Translate this page into:
Impact of ascorbic acid-rich phyto-extracts on growth, yield and physio-biochemistry of okra [Abelmoschus esculentus (L.) Moench.] subjected to drought stress
⁎Corresponding author. nudrataauaf@yahoo.com (Nudrat Aisha Akram)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
To overcome the negative effects of drought stress by exogenous application of natural [lemon juice (LJ) and orange juice (OJ)] and synthetic ascorbic acid (AsA) as sources of potential antioxidants was assessed in okra (A. esculentus) under field conditions. The experiment comprised of two cultivars (Sabz Pari and Bhindi Sanwali) those were subjected to different irrigation regimes including control (12 irrigations) as well as water deficit regimes such as D1 (eight irrigations) and D2 (six irrigations). The plants were sprayed with synthetic AsA (150 mg L-1), 50 % LJ and 50 % OJ to both control and water stressed plants after 30 days of seed germination. Foliar applied AsA obtained from varied sources significantly increased the plant biomass, yield characteristics and leaf photosynthetic pigments, total soluble proteins, proline, AsA, total amino acids, total phenolics, total soluble sugars, and activities of POD, CAT and SOD enzymes in okra plants under varying irrigations. Although, in contrast different AsA sources minimized the accumulation of H2O2 and MDA contents in okra plants. In conclusions, LJ and OJ (both 50 %) proved to be more effective than the synthetic AsA (150 mg L-1) in enhancing yield production, plant biomass and different physio-biochemical traits of okra plants subjected to varying water stress levels. Thus, a better organic source of AsA both LJ and OJ may be suggested to overcome the negative influence of water deficiency in plants.
Keywords
Okra
Water deficit stress
Foliar spray
Ascorbic acid
Lemon juice
Orange juice
1 Introduction
Due to sessile nature of plants, they face different environmental clues that disturb their yield production as well as quality (Sadiq et al., 2018; Nadeem et al., 2019). Of all these challenges, the most devastating constraint for crop production is water scarcity (Nalina et al., 2018; Ghafar et al., 2021). Moreover, unpredictable changes occur frequently in global climate aggravate water scarcity-induced adversity for crop production throughout the world (Khalid et al., 2019). Deficiency of water causes reduction in crop yield by affecting different physiological mechanisms involved in growth of plants such as cell turgor potential, O2/CO2 exchange, assimilation rate, protein synthesis, defense system, nutrients uptake, hormonal balance etc. (Chowdhury et al., 2016; Shafiq et al., 2019). Water stress induces closing of stomata and hence it negatively affects photosynthetic process, carbohydrates utilization and metabolism (Yang et al., 2006; Ashraf and Harris, 2013).
Water deficiency at any stage of plant life also disturbs some morphological characters of plants that include suppression in plant height, leaf area and fruit weight and number of seeds (Shahidan et al., 2018; Langeroodi et al., 2018). Roots initially sense the water deficiency and synthesize abscisic acid (ABA) significantly which acts as stress signal molecule (Malcheska et al., 2017). The roots transfer this signal to shoots and leaves via xylem vessels to regulate stomatal functioning. Moreover, ABA also biosynthesizes rapidly in leaves and maintains the plant growth by closing stomata under drought conditions (Zhang et al., 2018).
Earlier reports indicate that different vegetable crops have the potential to survive utmost and acclimatize under environmental stresses as well as swift changes in the climate (Dhankher and Foyer, 2018). Plants adapt several mechanisms to limit the harmful influence of water stress and tend to sustain their growth and metabolic activities. Due to water deficiency, plants tend to take more water and nutrients due to deeper root system, plasma membrane stability, high accumulation of osmoprotectants, fast generation of antioxidants (enzymatic/non-enzymatic), maintenance of hormonal and nutritional status and enhanced generation/accumulation of stress related proteins (Anjum et al., 2017; Todaka et al., 2017; Khalid et al., 2019).
