Translate this page into:
Impact of heat stress on agro-morphological, physio-chemical and fiber related paramters in upland cotton (Gossypium hirsutum L.) genotypes
⁎Corresponding authors. waheed_riaz2007@yahoo.com (Muhammad Waheed Riaz), melshikh@ksu.edu.sa (Mohamed S. Elshikh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The unpredictably changing climatic conditions, especially high temperatures, are putting a continuous threat to sustainable cotton production. The current study was designed to investigate the impact of heat stress on several morpho-physiological, biochemical, and fibre quality-related traits. The results revealed the presence of significant variations in agro-morphological, physio-chemical and staple length-related parameters for upland cotton genotypes and stress treatments. Further analysis of pooled data unveiled that heat stress had a detrimental impact on all studied plant traits. Severe reduction in plant height, nodes per plant, sympodial branches per plant, number of bolls per plant, ginning out-turn, and staple length were recorded under heat stress. A significant reduction in net photosynthetic rate (Pn) up to 28.6 % was observed in cotton genotype BH-200 (24.7 to 19.2 µmole m−2 s−1). The accumulation of hydrogen peroxide (H2O2) was increased from 7.1 % in BH-306 to 28.7 % in BH-200 under heat stress due to the incidence of oxidative stress. A substantial increase in the accumulation of antioxidants i.e., catalase (65 %–74 %), peroxidase (54 %–169 %), and superoxide dismutase (52 %–98 %) was seen under high-temperature stress conditions. The correlation coefficient analysis unveiled a significantly positive correlation of seed cotton yield with nodes per plant (r = 0.432*), net photosynthetic rate (r = 0.829**), peroxidase (r = 0.974**), and superoxide dismutase (r = 0.868**), under heat stress conditions. However, a negative but statistically significant correlation of seed cotton yield with ginning out turn (r = −0.466*), staple length (r = −0.898**), hydrogen peroxide (r = −0.955**) and catalase (r = −0.904**) was also observed. The overall results unveiled that cotton genotype BH-232 has a comparatively higher heat tolerance than other contesting genotypes while BH-306 showed the highest susceptibility to heat stress. Hence, BH-232 could be recommended after its approval for general cultivation in heat-prone areas of Pakistan.
Keywords
High temperature
Antioxidants
ROXs
Photosynthesis
Climate change
Staple length
1 Introduction
The transitional environmental conditions are posing a continuous menace to the productivity and sustainability of major field crops by altering their development behaviour and capacity to endure severe environmental conditions. The constant deterioration in croping areas due to urbanization, desertification, salinization, and uncontrolled increase in the human population exacerbated the effects of climate change. Several environmental factors determine the productivity of crop plants including heat, drought, precipitation, humidity, and sunshine hours. However, high temperature or heat stress is one of the major constrain in the growth and development of crops including cotton. Singh et al. (2007) reported that with an increase in temperature of even 1 °C over optimal growing temperature, the lint yield of the cotton crop could be reduced up to 110 kg ha−1.
Cotton is the most important industrial crop regarding natural fibre and oil (Salimath et al., 2021). During 2020–21, it was cultivated on 31.42 million hectares from which 111.48 million 480 lb bales were obtained, averaging 773 kg ha−1 in the World (USDA, 2022). In Pakistan, its cultivated area during 2021–22 was 1.937 million hectares, from which 8.329 million bales were produced, averaging 731 kg ha−1 (ESP, 2021-22). Although Pakistan’s per hectare cotton production is very near to the world’s per hectare production, it is far behind the major cotton-producing countries i.e., Australia (2217 kg ha−1), China (1976 kg ha−1), Turkey (1804 kg ha−1), Brazil (1720 kg ha−1) and United States (957 kg ha−1) (USDA, 2022). The major reasons for a lower yield of cotton in Pakistan are high input rates, unavailability of inputs, high diseases and insects-pest infestation rates, drought stress, heat stress, lack of mechanical harvesting, and unavailability of quality seed.
In cotton, one of the most heat-sensitive phases is the flowering stage, which leads to severe flower shedding, stunted plant growth, and reduced number of bolls and boll weight, resulting in significant yield losses (Xu et al., 2020). Higher temperatures (32–40 °C) negatively influence root development and stomatal conductance. Moreover, temperatures higher than 29 °C also reduce the boll weight (Lokhande and Reddy, 2014). The seed germination rate is highly affected by the higher temperatures (˃37 °C) along with the reduction in pollen tube germination and elongation (Burke et al., 2004). Salman et al. (2019) showed that the reduction in cotton yield under heat stress is resulted by the decrease in germination rate, net photosynthetic rate, relative cell injuries, sympodial branches, boll weight, and increase in flower, square, and boll abscission. Along with the cotton yield, lint quality is also degraded under heat stress due to the increase in short fibres, decrease in fibre fineness and fibre uniformity (Zafar et al., 2021). Therefore, it is a hard challenge for plant breeders to rigorously evaluate cotton genotypes under stressful conditions to identify tolerant genotypes. The current experimental study was designed to evaluate local cotton genotypes for their heat tolerance along with the identification of key traits that contribute to the heat tolerance in cotton.
