Translate this page into:
Impact of omega-3 fatty acids supplementation in children with sickle cell disease in Saudi Arabia
⁎Corresponding author at: Applied Medical Nutrition Group, King Fahd Medical Research Center, King Abdulaziz University, P.O. Box 80216, Jeddah 21589, Saudi Arabia. sakhan01@kau.edu.sa (Shahida Khan) shahidakhan2009@gmail.com (Shahida Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Though hydroxyurea (HU) is one of the most widely used FDA approved drug in sickle cell disease (SCD), it is associated with certain side effects. Therefore, safer and effective alternatives need to be tried out. Earlier researchers have shown abnormalities in the blood cell fatty acid composition, in patients with SCD. Omega-3 fatty acids being a safer nutritional supplement, possessing anti-adhesion, anti-aggregatory, and anti-depressive properties could be tried out.
Objective
To explore the impact of omega-3 fatty acids eicosa-pentaenoic acid (EPA) and docosa-hexaenoic acid (DHA) in reducing disease severity in children with SCD, in Saudi Arabia through a pilot interventional study.
Methods
Forty-three SCD patients, aged between 5 and 16 years, were supplemented omega-3 fatty acids for a period of six months. Their clinical complications and hematological markers were studied, pre and post supplementation.
Results and conclusion
Omega-3 supplementation resulted in overall favorable changes in the cell membrane and was found effective in reducing the number of vaso-occlusive crisis (VOC) as well as complications accompanying SCD.
Keywords
Sickle cell disease
Vaso occlusive crisis
Hydroxyurea
Omega-3fatty acids
- SCD
-
Sickle Cell Disease
- VOC
-
Vaso-occlusive crisis
- HU
-
Hydroxyurea
- EPA
-
Eicosa pentaenoic acid
- DHA
-
Docosa hexaenoic acid
- AA
-
Arachidonic acid
- GLA
-
Gamma linolenic acid
- RBC
-
Red blood cell
- Hb
-
Hemoglobin
- HCT
-
Hematocrit
- MCV
-
Mean corpuscular volume
- MCH
-
Mean corpuscular hemoglobin
- MCHC
-
Mean corpuscular hemoglobin concentration
- WBC
-
White blood cells
- RDW
-
Red cell distribution width
Abbreviations
1 Introduction
World Health Organization has designated sickle cell disease (SCD) as a public health priority, as it is one of the most common gene disorders in the world (Weatherall and Clegg, 2001). SCD is characterized by recurrent episodes of debilitating pain, which at times requires hospitalization and medical intervention, either with opioid analgesics, hydroxyurea, or in certain cases transfusion or bone marrow transplantation (Yale et al., 2000). HU represents one of the widely prescribed drugs in SCD. The only other FDA approved drug is L-glutamine that can be administered to SCD children above five years of age. Though HU induces an increase in fetal hemoglobin (Hb), that is beneficial to patients with SCD, there is yet a reliance upon opioids for relief from chronic pain. The lack of its perceived effect on daily symptomatic response like daily pain intensity, and increasing percentile days with analgesic use, may require researchers to look for alternative interventions (Smith et al., 2011). Studies regarding its teratogenic side effects, and the clinical applications for modifying disease outcomes are still ongoing (Maia Filho et al., 2019). As genetic diseases impose a heavy emotional and economic burden in Saudi Arabia, there is an impending need to expedite effective preventive strategies (El-Hazmi et al., 2011). Therefore, safe alternatives apart from HU need to be researched, to alleviate the painful episodes and relieve the suffering of patients with SCD (Cannas et al., 2017). One of the factors impacting SCD is malnutrition, and the obvious need to correct the situation through wholesome nutrition is implied (Kawchak et al., 2007; Khan et al., 2016; Kirkham and DeBaun, 2004).
The composition of phospholipids in the membranes of sickle cell erythrocytes exhibit irregularity, with replacement by saturated and certain unsaturated fatty acids. The role of dietary omega-3 fatty acids deserves distinct mention in SCD as they enhance the integrity of the erythrocyte membrane in vivo, thereby, inhibiting hemolysis which is the governing mechanism in this disease (Delesderrier et al., 2020). The various beneficial properties of omega-3 fatty acids viz; anti-aggregatory, anti-inflammatory, and anti-adhesiveness, increase their potential for preventing sickling and reducing the excruciating crisis associated with the disease (Daak et al., 2013).
