Translate this page into:
Impact of different pollen protein diets on the physiology of Apis mellifera L. (Hymenoptera: Apidae) workers from essential plant sources
⁎Corresponding authors at: Guangdong Key Laboratory of Animal Conservation and Resource Utilization, Guangdong Public Laboratory of Wild Animal Conservation and Utilization, Institute of Zoology, Guangdong Academy of Sciences, Guangzhou 510260, China. atif_entomologist@yahoo.com (Atif Idrees), junl@giabr.gd.cn (Jun Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Background
The primary food sources for honey bees in colonies are processed nectar and fermented pollen. However, there are relatively few blooming plants that provide full sustenance to the bees, and their impacts on the physiology of honey bees in Pakistan have not yet been assessed. Diet with meager nutritional contents can affect various physiological parameters like hypopharyngeal glands (HPGs) sizes, rectal weight contents (RWCs), food intake and longevity of worker bees which indirectly affects overall colony development and make it vulnerable to diseases and pests.
Methods
This study was designed to investigate the impact of protein diets in the form of different pollen on some physiological parameters of honey bee (Apis mellifera L.) from major plant sources serving as melliferous resources in the agro-climatic conditions of Dera Ismail Khan, Khyber Pakhtunkhwa, Pakistan.
Results
Results indicated Brassica napus L. as the most effective pollen diet which took the highest time for 50 % of individuals’ mortality. B. napus L. also gave the highest increase of HPG size (278.47 µm) followed by Trifolium alaxandrinum L. Acacia modesta L., and Zea mays L., in comparison to sugar syrup (control) with 261.73, 237.49, 124.38 and 107.65 µm, respectively. Pollen intake followed a similar pattern, with B. napus having the highest levels (3.03 mg/bee/day), and bee bread (3.37 mg/bee/day), with an overall mean of 25.279. The overall mean for RWC demonstrates how easily the pollen diet may be digested. It was negatively correlated with the volume of rectal contents. When compared to pollen diets, honeybee workers given bee bread had the lowest overall mean (3.95 mg) for rectal contents, suggesting the greatest degree of digestibility.
Conclusion
Diets from B. napus were taken and digested more quickly than those from A. modesta, Z. mays, and T. alaxandrinum.
Keywords
Apis mellifera
Food consumption
Hypopharyngeal Glands
Mortality
Physiology
Rectal weight contents
1 Introduction
The primary food sources for honey bees in colonies are processed nectar and fermented pollen. For bees, nectar is a source of energy, while pollen is a source of protein, lipids, minerals, and vitamins (Yang et al., 2013). Honey bees get all of these essential nutrients from angiosperm plants. However, there are relatively few blooming plants that can provide bees with all of their nutritional requirements. A diet that is deficient in nutritional content may reduce an individual's capacity to fight against and increase their susceptibility to diseases (Alaux et al., 2010). The ability of the nursing bees to convert the collected pollen into royal jelly is totally responsible for the fitness of a colony. The nursing bees eat fermented pollen known as “bee bread,” which is kept in comb cells. The kind of food, colony size, brood raising, season, and accessibility of other food sources both within the colony and beyond it are just a few of the variables that influence how quickly this fermented pollen is consumed (Brodschneider and Crailsheim, 2010). Individual workers' pollen consumption varies with their age and the tasks they do for the colony. Honey bee larvae don't eat much pollen whereas adults consume large quantities of pollen (Babendreier et al., 2004). Older workers, often known as foragers, are unable to consume or digest pollen.
One important aspect to take into account while raising bees is the lifespan of the worker bees. Productivity in the colony and an increase in the effectiveness of crop pollination are dependent on the information on what factors govern worker bees’ longevity. The effects of nutrition on the lifespan of caged bees have been investigated by several researchers and reported that pollen from bees prolongs the life of caged bees compared to hand-collected pollen (Paoli et al., 2014). Pollen of poor quality, inadequate quantity, or nonexistent causes baby bees to develop slowly and weigh poorly (Smart et al., 2016), suppress reproduction, brood-rearing and shortens workers’ life (Zheng et al., 2014). Colony production is influenced in the long term by these factors (Human et al., 2007). Longevity and the consumption of the right quantity of proteins or amino acids are connected and Higher pollen quality promoted the growth development and adult lifespan (de Groot, 1952). A few studies demonstrate how pollen from various plant species, with varying quantities of protein and fat increase lifespan of honey bee.