2 Materials and methods
2.1 Experimental materials and location
The current experimental study was conducted at the research area of Cotton Research Station (CRS), Bahawalpur (29° 22′ 29.2296′' N, 71° 38′ 16.1124′' E) and at the altitude of 115 m during two consecutive cotton growing seasons (2020-21, 2021–22). The experimental material was comprised of five upland cotton genotypes viz., BH-254, BH-234, CIM-600, BH-200, and BH-306.
2.2 Experimental layout
The experiment was carried out in the field under two treatments 1) control and 2) heat stress for two consecutive cotton-growing seasons 2020-21 and 2021-22. In the control treatment, the cotton crop was sown during the 2nd week of March while under stress treatment, the sowing was done during the 3rd week of April (Late sowing). In the late sown crop, the flowering and bud formation stage is expected to experience a high temperature (above 40 °C) as revealed by previous years’ metrological data. The research was carried out in an RCBD pattern in triplicates and treatments were laid out using split-plot arrangement.
2.3 Measurement/Recording of plant parameters
2.3.1 Morpho-Physiological and fibre-related parameters
Several plant morphological traits i.e., plant height (PH), nodes per plant (N/P), number of sympodial branches per plant (SB/P) and number of bolls per plant (NB/P) were recorded at the time of second picking while ginning out-turn (GOT) and seed cotton yield (SCY) were measured after crop harvest. Net photosynthetic rate (Pn; µmolem-2s−1) was recorded through CI-320, a handheld Near-Infrared Gas Analyzer as recommended and used by Yousaf et al. (2022). The staple or fibre length of cotton was measured through a high-volume instrument (HVI) and measured in millimetres.
2.3.2 Methodology for the measurement of hydrogen peroxide (H2O2) accumulation
The activity of H2O2 was measured through the protocol developed and used by Velikova et al. (2000).
3 Enzymatic antioxidant determination
3.1 Preparation of enzyme extract
Collected Fully extended and healthy leaves were thoroughly washed with distilled water and frozen under liquid nitrogen (N2) and stored at −80◦C for the assessment of the antioxidant activity of different enzymes. Leaf samples of 5 g from the stored samples were ground meticulously in chilled 10 ml potassium phosphate buffer (pH 7.8). The reaction mixture was then centrifuged at 14000 rpm for 10 min at 4 °C. The supernatant was used for the determination of antioxidant activities through their absorbance at different wavelengths in a UV–vis spectrophotometer (Sarwar et al., 2019).
3.2 Estimation of Catalase, Peroxidase and Super-oxidase dismutase activities (U mg−1 Protein)
The activity of these enzymes was measured through a UV–vis spectrophotometer at different wavelengths. The Catalase activity was measured at the wavelength of 240 nm as given by Liu et al., 2009. The Peroxidase activity was recorded according to Liu et al. (2009) at 470 nm wavelength. Similarly, superoxidase dismutase (SOD) activity was measured spectrophotometrically through the absorbance capacity of SOD at 560 nm wavelength by following the protocol given by Beauchamp and Fridovich (1971).
3.3 Statistical analysis
The obtained data of seed cotton yield and its associated morpho-physiological and biochemical traits were investigated for analysis of variance (ANOVA) and correlation coefficient analysis (Steel et al., 1997). To execute the above mention analysis, three statistical packages i.e., Statistix 8.1, XLSTAT 21.0, and Origin 21.0 were used. Furthermore, Microsoft Excel 2021 and Adobe Illustrator 22.0 were used for the illustration of the results.
4 Results
4.1 Climatic data
Climatic data for key factors, temperature (°C) and precipitation (mm) were recorded on daily basis from the date of sowing to square and boll development (60 days after sowing). The data was recorded for both treatments and two consecutive years (2020–21 and 2021–22). The data showed that the maximum temperature for the control treatment during the month of sowing was less, 34 °C (2020–21) and 36 °C (2021–22) than the maximum temperature for the heat stress treatment, 36 °C (2020–21) and 40 °C (2021–22) (Fig. 1a). Moreover, the mean daily maximum temperature under high-temperature treatment was meaningfully higher than maximum temperature in control (Fig. 1b).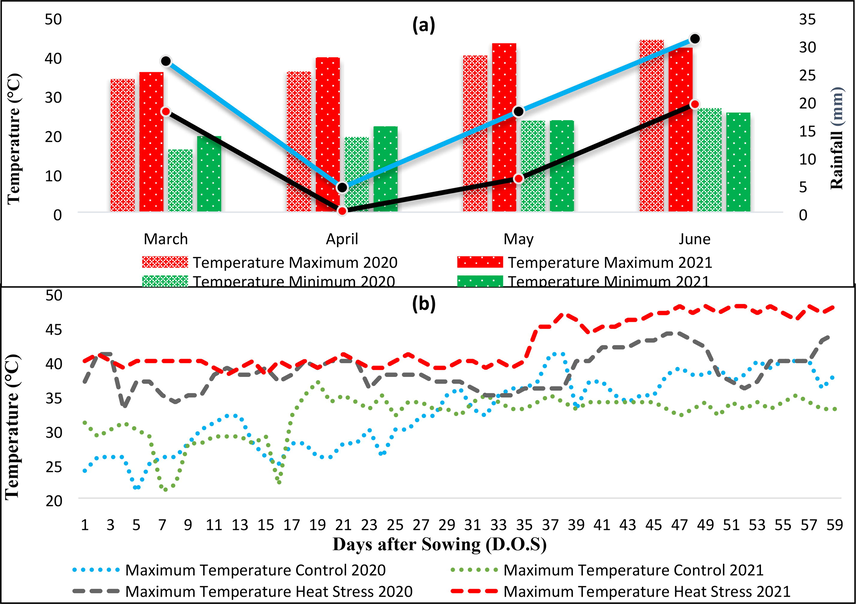
(a) Metrological data for the first four months of crop growth, (b) Variation in temperature under control and heat stress conditions.