Omega-3 fatty acids may score better than HU if we holistically consider their medicinal, mercantile and community perspectives. Moreover, they do not exhibit the adverse effects associated with the administration of HU. Omega-3 fatty acids from natural sources like egg, fish and chicken, are easily acceptable, because they are safely consumed as food globally. The direct beneficial effects on the various cells of the immune system by omega-3 fatty acids is well documented. Being an integral part of the cell membrane, omega-3 fatty acids are known to regulate its fluidity, lipid composition and act as important signaling molecules (Gutiérrez et al., 2019). DHA and EPA supplementation have been found to be beneficial in immuno modulation and reducing inflammation (Fenton et al., 2013). Animal studies have also shown that omega-3 fatty acids exhibit powerful immunomodulatory and anti-inflammatory action in many autoimmune diseases, and infections (Fritsche, 2006). As omega 3 fatty acids are affordable compared to drugs, their usage could be well appreciated in non-affluent countries, where the overwhelming majority of people affected by SCD live. Its usage therefore in reducing the complications of SCD, makes it effective and safe, in addition to being affordable (Wachira et al., 2014). We therefore investigated the effectiveness of omega-3 fatty acids to reduce the severity of disease symptoms in children with SCD in Saudi Arabia.
2 Materials and methods
2.1 Methods
A pilot interventional study on children with SCD was conducted at King Fahd Medical Research Center (KFMRC), King Abdulaziz University, Jeddah. The demographics and anthropological data of enrolled patients are presented in Tables 1 and 2 respectively. Criteria for sample selection were also based on practicality, availability of patients, procurement of clinical data and patient’s consent. Since we were conducting the study on children, the sample size of our patients mainly depended upon acceptance of patient’s guardians for participation. A control or placebo group could not be included in the study owing to non-compliance. Therefore, the patients’ pre supplementation values were considered as their controls.
n
Total screened
210
Patients fitting criteria
155
Total number of patients given consent for clinical data, supplementation and blood collection
43
Total number of patients completed the study
40
Number of VOC
≤ one----43
HbSS(only homozygous SS)
43
Hb SA
nil
Taking no medication
43
Parameter
Total number of patients
43
Male
23
Female
20
5–10 years
26
Average weights in Kg
19.89 ± 5.7 SD
Average height in cm
112.06 ± 10.03
11–16 years
17
Average weights in Kg
33.98 ± 8.85SD
Average height in cm
145.5 ± 15.20
Ethnicity
Saudi Arabia
24
Yemen
9
Sudan
4
Syria,Palestine,Jordan,Egypt
6
2.2 Ethical consideration
The study was approved by the institutional ethical committee of King Abdulaziz University (no.2/36/8390). Written consents were obtained from parents/guardians of the patients.
2.3 Inclusion criteria
Male and female children with SCD in a steady state, 5–16 years of age, approximately matched with weights were included in the study. Steady state was defined as steady hemoglobin and hematocrit levels for more than 3–4 weeks. There was no bias due to composite physical traits of different ethnicities contributing to the patient’s phenotype.
2.4 Exclusion criteria
Patients less than 5 years of age and above 16 years of age, having undergone any blood transfusions within the last ten weeks prior to getting enrolled for the study; presence of other chronic diseases; or diagnoses other than VOC, or prior treatment with HU, in the previous 10 weeks, were excluded.
2.5 Supplementation
Children were given oral omega-3 mango flavored syrup (BRAINWISE from SPB Laboratories, HP, India) according to their body weights. Children of body weight 11–24 kg were given 2 teaspoons oral omega-3 syrup every day and those having body weights 25 kg and above were supplemented with 3 teaspoons every day. Each teaspoon contained 190 mg of DHA, and EPA 250 mg. No other dietary recommendations were prescribed along with the supplement administered.