Resistance/tolerance against drought can be improved by foliar-applied different osmoprotectants, growth regulators, antioxidants and essential/beneficial nutrients (Kaya et al., 2020; Mehak et al., 2021). Ascorbic acid (AsA) famous as vitamin C, a potential metabolite for plants, plays role in oxidative defense, osmotic regulation and cell division in plants (Akram et al., 2017). Additionally, AsA as an antioxidant helps plant metabolic machinery to form and control scavenging of oxygen reactive species (Akram et al., 2017). Earlier reports indicated that the endogenous level of AsA might be enhanced by supplementation of AsA which can scavenge free oxygen species and protect the plants (Moradi and Ismail, 2007). AsA is the most valuable and abundant growth promoting chemical found in plants (Khan et al., 2011). The consumption of plant growth regulators (PGRs) for plants has been increased for many years for enhancing production and quality (Ertani et al., 2016; Naz et al., 2022). Different bioregulators directly extracted from plants are considered more effective in upregulating abiotic stress tolerance and are safe for the environment as compared to synthetic chemicals (Godlewska et al., 2019; Ben Mrid et al., 2021). Moreover, AsA as an antioxidant, has role in different physiological processes (Podgórska et al., 2017). AsA application can enhance the plant biomass and survival ability of plants (Ullah et al., 2016; Fatima et al., 2019; Ma et al., 2022). Increased levels of AsA cause removal of different free radicals e.g., O2•-, OH and H2O2 (Bilska et al., 2019; Sharma et al., 2019; Sarwar et al., 2022).
Okra (A. esculentus) is the important and delicious vegetable and consumed frequently in Asia (Temam et al., 2021). It is susceptible to different environmental challenges including water deficit stress (Bhusal et al., 2020). Water stress has been known to affect biochemical and physiological functions of okra thereby causing growth restrictions (Ali et al., 2022). Thus, the present study was conducted not only to introduce the natural sources of AsA but also to compare the effectivity of exogenous application of natural and synthetic AsA in okra plants under water stress. In this comparative study, the response of okra plants in terms of physio-biochemical, growth and yield production were examined under varying irrigations. uncover the responses of okra plants.
2 Materials and methods
A trial was organized under field conditions at village Mahar Sharif, Chishtian Punjab, Pakistan during year, 2019. In this trial the effectiveness of foliar-applied ascorbic acid (AsA) obtained from different sources including natural sources [extracted from fruits of two different plants, lemon (Citrus limon) and sweet orange (Citrus sinensis) as well as synthetic AsA on okra (A. esculentus) plants was determined. Two okra cultivars namely Sabz Pari and Bhindi Sanwali were subjected to control and drought (with-holding irrigations). The trial included three main plots, control (normal irrigations i.e., 12 irrigations during entire experimental period) and two drought stress regimes (one plot received eight and the other one received six irrigations). During the study, the average day temperature, 35.0C, average night temperature, 20.0C, light period, 12.1 h and relative humidity 57 %, were recorded. The soil texture was sandy loam with potassium, 164.45, nitrogen, 74 and phosphorus, 69 mg g−1 soil, respectively, pH, 8.1, EC, 2.14 dS m−1 and saturation percentage, 0.78 % were observed. Before sowing, the okra seeds were merged in dH2O for 2 h. The seeds were sown 2 cm deep and 2 cm difference in soil by hand drill-method. After 30 days of seed germination, four levels of AsA, 150 mg L−1 (synthetic AsA), 50 % orange juice and 50 % lemon juice and mixed with 0.1 % Tween-20, besides control (no spray) were foliar-applied at the vegetative stage of plant growth. Overall, 15 mL/plant of each concentration of AsA was sprayed using a plastic manual sprayer. The LJ and OJ were extracted by collecting the fresh lemon and sweet oranges from the local market. The peels of both fruits were removed, and the juice was extracted by using a machine followed by stored at 4.0C for 24 h. The required dilutions were prepared by adding distilled water just before their use. Following Mukherjee and Choudhuri (1983), AsA concentration in 50 % LJ (14 mg L-1) and 50 % OJ (24 mg L-1) were noticed. After one month of external application (applied once), two plants of similar size were uprooted, from each row (four rows/plot and four plants/row) washed their roots and noted their weights. For dry biomass, air and oven dried the samples at 80C till their constant dry weights. In addition, the leaves were collected and preserved in ultra-low freezer for the following analyses:
2.1 Chlorophyll contents
Leaf (250 mg) was homogenized in 80 % acetone (5.0 mL) and the mixture was stored for a day. The absorbance was measured at 645 and 663 nm following Arnon (1949).