4.2 Analysis of variance (ANOVA)
The combined analysis of variance revealed the presence of highly significant variations among cotton genotypes for morpho-physiological, biochemical, and fibre related parameters under control and high temperature conditions (Table 1). However, the variation for the year’s source of variance was non-significant for all studied traits except the number of bolls per plant. Due to this fact, the two-year data were pooled, and their average was taken for further analysis. *P ≤ 0.05, **P ≤ 0.01; ns: non-significant. P.H = Plant height (cm), N/P: Number of nodes per plant, SB/P: Number of sympodial branches per plant, NB/P: Number of bolls per plant, G.O.T: Ginning out turn (%), SL: Staple length (mm), Pn: Net photosynthetic rate (µmolem-2s−1), H2O2: Hydrogen peroxide (µmoleg−1), CAT: Catalase (U mg−1 protein), POXs: Peroxidase (U mg−1 protein), T-SOD: Total superoxide dismutase (U mg−1 protein), SCY: Seed cotton yield (Kg per ha−1).
Source of Variation
df
P.H
N/P
SB/P
NB/P
GOT
SL
Pn
H2O2
CAT
POXs
T-SOD
SCY
REP (A)
2
197.36
5.12
24.08
20.70
0.20
0.88
3.15
0.10
75.51
58.42
23.51
35,900
Year (B)
1
0.07NS
0.60NS
0.02NS
1.30*
0.32NS
0.50NS
0.14NS
1.33NS
0.48NS
0.51NS
0.14NS
1.67NS
Error A*B
2
0.68
0.35
2.13
0.55
0.22
0.38
0.22
1.42
0.54
0.38
0.32
2.92
Treatment (C)
1
1870.4**
98.8**
112.07**
148.8**
15.2**
0.46**
182.6**
194.8**
324283**
33443**
22136**
2054980**
B*C
1
0.02NS
0.82NS
0.07NS
0.94NS
0.42NS
0.10NS
1.49**
1.64NS
0.68NS
0.71NS
0.26NS
201.67NS
Error A*B*C
4
1.65
0.67
0.20
0.66
0.29
0.12
0.36
1.32
78.95
0.81
23.00
6379.27
Genotypes (D)
4
1430**
13.90**
42.4**
87.43**
14.54**
4.38**
49.18**
63.02**
6518.9**
2861.9**
930**
377000**
C*D
4
324.6**
10.77**
0.83NS
41.57*
5.50**
0.008NS
9.03**
0.80**
640.4**
122.65**
125**
165008**
Error A*B*C*D
32
98.65
4.39
10.77
19.10
1.99
0.12
0.92
0.07
47.17
8.47
10
24,562
Total
59
197.36
5.12
24.08
20.70
0.20
0.88
3.15
0.10
75.51
58.42
23.51
35,900
4.3 Seed cotton yield-associated morpho-agronomical traits
The correlation analysis unveiled a significant positive association between nodes per plant (N/P) and seed cotton yield (SCY) under normal (r = 0.465*) as well as heat stress conditions (r = 0.432*) (Table 2). However, seed cotton yield depicts a significantly negative correlation with GOT under normal (r = −0.548*) and heat stress conditions (r = −0.466**). The correlation of SCY with PH and NB/P was positive but non-significant under both normal (r = 0.96, r = 0.179) and heat stress (r = 0.235, r = 0.236) conditions, respectively. On the other hand, the correlation between SB/P and SCY was negative but non-significant under both stress conditions (r = −0.317, r = −0.270), respectively (Table 2). A significant decrease in seed cotton yield (SCY) and its associated morpho-agronomical traits i.e., PH, N/P, SB/P, NB/P and GOT was observed in heat stress treatment compared to control (Table 3). Note: Values in the upper diagonal are the correlation (rh) between different traits under heat stress while the values in the lower diagonal are the correlation (rn) between different plant traits under control/normal conditions. P.H = Plant height (cm), N/P: Number of nodes per plant, SB/P: Number of sympodial branches per plant, NB/P: Number of bolls per plant, G.O.T: Ginning out turn (%), SL: Staple length (mm), Pn: Net photosynthetic rate (µmolem-2 s-1), H2O2: Hydrogen peroxide (µmoleg−1), CAT: Catalase (U mg−1 protein), POXs: Peroxidase (U mg−1 protein), T-SOD: Total superoxide dismutase (U mg−1 protein), SCY: Seed cotton yield (Kg per ha−1). P.H = Plant height (cm), N/P: Number of nodes per plant, SB/P: Number of sympodial branches per plant, NB/P: Number of bolls per plant, G.O.T: Ginning out turn (%).