The use of HU may affect the frequency and severity of vaso-occlusive crisis. Therefore only those patients who were not taking hydroxyurea were enrolled for our study.
2.6 Clinical data and hematological parameters
Standard clinical parameters were assessed and recorded by the clinician at the hospital during their check-up, along the course of the study. Breathing problems include breathlessness due to chest infections and shortness of breath; abdominal pain—refers to stomach cramps and severe stomach pain at times; renal complications include renal papillary necrosis and renal medullary carcinoma; splenomegaly refers to spleen enlargement; bed wetting problems refer to the incontinence issues faced; eye problems include conjunctivitis, and itchy eyes; and dental problems include dental caries.
Five ml blood was collected before and after supplementation. A complete blood count was done using a Sysmex KX-21 automated cell counter (Sysmex Corporation, Kobe Hyogo, Japan).
2.7 Fatty acid analysis
Blood collected (3 ml) in non-EDTA tubes, was allowed to stand for half an hour and then centrifuged at 3000 rpm for 15 min. to obtain serum fraction as supernatant. Methyl esters were prepared using anhydrous methanolic HCl and the resultant esters extracted in hexane (Ichihara and Fukubayashi, 2010; Ren et al., 2013) and dried using nitrogen gas. The methyl esters were then re-suspended in 50 μl of chloroform and 1 μl sample injected and analyzed by gas chromatography performed on an Agilent 6890 gas chromatograph using a Omegawax®, column (30 m × 0.25 mm I.D., 0.25 µm). Helium was the carrier gas set at a flow rate of 1.0 ml/min. A flame ionization detector was used with a column oven temperature initially held at 140 °C for 1 min, programmed at 5 °C rise/minute up to 200 °C. After a 3.0 min hold, temperature was increased to 215 °C with a 5 °C /min heating ramp for 5 min. This temperature was finally increased to 240 0C with a 10 °C/min for 10.5 min. Injector and detector temperature were kept at 220 °C and 200 °C respectively. Injection was done in a split mode of (20:1) and the run time was 35 min. Peak identification was done by retention time matched to standards. The data was presented as percent of the total fatty acids, and compared with appropriate standards (Masood et al., 2005).
2.8 Psychological parameters
Assessment of Intensity of pain -The pain indices were checked using the Wong Baker faces pain scale. (Chambers et al., 1999; von Baeyer, 2006; Wong et al., 2001; Wong and Baker, 1988). Pain was measured on a scale of 1–10; where ‘0′ denotes - no pain, 1–3 denotes low pain, 4–6 denotes moderate pain, and 7–10 denotes extreme pain. Frequency of pain was measured by the number of episodes in the past six months in different ranges (range 1 with one pain episode; range 2 with 2–5 pain episodes and range 3 with 6–10 episodes).
2.9 Statistical analysis
Mean values of the variables along with their standard deviations were analyzed using the statistical software SPSS16 for a paired t-test. P value of <0.05 was considered as statistically significant.
3 Results
3.1 Clinical picture
At the inception of the study, all patients experienced the same complications, crisis and other clinical features (Table 3). Data on their hospitalizations could not be collected due to insufficient procurement procedure. No adverse reaction was observed by the children whatsoever, except for gastric upset in three children at the beginning of the study, which subsided in 2–3 days. Also, no bleeding events were observed throughout the supplementation period.
Clinical Record
6 Month pre suppl
(n = 40)6 Month post suppl
(n = 40)
No
little
high
No
little
High
Fever
10
4
26
25
5
10
Jaundice
17
5
18
18
5
17
VOC
3
No patients
37
30
7
3
Breathing Problem
16
2
22
12
12
16
Abdominal pain
17
2
21
21
2
17
Oedema
30
No patients
10
30
3
7
Tachycardia
15
2
23
19
4
17
Renal complications
40
No patients
No patients
40
No patients
No patients
Splenomegaly
29
4
7
32
7
1
Bed wetting
30
4
6
30
3
7
Eye problems
37
2
1
38
1
1
Dental problems
31
7
2
37
2
1
During the six months of omega-3 supplementation, patients reported lesser complications, tolerable pain, fewer infections, and fewer visits to the hospitals. Moreover, management for the fewer complications like head ache, body ache, slight fever, cold etc. could be done by the parents of patients at home by giving medications for a very short duration. No hospital admissions were required. A general positive influence of omega-3 supplementation on the clinical symptoms of the patients was visibly noticed.