Nickel (Carreck, 2016) initially characterized the hypopharyngeal glands. In addition to these names, they are often referred to as worker glands, food glands, larval food glands, or brood food glands (Al-Ghamdi et al., 2011). Age affects the size and production of the hypopharyngeal glands (HPG) (Suwannapong et al., 2010). Local, seasonal, and environmental factors also affect the size and production of HPG (Ali et al., 2019). There are inter and intraspecific variations in morphology and physiology of hypopharyngeal glands of eusocial insects (Amaral and Caetano, 2005). The creation of HPGs is a quality that is unique to nurse bees and deteriorates as the bees' work responsibilities change from nursing to foraging (Britto et al., 2004). This takes place around 18 days after the first employees have emerged. The milky secretion of HPGs known as royal jelly (RJ) is one of the most significant products that may be obtained through beekeeping. The growing larvae and adult queens depend on a diet consisting mostly of RJ since it is a complex blend of a wide variety of nutritious components. Due to their unique ability to produce RJ, several morphological and physiological research on these honey bee worker glands have been carried out (Gatehouse et al., 2004). The hypopharyngeal gland is absent in the queen or in male bees. The size of the acini is a reliable metric for determining the quality of the foods that nursing bees are provided with (Amro et al., 2015). In addition, the size of the nursing bees' HPG is employed as a criterion to evaluate their physiological states (DeGrandi-Hoffman et al., 2008). The grade of HPGs varies depending on the kind of food consumed (Al-Ghamdi et al., 2011). When the bees consumed varied pollen loads and different protein-containing substances, their HPGs were of different sizes (Al Mărghitaş et al., 2010). A linear rise in the amount of protein consumed suggests that protein has a significant role in how well honey bees operate physiologically (Roulston and Cane, 2000).
The RWCs are regarded as a crucial sign for figuring out if a diet is suitable for honey bees. It's used to determine how well honey bee colonies respond to various types of honey bee diets. Honey bee workers' natural diet of pollen is transported from the mouth to the stomach, where it is digested and absorbed. The bee's waste and excess water are stored in the hindgut until the bee leaves the colony, at which point the feces are expelled (Nicolson, 2009). These contents of the rectal cavity are distinct and are influenced by factors such as age, season, functional status, and the kind of nutrients consumed. When a honey bee is maturing, the rectum is not emptied until after search on the field has begun, which is three weeks after the bee has emerged from its cocoon. The majority of the fecal waste is made up of pollen coatings, pollen fat globules, and broken-up epithelial cells from the ventriculus. In the rectum, there is not going to be any more modification to this situation.
There is a dearth of studies in Pakistan that examine the effects of protein meals (pollen) from various plant sources on the physiology of honey bees. This study investigated the impacts of diet from various pollen sources on lifespan, HPGs development, and Rectal Weight of caged honey bees. The discovery will be used as a starting point for developing and using that pollen in alternative meals for the dearth period, which has been extended owing to climate change in Pakistan. Honey output, summer pollination efficiency, and winter colony strength may all be boosted by feeding the bees in an apiary the beneficial components that were isolated from their nectar and pollen.
2 Materials and Methods
2.1 Pollen loads trapping
Pollen trapping is a simple method to get pollen samples gathered by honey bee foragers (Dimou and Thrasyvoulou, 2006). Pollen traps have been used worldwide as a method to assess the pollen flora of any area (Andrada and Tellería, 2007). Twelve typical Afghani hives with rather uniform colonies of Apis mellifera L. were chosen for the current investigation, and they were placed in four different sites to catch pollen loads. Standard pollen traps obtained from the National Agriculture Research Center in Islamabad, with an extraction effectiveness of 50 % were used in the study. Every-two weeks, pollen-loads from coming foragers were captured for two days, providing the colonies with 13 days of inbound pollen. The pollen loads were collected from mid-January to the end of December. The weighted and colour sorted pollen loads were divided into groups, placed in polyethylene bags, and stored at −20 °C in a freezer.
2.2 Harvesting of bee bread
The bee bread was harvested in the active season by cutting the pieces of comb full of bee bread. These pieces were than stored in plastic boxes at 20 degreesC. For feeding bees the pieces were taken and placed for four hours in room temperature before feeding.
2.3 Determination of total protein and moisture content
For the purpose of analyzing pollen for protein content, the Kjeldahl technique was used (Table 1). Approximately-four hours were spent heating one gram of pollen and 20 ml of sulfuric acid (95–97 %) in the presence of a catalyst. A 90-ml solution of 30 % NaOH was used for distillation. For 2 min, the H3BO3 solution (4 %) volume was kept at 30 ml. The nitrogen content was then calculated after measuring the amount of HCl solution (0,1 M) consumed throughout the titration. The 5.60 factor was used for estimation of crude protein content and expressed as % dry matter (Rabie et al., 1983) was used for estimation of crude protein content and expressed as % dry matter. In addition to this, it is mandatory to know how much dry matter is still left and be able to quantify the protein value as a % dry matter. The level of moisture in the samples was used as a proxy for the dry matter content. The value of this parameter was determined by placing one gram of pollen in an evaporating dish made of porcelain and heating it in an oven set at 103 °C for two hours. It was determined that the moisture content of the sample was equal to the difference in weight between the samples after they had been dehydrated and before they were placed in the drying oven.