Traits
P.H
N/P
SB/P
NB/P
GOT
SL
Pn
H2O2
CAT
POXs
T-SOD
SCY
P.H
0.418
0.025
0.471
0.767
0.220
0.578
−0.160
−0.490
0.215
0.327
0.179
N/P
0.771
0.656
−0.285
0.185
−0.177
0.226
−0.576
−0.515
0.473
0.158
0.432
SB/P
0.186
0.423
−0.809
0.229
0.418
−0.466
0.153
0.300
−0.293
−0.631
−0.270
NB/P
0.411
0.040
−0.516
0.292
−0.212
0.555
−0.217
−0.506
0.344
0.684
0.236
GOT
0.772
0.295
0.255
0.210
0.732
−0.058
0.399
0.090
−0.379
−0.237
−0.466
SL
0.183
−0.104
0.609
−0.357
0.700
−0.590
0.884
0.732
−0.895
−0.804
−0.898
Pn
0.235
0.631
−0.216
−0.024
−0.347
−0.722
−0.700
−0.868
0.792
0.881
0.829
H2O2
−0.174
−0.582
0.246
−0.185
0.478
0.859
−0.940
0.920
−0.986
−0.831
−0.955
CAT
−0.330
−0.517
0.470
−0.495
0.289
0.865
−0.851
0.932
−0.953
−0.926
−0.904
POXs
0.470
0.709
−0.239
0.426
−0.185
−0.752
0.866
−0.939
−0.967
0.908
0.974
T-SOD
0.193
0.599
−0.243
0.160
−0.454
−0.842
0.956
−0.999
−0.929
0.938
0.868
SCY
0.096
0.465
−0.317
0.335
−0.548
−0.921
0.838
−0.972
−0.937
0.920
0.959
Variety
Treatment
P.H
N/P
SB/P
NB/P
GOT
BH-254
Control
115.7 ± 3.22abc
24.0 ± 0.47abc
18.7 ± 0.75ab
20.3 ± 1.09abc
41.3 ± 0.41a
Heat Stress
103.0 ± 3.45bcd
21.0 ± 0.53 cd
16.3 ± 0.73ab
18.0 ± 1.08bc
39.9 ± 0.38abcd
BH-232
Control
120.0 ± 3.23ab
25.0 ± 0.49ab
17.7 ± 0.73ab
26.0 ± 1.1a
40.0 ± 0.39abc
Heat Stress
110.0 ± 3.47abcd
21.7 ± 0.52 cd
15.0 ± 0.72b
22.0 ± 1.09abc
39.0 ± 0.39bcd
CIM-600
Control
125.0 ± 3.26a
26.3 ± 0.47a
20.7 ± 0.73a
20.7 ± 1.12abc
41.0 ± 0.41ab
Heat Stress
120.0 ± 3.45ab
23.3 ± 0.52abcd
17.7 ± 0.72ab
17.7 ± 1.09bc
40.7 ± 0.37ab
BH-200
Control
99.7 ± 3.24cde
24.0 ± 0.48abc
19.0 ± 0.74ab
18.7 ± 1.09abc
38.4 ± 0.40 cd
Heat Stress
80.0 ± 3.47e
22.0 ± 0.54bcd
17.0 ± 0.75ab
15.0 ± 1.10c
37.8 ± 0.37d
BH-306
Control
108.7 ± 3.26bcd
23.3 ± 0.45abcd
19.3 ± 0.75ab
23.3 ± 1.11ab
40.4 ± 0.39abc
Heat Stress
90.0 ± 3.45de
20.7 ± 0.53d
16.0 ± 0.74ab
19.3 ± 1.08abc
39.8 ± 0.38abcd
4.4 Staple length
Staple length (SL), which is one of the most important fibre traits from the textile industrial point of view, showed significant variance among cotton genotypes under normal and heat stress conditions (Table 1). The results revealed that the highest staple length was exhibited by cotton genotype BH-254 (29.2 mm) followed by BH-306 (29.1 mm) and CIM-600 (29.0 mm) (Fig. 2a). On the other hand, BH-232 showed minimum staple length under normal (28.2 mm) and heat stress treatment (27.9 mm) followed by BH-200 (28.5 mm, 28.2 mm), respectively. The correlation coefficient analysis indicated a strong negative and highly significant correlation between staple length (S.L) and seed cotton yield (SCY) under normal (r = −0.921**) and heat stress treatment (r = −0.898**) (Table 2).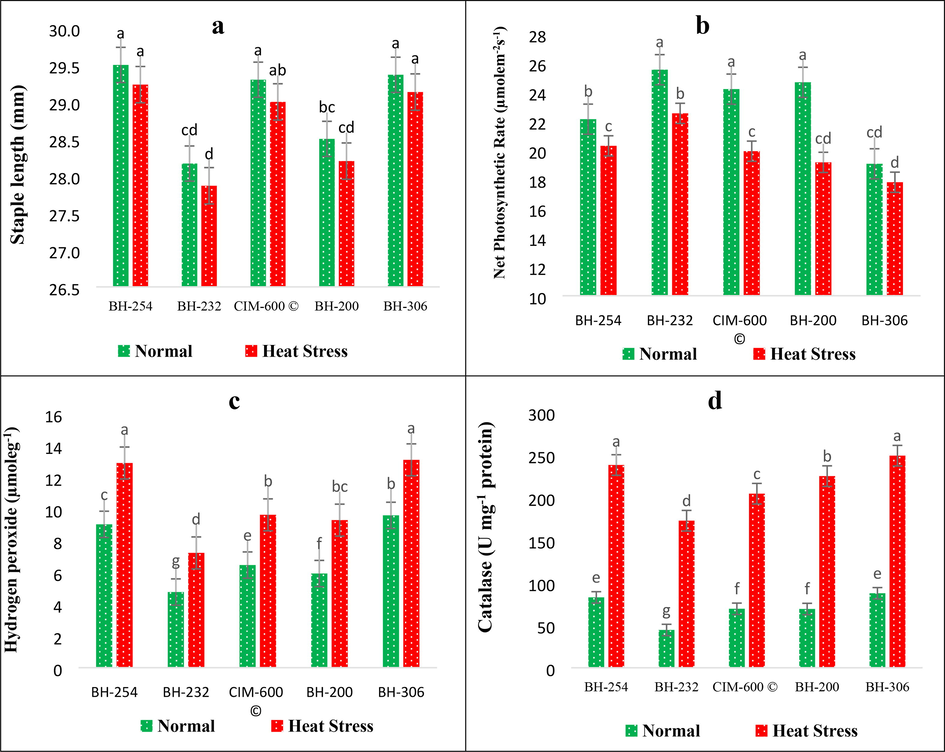