3.2 Hematological parameters
Most of the children enrolled were severely anemic (Hb < 8) with only a few being moderately anemic (Hb 8.0–10.9). Supplementation caused an increase in hemoglobin concentration significantly with a p value of 0.045 as seen in Table 4. The concentration of MCHC was increased significantly from 32.390 ± 1.500 to nearer the normal range 33.690 ± 2.19 with a p value of 0.02. Red cell distribution width (RDW) decreased significantly with a ‘p ‘value of 0.000. Other parameters like hematocrit (HCT), red blood cells (RBC), platelet count, mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), and white blood cells (WBC) did not alter significantly.
Parameters
n = 40Time of analysis
Levels
p-value
RBC(x1012/L)
Pre
post2.69 ± 0.46
2.83 ± 0.58NS 0.12
Hb(gm/dL/dl)
Pre
post7.26 ± 0.74
8.04 ± 1.590.04
HCT(%)
Pre
post22.85 ± 2.52
22.99 ± 4.87NS − 0.4
MCV(fl)
Pre
post83.94 ± 8.60
83.59 ± 8.43NS 0.38
MCH(pg)
Pre
post27.20 ± 3.61
30.02 ± 8.53NS 0.19
MCHC (%)
Pre
post32.39 ± 1.5
33.69 ± 2.190.02
Platelet-(×109/l)
Pre
post404.24 ± 154.86
431.66 ± 134.72NS 0.37
WBC(x109/L)
Pre
post12.38 ± 3.54
11.07 ± 3.92NS 0. 08
RDW(%)
Pre
post33.36 ± 2.08
20.60 ± 4.860.00
3.3 Omega-3 fatty acids
No appreciable reduction of saturated fatty acids was observed as seen in Table 5. Nonetheless, there was a subsequent decrease in the omega-6 to omega-3 fatty acid ratio. There was an increase in the omega-3 fatty acids EPA and DHA after supplementation, but it was not significant. A decrease in the omega-6 fatty acid arachidonic acid (AA) was significant with a p value of 0.03. But the reduction in total sum of omega-6 concentrations, was not significant. The only omega-6 fatty acid which increased was gamma linolenic acid (GLA) which is an anti-inflammatory fatty acid and is known to possess immense health benefits.
Parameters
n = 40Pre Suppl
Percentage of fatty acidsPost Suppl
Percentage of fatty acidsProbability
Sat
Myristic C14:0
1.04 ± 0.33
1.04 ± 0.22
0.33
Palmitic C16:0
30.70 ± 8.09
30.34 ± 7.13
0.83
Stearic C18:0
14.37 ± 3.56
14.21 ± 3.54
0.89
n-3
Alpha Linolenic C18:3
2.69 ± 1.48
3.11 ± 2.11
0.51
Eicosapentaenoic C20:5
1.88 ± 1.02
2.18 ± 1.49
0.48
Docosadienoic C22:0
1.48 ± 1.18
1.54 ± 1.44
0.94
Docosapentaenoic C22:5
1.10 ± 0.83
1.62 ± 1.02
0.09
Docosahexaenoic C22:6
0.44 ± 0.13
0.65 ± 0.76
0.24
Total omega-3 fatty acids
6.91 ± 3.45
7.51 ± 4.39
0.43
n-6
Linoleic C18:2
15.85 ± 4.88
13.04 ± 4.76
0.07
Gamma Linolenic C18:3
0.37 ± 0.13
0.41 ± 0.15
0.41
Eicosadienoic C20:2
2.89 ± 1.73
2.42 ± 2.30
0.58
Dihomo-gamma-linoleic C20:3
0.95 ± 0.32
0.76 ± 0.26
0.13
Arachidonic C20:4
4.18 ± 2.43
3.40 ± 1.63
0.03
Total omega-6 fatty acids
28.30 ± 7.00
25.58 ± 8.22
0.21
n-9
Oleic C18:1
14.67 ± 5.23
12.78 ± 5.23
0.32
Omega-6/omega-3 ratio
5.27 ± 4.37
4.86 ± 3.85
0.89
3.4 Psychological parameters