Diets
Components
Diet 1
Berseem pollen + Sugar power (1:1)
Diet 2
Maize pollen + Sugar power (1:1)
Diet 3
Pulai pollen + Sugar power (1:1)
Diet 4
Canola pollen + Sugar power (1:1)
Diet 5
50 % sucrose solution
Control
Bee bread harvested from colonies
2.4 Formulation of pollen diets
The impacts of the primary flora of Dera Ismail Khan, which is located in Khyber Pakhtunkhwa, Pakistan, were investigated and measured for their effects on lifespan. Pollen sources, i.e., Trifolium alaxandrinum L. (Berseem), Zea mays L. (maize), Acacia modesta L. (Pulai), and Brassica napus L. (Canola), were employed. Each diet was designed using a single sample drawn from a larger pool (Table 1). An initial mix of 6 gm divided into two portions measuring 3gm/ portion was provided to the caged bees. The diet container was placed on the floor of the cage. To create a wet, kneadable consistency, pollen, sugar, and lukewarm water (2:1:1, weight) were combined and soaked for 12 h. The creation of corbicular loads typically involves 30 % nectar mixing by the bees. We used the same methodology and added 30 % sugar by weight before combining with water to get a sucrose level that was almost identical to that which was typically provided by bees for the manufacture of pollen pellets.
2.5 Food consumption of pollen diets
Bees were selected from strong colonies at the apiary at Gomal University DIK's Faculty of Agriculture and hatched in a warm environment, gently brushed into a cage for about a day before being used in the experiment. After a day, the newly hatched bees were randomly placed in cages (with 60 bees per cage) at a temperature of 35 °C and 50 % relative humidity. Wood was used to construct the confinement cages (70 mm 90 mm 100 mm), and the Plexiglas door was designed to glide open and down to reveal feeding chambers where 5 ml plastic medical syringes could be hung for feeding. A pollen diet's intake was evaluated by weighing the residual food and subtracting it from the amount supplied, then adding the water evaporated from a comparable diet in a bee-free cage in the environmental chamber. Both the controls and the experimental groups were given sugar solution, thus that was the only noticeable difference. Each experiment was repeated three times with bees from three different colonies. In this case, pollen feeding continued for another 21 days until it stopped completely.
2.6 Pollen diets’ impact on longevity of Apis mellifera L.
The sucrose solution and water was diluted to a 1:1 (w/v) ratio and placed into the vials. A little piece of wax comb was hung from the ceiling of the hives' confinement chambers to familiarize the bees with their surroundings and encourage them to remain still (Williams et al., 2013). Caged honey bees live longer if they have access to a comb (Rinderer and Elliott, 1977), which also reduces stress and restless appetitive behavior (Craig, 1917). Each day, fresh water and a 50 % sucrose solution were provided to the bees.
By removing and counting the dead bees from each cage, the longevity of the workers from the trial's beginning was estimated for each treatment group. Three-day intervals were used for this examination until half of the test insects' original population had died (LT50).
2.7 Acini size measurement for determination of hypopharyngeal glands development
Ten honey bee workers were chosen at random between the ages of 3 and its multiples up to 18 days old to study the variance in morphometry of the feeding glands. Upon capturing bees in Epondwarf tubes, we chilled them for 10 min in the fridge. In the Plant Breeding & Genetics Lab at the DIK Faculty of Agriculture, the heads of carefully chosen worker bees were slashed off with a razor blade. Fine forceps were used to split the HPGs into their appropriate age groups. Overnight at 4 °C, the glands were preserved in 4 % formaldehyde in phosphate-buffered saline (PBS, pH 7.5). HPGs were washed three times in PBS to remove any debris. These were cleaned, then left in an incubator with 30 % glycerol in PBS at 40 °C for a full 24 h. The glands were re-incubated for 2 h at 40 °C with 50 % glycerol in PBS before to mounting. Slides were prepared from samples using a mounting medium of 50 % glycerol in PBS. Translucent nail polish was used to seal the transparency's edges so they wouldn't dry off. Under a binocular light microscope equipped with a 10x objective, we captured these pictures of acini from mounted gland specimens (M165C, Leica, Germany). Images captured with the high-resolution Vivo-S1 camera were analyzed using the image J software. Ten acini were randomly picked from glands in different places, and their pictures were analyzed using the Image J program. Following the method shown in, morphological measurements were extracted from these photographs. We measured the bees at 3, 6, 9, 12, 15, and 18 days of age. The selected acini/slide/bee had its length (L) and width (W) measured (W). Each group had 270 acini measured. The following formula was examined in order to calculate the acinar surface area (Maurizio and Hodges, 1951).
Where a is the full length and b is the full width of the acini and π has a constant value of 3.14.
2.8 Measurement of rectal weight contents (RWCs) of the workers
The same bees that were employed to gauge Hypopharyngeal gland maturation also weighed in on the rectal contents. Each bee had its whole digestive system removed with delicate forceps before its head was dissected. The rectum was carefully removed with a blade from the stomach and placed on a weighted coverslip. Once again, the coverslip was weighed, and the rectum's mass was determined by the following formula.
In this scenario, WC1 represents the beginning weight of coverslips without a rectum and WC2 represents the ultimate weight of coverslips with a rectum. In order to get the means, analysis of variance was utilized. The DMR test was used to compare means (Duncan, 1955).