Impact of Heat stress on (a) Staple length and (b) Net Photosynthetic rate (Pn) (c) Hydrogen peroxide (H2O2) and (d) Catalase (CAT) activity in cotton genotype.
4.5 Net photosynthetic rate (Pn)
Photosynthesis is the key physiological process in cotton which is responsible for optimum growth and development for higher productivity. The ANOVA revealed the presence of significant diversity for net photosynthetic rate (Pn) in cotton genotypes under normal and stress conditions (Table 1). The results further showed a significant decline in net photosynthetic activity (Pn) under heat stress compared to control in all-cotton genotypes (Fig. 2b). The highest reduction in Pn under heat stress was observed in BH-200 (28.6 %) from 24.7 µmolem-2s−1 in control to 19.2 µmolem-2s−1 in stress conditions (Fig. 2b). However, the minimum reduction in Pn (7.0 %) was reported in BH-306 under heat stress, even though the overall net photosynthetic rate of BH-306 under control and heat stress treatment was the lowest among the cotton genotypes under study (Fig. 2b). The correlation coefficient analysis unveiled a strong positive correlation of net photosynthetic rate (Pn) with seed cotton yield (SCY) under control (r = 0.838**) as well as heat stress treatment (r = 0.829**), respectively (Table 2).
4.6 Accumulation of reactive oxygen species (ROX)
4.6.1 Hydrogen peroxide (H2O2)
Results from combined ANOVA revealed a high degree of variation in cotton genotypes for the accumulation of hydrogen peroxide (H2O2) under heat stress conditions (Table 1). The further results revealed that the accumulation of hydrogen peroxide (H2O2) was quite significant under heat stress conditions compared to control in all-cotton genotypes, indicating the higher oxidative stress experienced by the plants under stress. Under heat stress, the maximum accumulation of H2O2 was recorded in BH-306 (13.1 µmoleg−1) in comparison to control (9.6 µmoleg−1) while the lowest H2O2 accumulation was observed in BH-232 under heat stress (7.2 µmoleg−1) in contrast to the control treatment (4.8 µmoleg−1) (Fig. 2c). Moreover, the hydrogen peroxide (H2O2) was found to have a very strong negative correlation with seed cotton yield under control (r = −0.972**) as well as heat stress conditions (r = −0.955**) (Table 2).