All children enrolled in our project experienced immense pain when assessed on a scale of 1–10 as well as the faces pain scale at the commencement of the study (Fig. 1). It was observed that the pain was accompanied with negativity and a feeling of hopelessness. The number of crises experienced was in the higher range at the start of the study. Post supplementation, most of the children were in the no pain range with the number of pain episodes also decreased (Fig. 2). None of the patients required hospitalization during the few pain crises experienced during the entire supplementation period.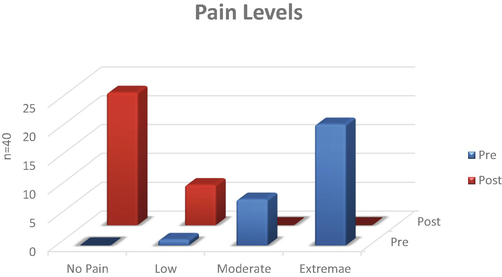
Pain levels in children with sickle cell disease supplemented with omega-3 fatty acids.
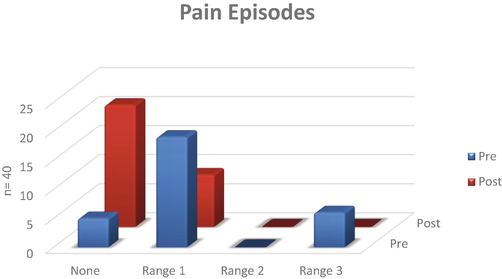
Pain episodes in children with sickle cell disease supplemented with omega-3 fatty acids.
4 Discussion
Supplementation with omega-3 fatty acids was effective in reducing the adverse clinical complications, VOC, and pain, apart from boosting their mental wellbeing. After 6 months of omega-3 supplementation, significant positive changes in the Hb, MCHC, and RDW values were observed. No apparent changes were seen in the HCT and MCV values, which could probably be due to the short time period of supplementation or low dosage. Nonetheless, the overall clinical benefits to the patients were very obvious. A higher hemoglobin level reflects better overall health and oxygen exchange capacity, which is brought about by positive alterations in the membrane. Lockdowns during pandemics further result in decreased oxygen availability to most of the people staying indoors with limited access to fresh air. As SCD children require more oxygen intake, the consumption of omega-3 fatty acids would improve their health status. Low levels of hemoglobin due to lack of oxygen causes fatigue and also results in low MCHC values. In consistence with previous studies, SCD children showed increased white blood cell and lowered platelet counts before supplementation, confirming that SCD is a chronic inflammatory disorder. Supplementation increased Hb, MCH, MCHC concentrations, and lowered the WBC count, which is in agreement with earlier research (Babiker et al., 2014). In SCD patients, a lack of production of enough RBC’s, is seen to result in macrocytic anemia as observed by larger RDW values. Reticulocytosis, or a possible nutritional deficiency of folate, B12 and iron results in macrocytic anemia which is reflected by larger RDW values. Though folate is normally prescribed to children with sickle cell disease, yet insufficiency or more requirements may reflect higher RDW values. This value reduced after supplementation. Though not significant, platelet counts increased and WBC counts lowered after supplementation. Probably a study conducted for a longer period or with larger doses etc., would give a clearer picture.