2.9 Statistical analysis
Diet and age of bees were included as independent factors into a general linear model with food intake, longevity, HPG sizes, and RWCs as the dependent variables. The post hoc method for mean comparisons was Tukey’s HSD. Two-way analysis of variance was used to determine statistically significant mean differences.
3 Results
3.1 Nutritional analyses for crude protein and moisture content
The nutritional assessments of the four species under investigation's mono-floral pollens are shown in Table 2 along with their moisture and crude protein contents. Maize had the lowest protein levels and canola had the highest protein levels. All of polen kinds that were investigated had protein values > 4 %, ranging from 4.06 % to 25.1 %. Similar results were reported for moisture contents, for maize showing the highest values (72.23 %) and canola showing the lowest (8.28 %). Berseem and phulai had protein contents of 23.45 % and 4.14 %, respectively. With readings of 19.89 % and 19.94 %, phulai and berseem practically had the same moisture content. Means followed by the same letter do not differ significantly at the 5% level of probability.
Species
Common Names
Family
Crude
Protein (%)
Moisture
(%)
Trifolium alaxandrinum L.
Berseem/Egyptian clover
Fabaceae
(Pea family)23.45 ± 0.07B
19.94 ± 0.22B
Zea mays L.
Makai/Maize
Poaceae
4.06 ± 0.09D
72.23 ± 0.21A
Acacia modesta L.
Pulai
Fabaceae
4.14 ± 0.08C
19.89 ± 0.23B
Brassica napus L.
Canola
Brassicaceae
25.1 ± 0.08A
8.28 ± 0.19C
3.2 Food consumption
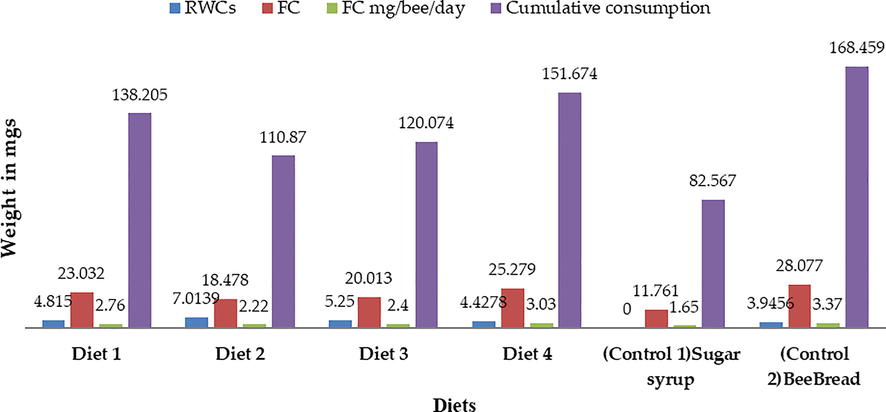
Table 3 and Fig. 1 show that there are statistically significant differences between the treatments at the P < 0.05 level. Sixty bees were monitored every-three days, and their food intake was recorded in milligrams per day. Experiments were conducted using freshly emerging bees given a variety of mono-floral pollen regimes as a comparison to two controls: Sucrose syrup, which is typically supplied during the dearth period, and bee bread, which was collected from hives during the active season. According to the average pollen intake, confined bees fed bee bread consumed 1.46 gm of pollen in the first three days after emergence, and this number grew to 1.81–2.01 gm/50 bees in the next three-day interval. After that, between 16 and 18 days, the quantity dropped to a minimum of 0.50gm/60 bees. The amount of each experimental diet was consumed in the same way by the nursing bees. Over the course of the 9 days, the nursing bees gradually ate less and less of their food. It continued till the experiment was completed. For all experimental diets, the shortest period of time between the onset of starvation and the first occurrence of food consumption was 16–18 days. Means followed by the same letter do not differ significantly at the 5% level of probability.
Diets
Mean of Food Consumption
Cumulative consumption mg/bee/18 days
Pollen consumption mg/bee/day
General Mean
1–3
Days
4–6
Days
7–9
Days
10–12 Days
13–15 Days
16–18
Days
Diet 1
15.37 M
22.97 HI
29.52 DE
24.34 GH
23.10 GH
22.00 I
138.21C
2.76C
23.03C
Diet 2
8.59P
18.27 L
24.84 JK
20.31 GH
23.84 FG
15.02 MN
110.87 E
2.22 E
18.48 E
Diet 3
8.65 OP
18.91 JKL
24.12 I
22.17 GH
25.97F
20.26 JK
120.07 D
2.40 D
20.01 D
Diet 4
20.52 J
28.71 E
33.43 D
30.30C
20.30 L
18.42 JK
151.67B
3.03B
25.28B
Diet 5
4.07 Q
8.23P
22.17H
18.75 N
15.37 M
13.98 KL
82.57F
1.65F
11.76F
Diet 6
29.16 DE
36.25B
40.27 A
32.72C
20.04 IJK
10.01O
168.46 A
3.37 A
28.08 A
General Mean
14.39 E
22.23B
25.31 A
25.41 A
21.44C
17.72 D
Effects of different pollen diets on FC & RWCs of Apis mellifera L.