2.2 Free proline contents
Leaf (250 mg) was mixed in 3 % sulfo-salicylic acid. The filterate (1.0 mL), GAA (1.0 mL) and acid ninhydrin (1.0 mL) were mixed. This solution was boiled at 100C, cooled in ice and toluene was added. The absorbance of upper layer was noted at 520 nm (Bates et al., 1973).
2.3 Glycine betaine (GB)
Leaf (0.25 g) was ground in 5 mL dH2O, and one mL filtrate was mixed with one mL of 2 N H2SO4 along with potassium tri-iodide (200 μL) and cooled at 4C (Grieve and Grattan, 1983). Moreover, 2.8 mL dH2O and 1, 2-dichloroethane (6.0 mL) homogenized together. After vortexing the mixture, the OD was determined at 365 nm.
2.4 Hydrogen peroxide (H2O2)
Leaf (0.25 g) was homogenized in 0.1 % ice-cold TCA (5 mL) and centrifuged (Velikova et al., 2000). Then, 500 μL supernatant was mixed with K-P buffer (10 mM; 7.0 pH) and 1.0 mL of 1 M KI2 solution. The optical density was noted at 390 nm for the determination of H2O2 contents.
2.5 Malondialdehyde (MDA)
Leaf tissue (250 mg) was extracted with TCA and centrifuged (Heath and Packer, 1968). An aliquot (0.5 mL) was mixed with TBA (0.5 %). The samples boiled for 30 min at 80C followed by cooling. The reading was taken at 532 and 600 nm.
2.6 Total free amino acids
Leaf (100 µL) was homogenized in acidic ninhydrin and pyridine (10 %) following Hamilton et al. (1943). Heated the reaction mixture by using water bath and cooled. The final volume prepared up to 7.5 mL using dH2O and OD (570 nm) was noted.
2.7 Total soluble sugars (TSS)
Fresh leaf (0.25 g) was mixed in 5 mL ethanol (80 %) (Yemm and Willis, 1954). The aliquot (100 μL) was mixed with 3 mL anthrone solution. After incubation, the solution was cooled and OD was read at 625 nm.
2.8 Ascorbic acid
Leaf (250 mg) was extracted in TCA (5 mL; 6 %) (Mukherjee and Choudhuri, 1983). Then, 2.0 mL filtrate was added to one mL of 2, 4-dinitrophenylhydrazine (2 %) along with 1 mL 10 % thiourea. The homogenate was heated at 95C by using water bath and cooled. To it, 2.5 mL of sulfuric acid (80 %; v/v) mixed and reading was observed at 530 nm.
2.9 Total phenolics
A frozen leaf (0.25 g) was extracted in acetone (5 mL; 80 %) (Julkenen-Titto, 1985). Then mixed sample (100 μL), Folin-Ciocalteu phenol (1.0 mL) and 2.0 mL dH2O. Then, 20 % sodium carbonate (5.0 mL) taken and finalized up to 10 mL by adding dH2O and noted OD at 750 nm.
2.10 Activities of antioxidant enzymes
For enzymes, a leaf (0.5 g) was crushed in ice-cooled potassium phosphate buffer (50 mM; 7.8 pH) (Akram et al., 2017).
2.11 Superoxide dismutase (SOD)
To estimate the activity of SOD enzyme, Van Rossum et al. (1997) protocol was followed. The reaction mixture was subjected to light for 5 min and OD was noted at 560 nm by using spectrophotometer.
2.12 Peroxidase (POD)
To determine the activity of POD enzyme, the protocol proposed by Chance and Maehly (1955) was followed. Three mL of peroxidase reaction solution were mixed with 0.1 mL enzyme extract and then 40 mM H2O2, 20 mM guaiacol and 50 mM phosphate buffer (pH 5 0.0) were added to it. The changes in absorbance were recorded at 470 nm after every 30 sec. A change in the absorbance per min was considered equal to one unit of POD activity.
2.13 Catalase (CAT)
The CAT activity was measured by making a reaction mixture following the protocol outlined by Chance and Maehly (1955). Three mL reaction solution used for the determination of CAT activity contained 5.9 mM H2O2, 50 mM phosphate buffer having pH 7.8, and 0.1 mL enzyme extract. The reaction was initiated by adding the enzyme extract and change in absorbance of the reaction solution after every 20 sec were measured at 240 nm. A change of 0.01 units per min in absorbance was considered equal to one unit CAT activity.