4.6.2 Accumulation of enzymatic antioxidants
The combined analysis of variance unveiled the existence of significant diversity for CAT, POXs and SOD under stress treatments (Table 1). However, the variations for these antioxidants across years were found non-significant and their interactions too. Further results revealed that heat stress triggered the accumulation of several antioxidants including CAT, POXs and SOD in cotton genotypes (Fig. 2d, Fig. 3a & b). The maximum increase in CAT accumulation was observed in BH-254, where CAT accumulated 189 % more under heat stress (238.0 U mg−1 protein) than control (82.6 U mg−1 protein). However, the highest CAT accumulation under heat stress was recorded in BH-306 (248.9 U mg−1 protein). A strong, negative but highly significant correlation was observed between CAT accumulation and seed cotton yield under both conditions (rn = −0.937**, rh = −0.904**) in studied cotton genotypes (Table 2). The highest accumulation of POXs was recorded in BH-232 (115.9 U mg−1 protein) while the lowest POXs accumulation was observed in BH-306 (83.6 U mg−1 protein), respectively. However, the percentage increase in POXs accumulation was found highest in BH-306, where POXs accumulated 168 % higher under heat stress conditions compared to control (Fig. 3a). The correlation coefficient analysis disclosed a strong positive and highly significant correlation between POXs accumulation and seed cotton yield under control (r = −0.920**) heat stress conditions (r = −0.974**), respectively (Table 2). Similarly, the highest SOD activity was observed in cotton genotype BH-232 (109.8 U mg−1 protein) while the lowest activity was recorded in BH-306 (84.8 U mg−1 protein) under heat stress conditions. The maximum percentage increase in T-SOD activity was recorded in BH-306 (97.8 %) from 42.9 U mg−1 protein in control to 84.8 U mg−1 protein in heat stress conditions, respectively.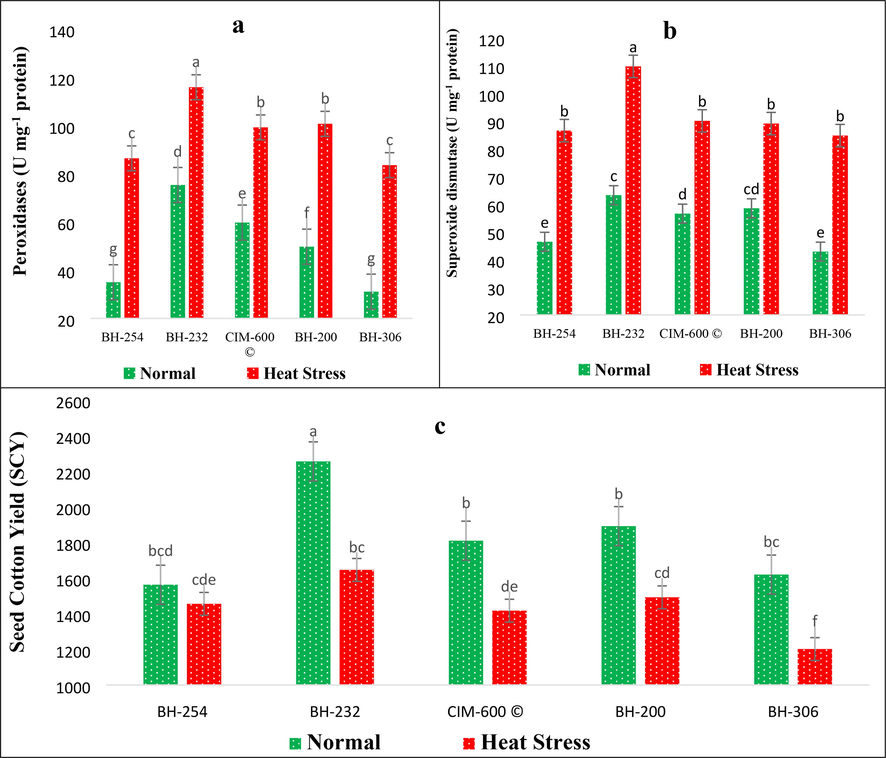
Impact of Heat stress on (a) Peroxidases (POXs) and (b) Total superoxide dismutase (T-SOD) activity (c) seed cotton yield in cotton genotypes.
4.6.3 Seed cotton yield (SCY)
Seed cotton yield is the most important trait and the final product ready for usage in different industries. In the current study, significant differences were observed among cotton genotypes, stress treatments, and their interactions with seed cotton yield (Table 1). The mean data of five cotton genotypes revealed the drastic impact of heat stress on seed cotton yield (Fig. 3c). Results revealed that heat stress reduced SCY from 15.1 % in BH-254 to 27.0 % in BH-232. Maximum SCY under heat stress was recorded in BH-232 (1646.7 kg ha−1) followed by BH-200 (1492.7 kg ha−1) while the lowest seed cotton yield was recorded in BH-306 (1202 kg ha−1) (Fig. 3c). The correlation coefficient analysis unveiled the presence of highly significant and positive correlation of seed cotton yield with N/P (r = 0.432*), Pn (r = 0.829**), POXs (r = 0.974**) and T-SOD (r = 0.868**) under heat stress conditions. However, under heat stress conditions, SCY had a strong negative correlation with GOT (r = −0.466**), SL (r = −0.898**), H2O2 (r = −0.955**) and CAT (r = −0.904**) (Table 2).
5 Discussion
Heat stress is one of the major constrain in improving cotton yield and fibre quality as it affects several yields and quality-associated physio-chemical and metabolic processes (Xu et al., 2020). The development of new cotton genotypes and improving the existing ones with high-temperature tolerance along with higher cotton yield is the primary focus of plant breeders to combat climate change. To achieve this target, screening and evaluation of existing germplasm for seed cotton yield and its associated physio-chemical, agronomical and fibre-related traits under stress conditions is pivotal to assess the magnitude of genetic diversity for these traits (Yousaf et al., 2022). The results obtained through combined ANOVA showed the existence of sufficient genotypic variations among cotton genotypes for studied traits, which indicates the extent of genetic variability present in the studied genotypes and could be used to develop heat stress-tolerant cotton genotypes as suggested by Riaz et al., 2021. However, the variations in the studied traits were non-significant for years as a source of variation, indicating the minute impact of different growing seasons, suggesting the pooling of data as recommended and used by Saroj et al., (2021).