Patients with SCD have shown to possess higher levels of the omega-6 fatty acid AA as compared to the omega-3 fatty acid DHA in the RBC membrane. AA favors the formation of the pro-inflammatory eicosanoid viz; thromboxane A2 thereby facilitating inflammation, platelet aggregation and vaso-occlusion in SCD (Daak et al., 2013). In accordance with this, lower amounts of omega-3 concentrations and higher AA concentrations were observed in our group of patients (Table 5) prior to supplementation. The only omega-6 fatty acid increased after supplementation (though not significant) in our study was GLA which is anti-inflammatory in nature (Kapoor and Huang, 2006). GLA is known to produce the eicosanoids thromboxane and prostaglandin of 1 series (thromboxane X1 and prostaglandin E1) and leukotriene of the three series. Prior to supplementation, omega-6: omega-3 ratio imbalance was noticed in all patients which is known to predict the asymmetry, aggregation, blood cell adhesion and, vaso-occlusion in SCD (Ren et al., 2005). Supplementation with omega-3 fatty acids lowers this omega-6: omega-3 ratio, thereby favoring the anti-inflammatory state. Earlier studies have shown an association between the dietary intake, lipid profile and saturated fatty acid content in young children. (Cheng et al., 2003). We observed that our study children had low body mass index with low-calorie intakes. It has also been observed in one of our previous studies that omega-3 fatty acids normalize the lipid profile in patients with hyperlipidemia (Khan et al., 2017). As the blood saturated fatty acid content in these SCD children was not high before supplementation, the only requirement was an increase in the anti-inflammatory omega-3fatty acids and a reduction in the inflammatory omega6 fatty acids. This could explain for minimal changes in saturated fatty acids and the selective shift of omega-6: omega-3 fatty acids ratio in the present study.
Omega 3 supplementation resulted in reduced frequency and severity of vaso-occlusive pain episodes, probably due to the omega-3 fatty acid dependent inhibition of thrombotic events (Babiker et al., 2014; Tomer et al., 2001). As none of the patients needed hospitalization during the supplementation period, there was lesser stress on the patient as well as the caretaker, depicting a great social and economic impact.
A recent trial observed that after 8 weeks of treatment with omega-3 fatty acids, all the tested doses were found safe and well tolerated (Daak et al., 2018) making it suitable for therapeutic usage. Omega-3 fatty acids are safe, naturally available and affordable too. Moreover, there are concerns about infertility, and its safety during pregnancy and lactation with HU usage (Adeyemo et al., 2019). Most drugs show a negative interaction with omega-3 fatty acids (Di Minno et al., 2010) excepting large doses of anticoagulants (Harris, 2007), thereby making it a molecule of choice for interventional studies. They have also been shown to exhibit antioxidant potential, apart from their anti-sickling effects (Kotue et al., 2019).
The diversity of options of consumption of EPA and DHA omega-3 fatty acids by way of egg, fish, fish oil, chicken etc. makes it an ideal candidate for consumption during pandemics and also otherwise by all age groups. This could be adopted in non-affluent countries where the number of people affected by SCD is higher. Alternatively, a supplementation protocol, incorporating the benefits of hydroxyurea along with omega-3 fatty acids could bring about immense health improvements in these SCD patients.
4.1 Limitations of the study
One of the biological variables that modulates the severity of this disease is the concentration of HbF in blood, which was not analyzed in our study.
4.2 Future follow up studies
The increases in active vitamin D by omega-3 fatty acids when supplemented along with vitamin D to patients with cardiovascular problems, could possibly be responsible for the subsequent cardio protective effects observed (Lee et al., 2015; Manson et al., 2012). Also the role of vitamin D in selectively targeting cancer cells by its interaction with Cu(II) ions (Rizvi et al., 2015) and bio-synthesized silver nanoparticles (Azmi et al., 2021) are worth mentioning. These studies could give insights in planning future researches.
It would be interesting to note the differences if any, are observed, in the blood as well as clinical picture of SCD patients of varying genotypes. More structured follow up studies with a control group, different dosage, larger sample size, and different time periods of supplementation of omega-3 fatty acids are warranted, for consolidating the results obtained.
5 Conclusion
Supplementation not only increased the blood concentrations of the anti-inflammatory omega-3 fatty acids in blood, but also decreased the concentration of the inflammatory fatty acid AA. Furthermore, an improvement in hematological parameters and adverse clinical events was experienced by children with SCD. Omega 3 fatty acids exhibit great potential in alleviating the complications accompanying the disease.