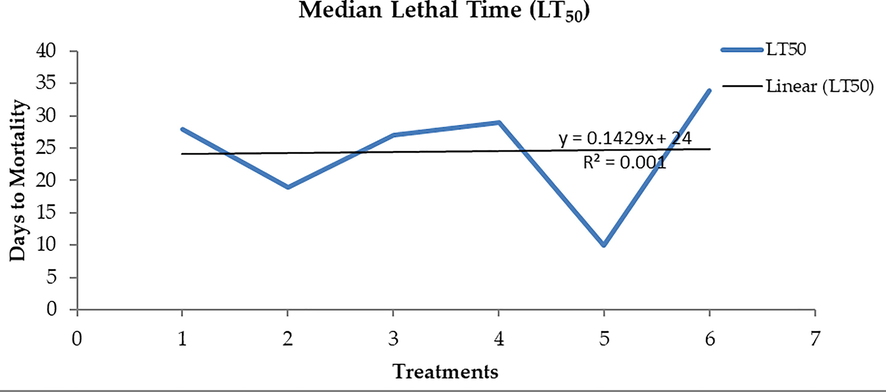
With a mean of 25.279 mg, this study found that worker bees consumed 3.03 mg of pollen per bee per day from canola which is comparable to the 3.37 mg of pollen per bee per day found in Bee bread. Berseem's pollen diet came in second with a consumption of 2.76 mg per bee per day, followed by phulai with a consumption of 2.40 mg per bee per day. The confined bees were found to ingest the least amount of maize pollen diet. In contrast to pollen diets, control 1′s sugar syrup content (1.65 mg/bee/day) was not as high. The current study calculated the average longevity of confined workers that consumed various mono-floral pollen diets. Four different pollen loads were fed to honey bee workers as protein-rich meals when they were a day old (Table 1). Every subject was housed in a little cage, and freshly emerging workers were given experimental meals with sucrose solution (1:1). Both the overall death rate and the bees' life cycle in each experiment were recorded. LT50 is the expected number of days needed for 50 % of the bees to perish. Through the use of Probit analysis, the lethal time for 50 % mortality was determined (Table 4, Fig. 2). In contrast to the negative control sugar syrup, which required 10 days, diet 4 took the longest time for 50 % mortality, followed by diets 1, 3, and 2. The positive control, which consisted of a bees' normal diet, required the longest time to reach 50 % mortality (34 days). Means followed by the same letter do not differ significantly at the 5% level of probability.
Treatment
LT50
LT90
Trifolium alaxandrinum L.
28C
65 A
Zea mays L.
19 E
48 D
Acacia modesta L.
27 D
60C
Brassica napus L.
29B
60C
Sugar syrup
10F
27 E
Beebread
34 A
62B
Graph showing lethal time for 50% mortality of worker bees fed on different pollen diets.
3.3 Hypo-Pharyngeal glands (HPGs) sizes and rectal weight contents (RWCs)
Fig. 3 illustrate the influence of various pollen diets at various time intervals (3,6,9,12,15, and 18 days), in regard to the sizes of HPG. The three treatments produced statistically significant changes (P 0.05) when comparing the HPG diameters of worker bees. HPGs had sizes ranging from 107.65 to 332.77 µm. Natural diet bee bread had the largest size (332.77 µm), followed by diets including canola, berseem, phulai, maize, and sugar syrup, which had respective sizes of 278.47, 261.73, 237.49, 124.38, and 107.65 µm.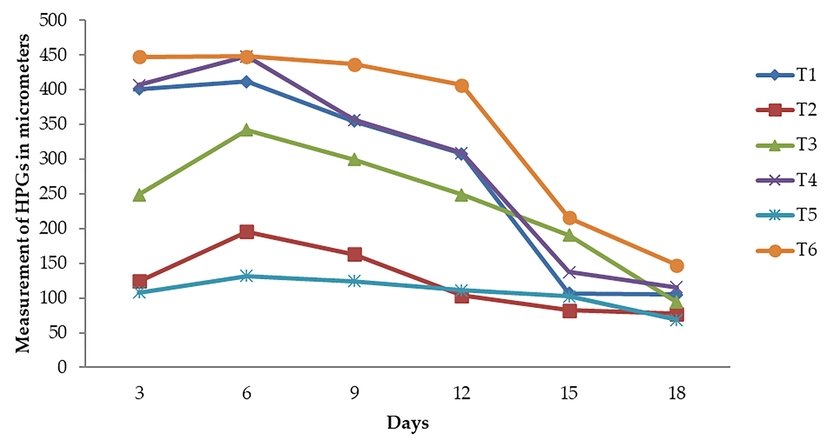
Hypopharyngeal glands development on different days.