Results revealed that the performance of seed cotton yield-associated traits was greatly reduced under heat stress (Fig. 4). Plant height, nodes per plant and sympodial branches per plant were significantly reduced under stress. This might be due to a decrease in the internodal distance, and photosynthate availability because of the reduction in chlorophyll contents and net photosynthesis (Abro et al., 2021). Cotton genotype CIM-600 was the best performer due to higher plant height, nodes per plant, and sympodial branches per plant. Several other studies also categorize CIM-600 as a heat-tolerant cotton variety (Majeed et al., 2021). Fibre quantity and quality are also greatly affected by the increase in temperature. In the current study, ginning out turn (GOT) and staple length were significantly reduced under stress conditions.
Impact of Heat Stress on Cotton Genotypes under Current study.
The reduction in GOT and staple length is associated with a decrease in cell division and photosynthetic ability (Abro et al., 2021). Correlation analysis also showed a negative but strong correlation of seed cotton yield with GOT and staple length under control as well as heat stress conditions as shown by Bhatti et al. (2020), Abro et al. (2021), Aslam et al. (2022).
Photosynthesis is one of the key physiological processes which is responsible for yield potential and sustainability. However, heat stress adversely affects plant growth and development by halting and ceasing plant photosynthesis (Hassanuzzaman et al., 2013). Results revealed a differential response of net photosynthesis under heat stress conditions (Fig. 3). It was observed that heat stress significantly reduced the net photosynthetic rate in cotton genotypes. The reduction or sometimes inhibition of net photosynthetic rate under stress conditions might be attributed to the reduction in chlorophyll contents, increase in ionic conductance of thylakoid membranes, and disruption in RuBisCo activity (Crafts-Brandner and Law, 2000; Karademir et al., 2018).
Heat stress in cotton is accompanied by oxidative stress, which disrupts the composition and concentration of biochemical and metabolic compounds. One of the major impacts of oxidative stress in cotton is the increased accumulation of reactive oxygen species (ROXs), especially hydrogen peroxide, which increases the peroxidation of lipid bilayers and the degradation of cellular membranes. Therefore, a lower concentration of H2O2 in the cotton plants could serve as an indicator of heat stress tolerance (Majeed et al., 2019). In the current study, the concentration of H2O2 was increased in all-cotton genotypes under heat stress conditions. However, the highest accumulation of H2O2 was observed in BH-306, which appeared to be the least productive and heat susceptible cotton genotype. This was evident that a higher concentration of ROS species like H2O2 had a strong negative correlation with seed cotton yield under heat stress as shown by correlation analysis. Similar findings were reported by Zhang et al. (2014), Majeed et al. (2019), who reported a highly negative correlation between seed cotton yield and hydrogen peroxide concentration under stress conditions.
To check, minimize or detoxify the adverse effects of reactive oxygen species, several enzymatic and non-enzymatic antioxidants are produced in cotton plants under heat stress, which act as detoxifying or scavenging agents (Gür et al., 2010; Sekmen et al., 2014). The highest accumulation of CAT was observed in BH-306 while the lowest CAT activity was recorded in BH-232. However, the accumulation of POXs and T-SOD was found maximum in BH-232, respectively under heat stress conditions, and these were the main reasons for the lowest accumulation of H2O2 and highest seed cotton yield in BH-232 under stress conditions. The same results were also shown by many other researchers who revealed that an increase in antioxidant activity decreases the accumulation of ROXs in stress-tolerant genotypes, which has a very significant and positive impact on economical yield (Gür et al., 2010; Sekmen et al., 2014). However, the accumulation of SOD decreases at the temperature of 45 °C while the concentration of CAT, POXs, and APX kept on increasing with the increase in temperature (Sarwar et al., 2018).
6 Conclusion
In this study, heat stress was found to have a very negative impact on plant growth and development by significantly reducing plant height (PH), nodes per plant (N/P), sympodial branches per plant (SB/P), number of bolls per plant (NB/P), ginning out-turn (GOT), net photosynthetic rate (Pn), staple length (S.L) and seed cotton yield (SCY). However, the concentration of hydrogen peroxide (H2O2) was increased under heat stress as an indication of oxidative stress. To combat the negative impact of heat stress and higher concentration of H2O2, cotton genotypes increased the accumulation of certain enzymatic antioxidants i.e., CAT, POXs, and T-SOD. Based on the results, it is therefore recommended to cultivate the heat-tolerant cotton genotype BH-232 in heat-prone areas of the country.
Funding
The research was supported by the Cotton Research Station, Bahawalpur under Agriculture Grant #18 by the Govt of Punjab.
Acknowledgment
The authors extend their appreciation to the Researchers supporting project number (RSP-2021/173) King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Identification of Heat Tolerant Cotton Lines Showing Genetic Variation in Cell Membrane Thermostability, Stomata, and Trichome Size and Its Effect on Yield and Fiber Quality Traits. Front. Plant Sci.. 2021;12
- [CrossRef] [Google Scholar]
- Impact of Heat Stress on Agro-Morphometric and Fiber related Traits In Indigenous Upland Cotton Genotypes Under Semi-Arid Conditions. Biol. Clin. Sci. Res. J.. 2022;2022(1)
- [Google Scholar]
- Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem.. 1971;44:276-287.