Funding
Authors would like to thank King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia, for funding a large research grant bearing the number: AT35-25, to pursue a project titled- “Resolving malnutrition status in children affected with sickle cell disease for alleviating health complications”.
CRediT authorship contribution statement
Shahida Khan: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Supervision, Data curation, Investigation, Formal analysis, Validation, Writing – original draft, Writing – review & editing. Ghazi Damanhouri: Conceptualization, Methodology, Project administration, Resources, Supervision, Investigation, Formal analysis, Validation, Writing – review & editing. Tahir Jameel Ahmed: Methodology, Project administration, Resources, Investigation, Formal analysis, Validation, Writing – review & editing. Saeed Halawani: Resources, Investigation, Formal analysis, Validation, Writing – review & editing. Ashraf Ali: Data curation, Investigation, Formal analysis, Validation, Writing – review & editing. Ahmad Makki: Data curation, Investigation, Formal analysis, Validation, Writing – review & editing. Sarah Khan: Methodology, Data curation, Investigation, Formal analysis, Validation, Writing – review & editing.
Acknowledgments
The authors are grateful to King Fahd Medical Research Center, King Abdulaziz University Jeddah, Saudi Arabia, for the facilities provided to conduct the research work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Barriers to the use of hydroxyurea in the management of sickle cell disease in Nigeria. Hemoglobin. 2019;43(3):188-192.
- [CrossRef] [Google Scholar]
- Optimization for synthesis of silver nanoparticles through response surface methodology using leaf extract of Boswellia sacra and its application in antimicrobial activity. Environ. Monit. Assess.. 2021;193(8):497.
- [CrossRef] [Google Scholar]
- Assessment of the effect of omega-3 fatty acid supplementation in sudanese patients with sickle cell anemia; khartoum, sudan. Int. J. Multidiscip. Curr. Res.. 2014;2:1133-1138.
- [Google Scholar]
- Hydroxycarbamine: from an Old Drug used in malignant hemopathies to a current standard in sickle cell disease. Mediterr. J. Hematol. Infect. Dis.. 2017;9(1):e2017015
- [CrossRef] [Google Scholar]
- A comparison of faces scales for the measurement of pediatric pain: children's and parents’ ratings. Pain. 1999;83(1):25-35.
- [Google Scholar]
- Serum fatty acid composition in primary school children is associated with serum cholesterol levels and dietary fat intake. Eur. J. Clin. Nutr.. 2003;57(12):1613-1620.
- [CrossRef] [Google Scholar]
- Double-blind, randomized, multicenter phase 2 study of SC411 in children with sickle cell disease (SCOT trial) Blood Adv.. 2018;2(15):1969-1979.
- [CrossRef] [Google Scholar]
- Effect of omega-3 (n-3) fatty acid supplementation in patients with sickle cell anemia: randomized, double-blind, placebo-controlled trial. Am. J. Clin. Nutr.. 2013;97(1):37-44.
- [CrossRef] [Google Scholar]
- Antioxidant nutrients and hemolysis in sickle cell disease. Clin. Chim. Acta. 2020;510:381-390.
- [CrossRef] [Google Scholar]
- Exploring newer cardioprotective strategies: ω-3 fatty acids in perspective. Thromb. Haemost.. 2010;104(4):664-680.
- [CrossRef] [Google Scholar]
- Sickle cell disease in Middle East Arab countries. Indian J. Med. Res.. 2011;134(5):597-610.
- [CrossRef] [Google Scholar]
- Immunomodulation by dietary long chain omega-3 fatty acids and the potential for adverse health outcomes. Prostaglandins Leukot. Essent. Fatty Acids. 2013;89(6):379-390.
- [CrossRef] [Google Scholar]
- Fatty acids as modulators of the immune response. Annu. Rev. Nutr.. 2006;26:45-73.
- [CrossRef] [Google Scholar]
- Effects of omega-3 fatty acids on immune cells. Int. J. Mol. Sci.. 2019;20(20)
- [CrossRef] [Google Scholar]
- Expert opinion: omega-3 fatty acids and bleeding—cause for concern? Am. J. Cardiol.. 2007;99(6):S44-S46.