On HPGs of worker bees, the interactional effect revealed substantial changes in size based on both the days and the treatment. The sizes varied from 68.62 to 448.47 µm. The largest size was measured on day 6 of treatment 6 (Bee bread), with the same treatment producing the largest size on day 9 (437.04 m), and treatment 4 producing the smallest size on day 6 (429.48 m). On day 18, the treatment 5 reading of 68.62 m was recorded as having the lowest value.
On various days, there was a very significant fluctuation in the HPG sizes of the worker bees. According to the research, HPGs may have any size between 101.33 and 326.59 µm. In 6 day old bees, maximum sizes (326.59 µm) were noted. After six days, HPG sizes began to decline, reaching 289.21 µm in nine days, 246.28 µm in twelve days, 139.24 µm in fifteen days, and 101.33 µm in eighteen days old workers, at which point the bees began to act as foragers. The acinal size is age-related, according to the data above. Up to the age of six days, glands grow larger; after that, they begin to contract. At six days, HPGs have a diameter that is double that of freshly emerging bees.
3.4 RWCs as indicators of protein utilization
In Table 5 and Fig. 1, the findings of the current study that demonstrate the impact of various pollen diets at various time intervals (3, 6, 9, 12, 15, and 18 days) in relation to RWCs are shown. For worker bees' rectal weight contents, various treatments showed significant changes (P < 0.05). The information in Table 5 compares the RWCs (mg/bee/3 days) of freshly emerging bees fed various mono-floral pollen diets to a natural diet of bee bread, which served as the control. The data were taken from the combs of honeybee colonies during the active season. The mean RWC value demonstrates the digestibility of the pollen diet and is inversely related to the weight of the rectal contents. Starting on day one, the weight would grow until finally, on day fifteen, feces flights would be conducted and employees would be required to empty their rectums. Honeybee workers fed bee bread had the lowest average rectal contents (3.95 mg), indicating the highest digestibility, while in pollen diets, canola diet was consumed and digested more efficiently than berseem (4.81), maize (7.01), and phulai (11.01 mg), indicating the highest level of digestibility (5.25). The mean number of days showed a peak at about day 3, with a gradual decline afterwards with age. Means having different lettering (between treatments) are statistically significant at 5% of probablity.
Diets
Mean of Rectal Weight Contents
General Mean
1–3
Days
4–6
Days
7–9
Days
10–12
Days
13–15
Days
16–18 Days
Diet 1
0.32 T
1.29 S
4.66 L
7.53 E
8.79B
6.28 I
4.82C
Diet 2
3.51 Q
6.76 G
7.36F
7.64 E
10.60 A
6.20 IJ
7.01 A
Diet 3
1.24 S
2.58 R
6.49H
8.13 D
8.62C
4.42 M
5.25B
Diet 4
1.27 S
2.62 R
3.66P
5.66 K
6.78 G
6.56H
4.43 D
Diet 5 (Control)
0.33 T
3.94O
4.30 MN
4.79 L
6.10 J
4.20 N
3.95 E
General Mean
1.33F
3.44 E
5.29 D
6.75B
8.18 A
5.53C
The contents of the rectal cavity varied in weight from 10.60 mg to 1.24 mg. Overall, maize pollen was digested the least (7.01 mg), followed by phulai (5.25 mg), berseem (4.82 mg), canola (4.43 mg), and finally, natural diet bee bread (3.95 mg), which showed the most digestion of pollen. Rectal weight contents of worker bees differed significantly (P0.05) among days. In the research it was found that the rectums' weight might be anything from 1.33 mg to 8.18 mg. Bees between the ages of 13 and 15 days were measured to be the largest (8.18). The rectums' weights began falling after day 15, and by day 16–18, they had dropped by 5.53 mg.
4 Discussion
The results of our research showed that the protein content of different kinds of pollen has an effect on the amount of pollen that bees eat. According to previous research, confined bees consumed the most protein from day 1 to day 9, the same time period in which bees exhibit brood nursing behavior (Basualdo et al., 2013). On the other hand, there was hardly any consumption till day 18. In agreement with prior research, the present study concluded that bees continue eating pollen until they are 15–18 days old (Zherebkin, 1965). When worker bees are between three and five days old, they ingest the most pollen of their lives.
Canola pollen was the most popular among worker bees in this research, with a mean of 25.279, suggesting a pollen intake of 3.03 mg/bee/day, which is similar to the consumption of 3.37 mg/bee/day among those fed on Bee bread. In terms of pollen intake, berseem placed second, with 2.76 mg per honeybee per day, while phulai ranked third, with 2.40 mg per bee per day. Caged bees were shown to eat the least when fed a diet of maize pollen. Our findings that pollen from the Fabaceae and Brassicaceae families predominates in honey bee crops are supported by those of a previous study (Crailsheim et al., 1992). According to our findings, workers eat more pollen from the Brassica and Trifolium families than they do from the Acacia and Maize families. The nutritional examination of these pollen yielded the same findings (unpublished data), showing that the protein amount was greatest in brassica and Trifolium pollen and lowest in Acacia and maize pollen. The effects of pollen nutrition on worker bees may be evaluated using the results of these simple experiments.