2.14 Total soluble proteins (TSP)
Protein concentration of the extract was measured following Bradford (1976). An aliquot (2 mL) of the Bradford reagent and 100 µL sample were added to a test tube, kept for 20 min at room temperature and absorbance read at 595 nm.
2.15 Yield attributes
The capsule yield (dry/fresh weights) and the number of capsules per plant were calculated.
2.16 Statistical analysis
All the parameters measured were subjected to ANOVA to obtain mean square values and their differences were calculated on the basis of LSD at 5 % level.
3 Results
Withholding of irrigations such as D1 (eight irrigations) and D2 (six irrigations) after seed germination to 60-day of plant growth significantly suppressed the fresh and dry weights of okra shoot and root as compared to control plants. However, external applied 150 mg L-1 synthetic AsA and different phytoextracts enriched with AsA (50 % lemon juice and 50 % orange juice) considerably promoted (P ≤ 0.001) the weights of all okra plants. Of all foliar applications, 50 % LJ was shown to be markedly enhanced biomass (dry and fresh) of okra plants particularly under water deficit stress (Fig. 1). The cv. Bhindi Sanwali performed better in terms of growth attributes under deficit regimes.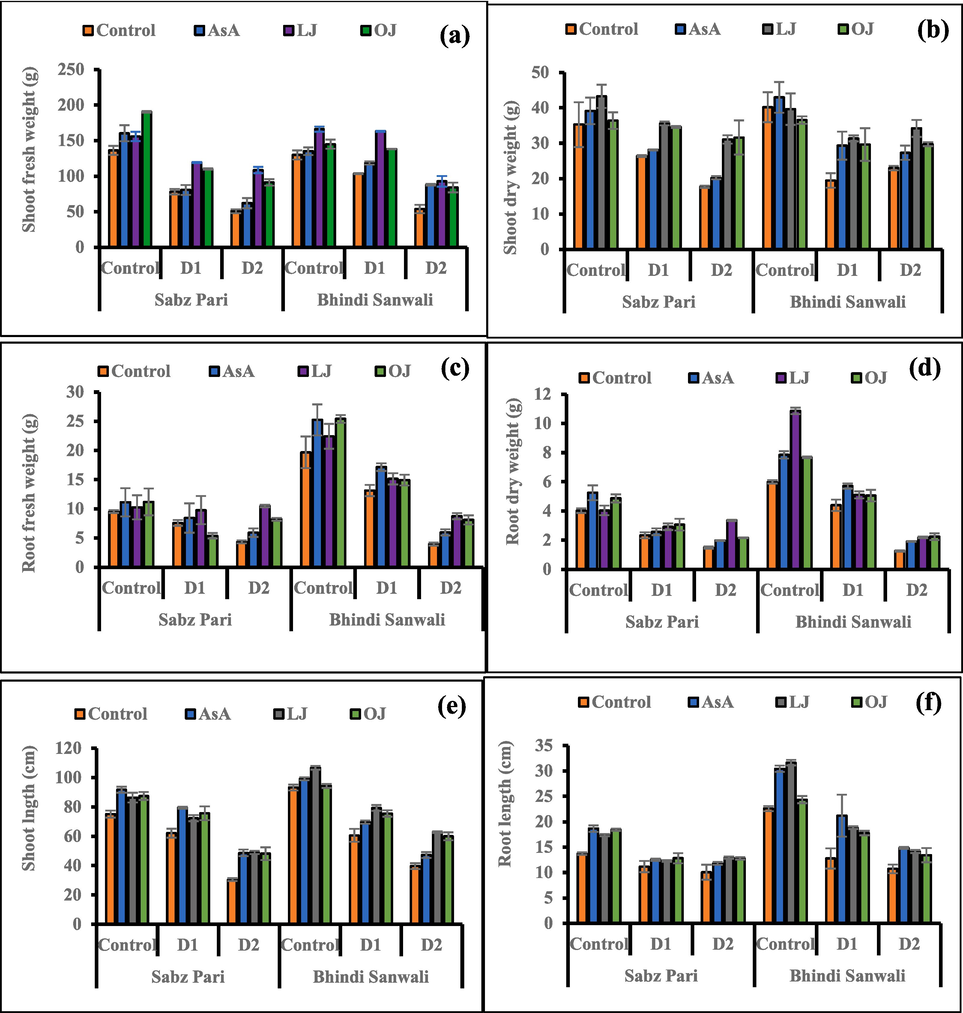
Shoot fresh weight (a), shoot dry weight (b), root fresh weight (c), root dry weight (d), shoot length (e) and root length (f) of 60 day-old plants of two cultivars of okra (Abelmoschus esculentus L.) subjected to foliar-applied different sources of ascorbic acid under varying irrigation regimes (Mean ± S.E.; n = 4). Control (received 12 irrigations); D1 (8 irrigations) and D2 (6 irrigations); AsA, Ascorbic Acid; LJ, Lemon Juice; OJ, Orange Juice.