- [CrossRef] [Google Scholar]
- Genetic variation and association among upland cotton genotypes under semi-arid conditions. Int. J. Biol. Biotech.. 2020;17:693-699.
- [Google Scholar]
- In vitro analysis of cotton pollen germination. Agron. J.. 2004;96:359-368.
- [CrossRef] [Google Scholar]
- Effect of heat stress on the inhibition and recovery of the ribulose-1, 5-bisphosphate carboxylase/oxygenase activation state. Planta. 2000;212(1):67-74.
- [CrossRef] [Google Scholar]
- ESP, 2021-22. Economic Survey of Pakistan 2021-22. Ministry of Finance, Govt. of Pakistan. Islamabad
- Diurnal gradual heat stress affects antioxidant enzymes, proline accumulation and some physiological components in cotton (Gossypium hirsutum L.) Afr. J. Biotechnol.. 2010;9:1008-1015.
- [Google Scholar]
- Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci.. 2013;14:9643-9684.
- [CrossRef] [Google Scholar]
- Correlations between canopy temperature, chlorophyll content and yield in heat-tolerant cotton (Gossypium hirsutum L.) genotypes. Fresen. Environ. Bull.. 2018;27:5230-5237.
- [Google Scholar]
- Uptake and accumulation and oxidative stress in garlic (Allium sativum L.) under lead phytotoxicity. Ecotoxicology. 2009;18:134-143.
- [CrossRef] [Google Scholar]
- Quantifying temperature effects on cotton reproductive efficiency and fibre quality. Agron. J.. 2014;106:1275-1282.
- [Google Scholar]
- Antioxidant and physiological responses of upland cotton accessions grown under high-temperature regimes. Iran. J. Sci. Technol. Trans. A. Sci.. 2019;43:2759-2768.
- [Google Scholar]
- Heat stress in cotton: a review on predicted and unpredicted growth-yield anomalies and mitigating breeding strategies. Agronomy. 2021;11:1825.
- [CrossRef] [Google Scholar]
- Effects of heat stress on growth, physiology of plants, yield and grain quality of different spring wheat (Triticum aestivum L.) genotypes. Sustainability. 2021;13:2972.
- [CrossRef] [Google Scholar]
- Production of tocotrienols in seeds of cotton (Gossypium hirsutum L.) enhances oxidative stability and offers nutraceutical potential. Plant Biotechnol. J.. 2021;19:1268-1282.
- [Google Scholar]
- Salman, M., Majeed, S., Rana, I.A., Atif, R.M., Azhar, M.T., 2019. Novel breeding and biotechnological approaches to mitigate the effects of heat stress on cotton. In Recent approaches in omics for plant resilience to climate change Springer. 251–277. doi: 10.1007/978-3-030-21687-0_11
- Unraveling the relationship between seed yield and yield-related traits in a diversity panel of Brassica juncea using multi-traits mixed model. Front. Plant Sci.. 2021;12:651936
- [CrossRef] [Google Scholar]
- Exogenously applied growth regulators protect the cotton crop from heat-induced injury by modulating plant defense mechanism. Sci. Rep.. 2018;8:1-15.
- [CrossRef] [Google Scholar]
- Role of mineral nutrition in alleviation of heat stress in cotton plants grown in glasshouse and field conditions. Sci. Rep.. 2019;9:1-17.
- [CrossRef] [Google Scholar]
- Reactive oxygen species scavenging capacities of cotton (Gossypium hirsutum L.) cultivars under combined drought and heat induced oxidative stress. Environ. Exp. Bot.. 2014;99:141-149.
- [CrossRef] [Google Scholar]
- Influence of high temperature and breeding for heat tolerance in cotton: a review. Adv. Agron.. 2007;93:313-385.
- [CrossRef] [Google Scholar]
- Principles and procedures of statistics: A biometrical approach. Inc, New York NY: McGraw-Hill Co.; 1997.
- USDA, 2022. Cotton: World Markets and Trade. Foreign Agricultural Service, Global Market Analysis, United States Department of Agriculture, USA
- Oxidative stress and some antioxidant systems in acid rain-treated bean plants: protective role of exogenous polyamines. Plant Sci.. 2000;151:59-66.
- [CrossRef] [Google Scholar]
- The difference in the formation of thermotolerance of two cotton cultivars with different heat tolerance. Arch. Agron. Soil Sci.. 2020;66:58-69.
- [Google Scholar]
- Concurrent Effects of Drought and Heat Stresses on Physio-Chemical Attributes, Antioxidant Status and Kernel Quality Traits in Maize (Zea mays L.) Hybrids. Front. Plant Sci.. 2022;13:898823
- [CrossRef] [Google Scholar]
- Unraveling Heat Tolerance in Upland Cotton (Gossypium hirsutum L.) Using Univariate and Multivariate Analysis. Front. Plant Sci.. 2021;12:727835.
- [CrossRef] [Google Scholar]
- Morphological and physiological responses of cotton (Gossypium hirsutum L.) plants to salinity. PLoS ONE. 2014;9:112807
- [CrossRef] [Google Scholar]