In terms of growth and survival, nutritional availability is crucial. Poor nutrition reduces bees' physical capacity and leaves them susceptible to a wide range of pathogens (Li et al., 2021). Lifespan of workers was obviously affected by pollen diet. The greatest single pollen, and the one that came closest to mimicking the bees' normal diet 6 (bee bread), was berseem which pushed their LT50 out by 39 days. In experiments where canola was provided, a 38-day increase in lifespan was seen, but all other mono-pollen diets resulted in just a 10-day rise in LT50 relative to a 10-day increase on a sugar diet. Previous research had similar findings (Schmidt et al., 1987). The combination of berseem pollen with maize and phulai pollen was proven to be quite advantageous. These findings agree with those of a previous research (Di Pasquale et al., 2013). Research like this supports the idea that honeybees' health may be improved by feeding them foods high in protein and other nutrients. Workers of honey bee colonies fed a variety of pollen diets showed the most statistically significant growth in HPG size from the time of their emergence to the time of their sixth day of life. As they aged, HPGs shrank until they were 18 days old. Canola pollen-fed workers showed the highest HPG development value (429.48 µm) at day 6, followed by berseem pollen-fed workers (412.02 µm). Canola and berseem-fed animals had more fully formed glands than those given phulai and maize pollen.
Our findings corroborated previous research showing that feeding bees with a high-protein diet increases the size of their HPGs as measured on day 6. This matches the age at which worker bees' HPGs reach their full maturity (Al-Ghamdi et al., 2011). Bees taken on days 12, 15, and 18 exhibited smaller acini than bees sampled on day 6, indicating the age-dependent growth of HPGs, with acini reaching the maximum size around days 6–8 to correlate to the nursing job performed in the hive (Ali et al., 2019). The workers' activity, measured by how often they fed the larvae royal jelly, was shown to be positively correlated with the peak size the larvae reached at about day 6 (Hrassnigg and Crailsheim, 1998).
We observed decline in acini size for 12-days age that continued until adulthood. The finding jibes with those of previous research (Brouwers, 1982). At day 12, the quality of the bees' food had no effect on the shrinking acini. This may be because, as previously documented, HPG acini in confined bees shrink to an abnormally small size at an unusually early age (Lass and Crailsheim, 1996) despite the pollen diet's quality, which is presumably because there isn't any brood being raised under artificial settings (DeGrandi-Hoffman et al., 2010).
Pollen's protein composition varies across plant species and geographical areas (2.5–61 %) as well (Roulston and Cane, 2000). Pollen is the honey bees' primary supply of protein, hence its quality and digestibility are crucial to their well-being. The amount of pollen in a honey bee's rectal weight reflects how well it supports the worker bee's metabolism. Those findings suggest that if a pollen diet is not digestible, the weight of the rectal contents will grow with age. This is because undigested pollen material is heavier than digested ones. High digestion of pollen meals is reflected in a light rectum. The pollen of Brassica napus L. and Trifolium alaxandrinum L., both of which were shown in our study, may be fed or mixed with replacements and supplements to improve their digestibility with respect to their rectal weight contents.
Funding
This research work was supported by the Higher Education Commission of Pakistan, Start-Up Research Grant Program (SRGP No. 2345); GDAS Special Project of Science and Technology Development (No. 2020GDASYL-20200301003); GDAS Action Capital Project to build a compre‐ hensive industrial technology innovation center (No. 2022GDASZH‐2022010106); Agricultural scientific research and technology promotion project of Guangdong Province (No. 2021KJ260); Science and Technology Planning Project of Guangdong (2018B030324003); Science and Technology Planning Project of Guangzhou (202103000009). The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/193), King Saud University, Riyadh, Saudi Arabia.
Acknowledgement
The authors extend their appreciation to the Researchers Supporting Project number (RSP-2021/193), King Saud University, Riyadh, Saudi Arabia.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Al Mărghitaş, L., Bobiş, O., Tofalvi, M., 2010. The Effect of Plant Supplements on the Development of Artificially Weaken Bee Families, Scientific Papers: Animal Science and Biotechnologies.
- Consumption rate of some proteinic diets affecting hypopharyngeal glands development in honeybee workers. Saudi J. Biol. Sci.. 2011;18:73-77.
- [CrossRef] [Google Scholar]
- Effect of season and behavioral activity on the hypopharyngeal glands of three honey bee Apis mellifera L. Races under stressful climatic conditions of central Saudi Arabia. J. Hymenopt. Res.. 2019;68:85-101.
- [CrossRef] [Google Scholar]
- The hypopharyngeal gland of leaf-cutting ants (Atta sexdens rubropilosa)(Hymenoptera: Formicidae) Sociobiology. 2005;46(3):515-524.