- [Google Scholar]
- Preparation of fatty acid methyl esters for gas-liquid chromatography. J. Lipid Res.. 2010;51(3):635-640.
- [CrossRef] [Google Scholar]
- Gamma linolenic acid: an antiinflammatory omega-6 fatty acid. Curr. Pharm. Biotechnol.. 2006;7(6):531-534.
- [CrossRef] [Google Scholar]
- Adequacy of dietary intake declines with age in children with sickle cell disease. J. Am. Diet. Assoc.. 2007;107(5):843-848.
- [CrossRef] [Google Scholar]
- Precipitating factors and targeted therapies in combating the perils of sickle cell disease – a special nutritional consideration. Nutr. Metab. (Lond.). 2016;13:50.
- [CrossRef] [Google Scholar]
- Effects of short term supplementation of fish oil capsules on the blood fatty acid profile of vegetarians: a pilot study. Int. J. Med. Res. Health Sci.. 2017;6(5):19-23.
- [Google Scholar]
- Stroke in children with sickle cell disease. Curr. Treat Options Neurol.. 2004;6(5):357-375.
- [CrossRef] [Google Scholar]
- Antisickling and antioxidant properties of omega-3 fatty acids EPA/DHA. Nutri. Food Sci. Int J.. 2019;9(1)
- [CrossRef] [Google Scholar]
- The effects of omega-3 fatty acid on vitamin D activation in hemodialysis patients: a pilot study. Mar. Drugs. 2015;13(2):741-755.
- [CrossRef] [Google Scholar]
- Is chronic use of hydroxyurea safe for patients with sickle cell anemia? An account of genotoxicity and mutagenicity. Environ. Mol. Mutagen.. 2019;60(3):302-304.
- [CrossRef] [Google Scholar]
- The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159-171.
- [CrossRef] [Google Scholar]
- A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. J. Lipid Res.. 2005;46(10):2299-2305.
- [CrossRef] [Google Scholar]
- Blood mononuclear cells and platelets have abnormal fatty acid composition in homozygous sickle cell disease. Ann. Hematol.. 2005;84(9):578-583.
- [CrossRef] [Google Scholar]
- Total serum fatty acid analysis by GC-MS: assay validation and serum sample stability. Curr. Pharm. Anal.. 2013;9(4):331-339.
- [CrossRef] [Google Scholar]
- Cu(II)-vitamin D interaction leads to free radical-mediated cellular DNA damage: a novel putative mechanism for its selective cytotoxic action against malignant cells. Tumour Biol.. 2015;36(3):1695-1700.
- [CrossRef] [Google Scholar]
- The association between hydroxyurea treatment and pain intensity, analgesic use, and utilization in ambulatory sickle cell anemia patients. Pain Med.. 2011;12(5):697-705.
- [CrossRef] [Google Scholar]
- Reduction of pain episodes and prothrombotic activity in sickle cell disease by dietary n-3 fatty acids. Thromb. Haemost.. 2001;85(6):966-974.
- [Google Scholar]
- Children’s self-reports of pain intensity: scale selection, limitations and interpretation. Pain Res. Manage.: J. Canad. Pain Soc.. 2006;11(3):157.
- [Google Scholar]
- n-3 Fatty acids affect haemostasis but do not increase the risk of bleeding: clinical observations and mechanistic insights. Br. J. Nutr.. 2014;111(9):1652-1662.
- [CrossRef] [Google Scholar]
- Inherited haemoglobin disorders: an increasing global health problem. Bull. World Health Organ.. 2001;79(8):704-712.
- [Google Scholar]
- Wong, D., Hockenberry-Eaton, M., Wilson, D., Winkelstein, M., Schwartz, P. 2001. Wong's essentials of pediatric nursing Mosby, St. Louis (Mo).
- Pain in children: comparison of assessment scales. Pediatr. Nurs.. 1988;14(1):9-17.
- [Google Scholar]
- Approach to the vaso-occlusive crisis in adults with sickle cell disease. Am. Fam. Physician. 2000;61(5):1349-1356. 1363-1344
- [Google Scholar]







