron. Sustain. Dev.. 2013;34:649-656.
- [CrossRef] [Google Scholar]
- Rejuvenation of biofertilizer for sustainable agriculture and economic development. Consilience: The Journal of. Sustainable Development. 2014;11(1):41-61.
- [Google Scholar]
- Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS One. 2020;15(4):e0231426
- [CrossRef] [Google Scholar]
- A photometric adaptation of the Somogyi method for the determination of glucose. J. Biol. Chem.. 1944;153:375-380.
- [Google Scholar]
- Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol. Plant. Pathol.. 2006;7(5):303-324.
- [CrossRef] [Google Scholar]
- Plant growth-promoting rhizobacteria (PGPR) improve the growth and nutrient use efficiency in maize (Zea mays L.) under water deficit conditions. Heliyon.. 2020;25(6)
- [CrossRef] [Google Scholar]
- Influence of Arbuscular mycorrhizal fungi on the growth of green gram (Vigna radiata L.) grown under water stress conditions. World Appl. Sci. J.. 2013;25(4):561-567.
- [CrossRef] [Google Scholar]
- Identification and characterization of genes responsible for drought tolerance in rice mediated by Pseudomonas fluorescens. Rice Sci.. 2017;24(5):291-298.
- [CrossRef] [Google Scholar]
- Sankaralingam, S., Eswaran, S., Balakan, B., Sundaram, V.M., Shankar, T. 2014. Screening and growth characterization of phosphate solubilizing bacterium Pseudomonas aeruginosa. Adv. Environ. Biol. 8(13), 673–680: http://www.aensiweb.com/AEB/.
- Santos, A.F.J., Esposito, E., 2014. Interaction between microorganisms and socio-environmental biotechnology. In: Public policies: studies and cases (Panhoca, I., Bonini, L.M.M., Eds.). Org. Ciancarullo, T.I., Ícone, São Paulo.
- Change to amount of chlorophyll on leaves depend on insolation in some landscape plants. Int. J. Environ. Sci.. 2012;3(3):1057-1064.
- [CrossRef] [Google Scholar]
- Purification and characterization of carboxymethylcellulase from Bacillus pumilus EWBCM1 isolated from earthworm gut (Eudrilus eugeniae) J. King Saud Uni –Sci.. 2021;33(1):101261
- [CrossRef] [Google Scholar]
- Effect of organic, inorganic and biofertilizers on uptake of nutrients by onion (Allium cepa L.) grown under western Maharashtra conditions. J. Agric. Res. Technol.. 2013;38(2):192.
- [Google Scholar]
- Phosphorus sorption and desorption indices in soil. Commun. Soil Sci. Plant Anal.. 1994;25(9–10):1483-1494.
- [CrossRef] [Google Scholar]
- Effect of liquid formulation of Symbion – N (Azospirillum) and Symbion - P (Phosphobacter) on the growth and yield of okra. An Asian Journal of Soil Science. 2008;3(2):261-263.
- [Google Scholar]
- Biological control of root rot fungus Macrophomina phaseolina and growth enhancement of Pinus roxburghii (Sarg.) by rhizosphere competent Bacillus subtilis BN1. World J. Microbiol. Biotechnol.. 2008;24:1669.
- [CrossRef] [Google Scholar]
- Can we use maize (Zea mays) rhizobacteria as plant growth promoter?.Vegetos- An International. Journal of Plant Research. 2015;28(1):86-99.
- [CrossRef] [Google Scholar]
- Significance of Plant Growth Promoting Rhizobacteria in Grain Legumes. Growth Promotion and Crop Production. Plants. 2020;9(11):1596.
- [Google Scholar]
- In vitro antagonistic activity of a root endophytic fungus towards plant pathogenic fungi. J. Appl. Biol. Biotechnol.. 2017;5(2):068-071.
- [CrossRef] [Google Scholar]
- Effect of integrated nutrient nourishment on yield attributes and economics of papaya (Carica papaya L.) cv. Pusa Dwarf. Plant Archives. 2011;11(1):327-329.
- [Google Scholar]