- [Google Scholar]
- Physiological effects of selected pollen loads types on honey bee workers (Apis mellifera l.) J. Int. Academic Research Multidisc. Impact Factor. 2015;1
- [Google Scholar]
- Andrada, A.C. and Tellería, M.C., 2007. Pollen collected by honey bees (Apis mellifera L.) from south of Caldén district (Argentina): botanical origin and protein content. Taylor & Francis 44, 115–122. https://doi.org/10.1080/00173130510010459.
- Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants Pollen consumption in honey bee larvae: a step forward in the risk assessment of transgenic plants. Apidologie. 2004;35:293-300.
- [CrossRef] [Google Scholar]
- Conversion of high and low pollen protein diets into protein in worker honey bees (Hymenoptera: Apidae) J. Econ. Entomol.. 2013;106:1553-1558.
- [CrossRef] [Google Scholar]
- Comparative analysis of morphological, structural and morphometric patterns of Polistes versicolor (Olivier)(Hymenoptera: Vespidae) hypopharyngeal glands. Neotrop. Entomol.. 2004;33:321-326.
- [Google Scholar]
- Measurement of hypopharyngeal gland activity in the honeybee. J. Apic. Res.. 1982;21:193-198.
- [CrossRef] [Google Scholar]
- Carreck, N.L., 2016. Ronald Ribbands and “The behavior and social life of honeybees”. Bee World 93, 26–26. https://doi.org/10.1080/0005772x.2016.1177280.
- Pollen consumption and utilization in worker honeybees (Apis mellifera carnica): dependence on individual age and function. J. Insect Physiol.. 1992;38:409-419.
- [CrossRef] [Google Scholar]
- Amino acid requirements for growth of the honeybee (Apis mellifica L.) Experientia. 1952;8:192-194.
- [CrossRef] [Google Scholar]
- Comparisons of pollen substitute diets for honey bees: consumption rates by colonies and effects on brood and adult populations. J. Apic. Res.. 2008;47:265-270.
- [CrossRef] [Google Scholar]
- The effect of diet on protein concentration, hypopharyngeal gland development and virus load in worker honey bees (Apis mellifera L.) J. Insect Physiol.. 2010;56:1184-1191.
- [CrossRef] [Google Scholar]
- Influence of pollen nutrition on honey bee health: do pollen quality and diversity matter? PLoS One. 2013;8
- [CrossRef] [Google Scholar]
- Efficient use of pollen traps to determine the pollen flora used by honey bees. J. Apicultural Res. 2006
- [CrossRef] [Google Scholar]
- Amylase activity in honey bee hypopharyngeal glands reduced by Rna interference. J. Apic. Res.. 2004;43:9-13.
- [CrossRef] [Google Scholar]
- The influence of brood on the pollen consumption of worker bees (Apis mellifera L.) J. Insect Physiol.. 1998;44:393-404.
- [CrossRef] [Google Scholar]
- Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata) J. Insect Physiol.. 2007;53:649-655.
- [CrossRef] [Google Scholar]
- Influence of age and caging upon protein metabolism, hypopharyngeal glands and trophallactic behavior in the honey bee (Apis mellifera L.) Insect. Soc.. 1996;43:347-358.
- [CrossRef] [Google Scholar]
- Amino acids and immune function. Amino acids and immune function.. 2021;98(2):237-252.
- [Google Scholar]
- Water homeostasis in bees, with the emphasis on sociality. J. Exp. Biol.. 2009;212(3):429-434.
- [Google Scholar]
- The dietary proportion of essential amino acids and Sir2 influence lifespan in the honeybee. Age (Omaha). 2014;36:1239-1247.
- [CrossRef] [Google Scholar]
- The effect of a comb on the longevity of caged adult honey bees 1,2,3. Ann. Entomol. Soc. Am.. 1977;70:365-366.
- [CrossRef] [Google Scholar]
- Pollen nutritional content and digestibility for animals. Plant Syst. Evol.. 2000;222:187-209.
- [CrossRef] [Google Scholar]
- Survival of Honey Bees, Apis mellifera (Hymenoptera: Apidae), Fed Various Pollen Sources. Ann. Entomol. Soc. Am.. 1987;80:176-183.
- [CrossRef] [Google Scholar]
- Linking measures of colony and individual honey bee health to survival among apiaries exposed to varying agricultural land use. PLoS One. 2016;11:e0152685.
- [CrossRef] [Google Scholar]
- Histochemical comparison of the hypopharyngeal gland in Apis cerana fabricius, 1793 workers and Apis mellifera linnaeus, 1758 workers. Psyche (London). 2010;2010:1-7.
- [Google Scholar]
- Standard methods for maintaining adult Apis mellifera in cages under in vitro laboratory conditions. J. Apic. Res.. 2013;52(1):1-36.
- [Google Scholar]
- Comparative sucrose responsiveness in Apis mellifera and A. cerana Foragers. PLoS One 2013:8.
- [CrossRef] [Google Scholar]
- The effects of dietary protein levels on the population growth, performance, and physiology of honey bee workers during early spring. J. Insect Sci.. 2014;14
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2022.102511.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