Lengths (root and shoot) of okra plants significantly (P ≤ 0.001) reduced on exposure of plants to drought [D1 (66 % irrigations) and D2 (50 % irrigations)]. However, foliar applied ascorbic acid, LJ and OJ exhibited beneficial effects on both okra cultivars (Fig. 1) and the maximum improvements in lengths of okra plants were caused by the foliar applications of AsA and LJ under non-stress conditions (Table 1; Fig. 1). ns = no-significant; *, ** and *** = significant at 0.05, 0.01 and 0.001 levels, respectively.
Source of variations
df
Shoot fresh weight
Shoot dry weight
Root fresh weight
Root dry weight
Cultivars (Cvs)
1
929.08**
2.607 ns
1003.82***
81.881***
Drought (D)
2
43155.39***
1357.23***
785.01***
145.08***
Ascorbic Acid (AsA)
3
8707.07***
331.48***
46.357**
9.293***
Cvs x D
2
5251.76***
107.54*
352.42***
31.865***
Cvs x AsA
3
590.26**
48.831 ns
13.291 ns
1.954***
D x AsA
6
401.06**
79.570*
19.704*
1.287***
Cvs x D x AsA
6
683.17***
17.517 ns
2.288 ns
4.509***
Error
72
108.09
34.418
8.774
0.229
Shoot length
Root length
Fresh weight of pods
Dry weight of pods
Cultivars (Cvs)
1
1108.06***
774.07***
462.31***
88.128***
Drought (D)
2
15192.35***
794.99***
1430.40***
81.128***
Ascorbic Acid (AsA)
3
1206.09***
110.97***
56.576**
9.948***
Cvs x D
2
421.88***
156.31***
2.578 ns
0.440 ns
Cvs x AsA
3
230.90***
29.524**
5.811 ns
1.262 ns
D x AsA
6
74.179**
7.653 ns
10.541 ns
1.393*
Cvs x D x AsA
6
26.754 ns
8.576 ns
9.972 ns
0.491 ns
Error
72
24.001
5.133
12.700
0.565
No. of pods
Chlorophyll a
Chlorophyll b
Total chlorophyll
Cultivars (Cvs)
1
8.760**
2.236***
2.963***
10.349***
Drought (D)
2
102.01***
0.766***
0.360***
2.111***
Ascorbic Acid (AsA)
3
10.621***
0.063***
0.049***
0.223***
Cvs x D
2
7.822***
0.015 ns
0.011 ns
0.001 ns
Cvs x AsA
3
0.677 ns
0.007 ns
0.002 ns
0.014 ns
D x AsA
6
0.538 ns
0.005 ns
0.001 ns
0.010 ns
Cvs x D x AsA
6
0.906 ns
0.003 ns
0.002 ns
0.001 ns
Error
72
0.795
0.006
0.005
0.014
Chl. a/b ratio
Proline
Glycinebetaine
H2O2
Cultivars (Cvs)
1
12.987***
3.277***
24.440 ns
100371.78***
Drought (D)
2
1.642**
0.107 ns
259.77**
41216.14***
Ascorbic Acid (AsA)
3
0.080 ns
0.827**
270.17***
10433.19***
Cvs x D
2
0.356 ns
0.089 ns
78.144 ns
868.40 ns
Cvs x AsA
3
0.087 ns
0.145 ns
17.108 ns
100.44 ns
D x AsA
6
0.066 ns
0.009 ns
18.905 ns
280.71 ns
Cvs x D x AsA
6
0.176 ns
0.026 ns
9.680 ns
780.44 ns
Error
72
0.264
0.141
34.323
1496.25
Malondialdehyde
Total phenolics
Ascorbic acid
Total soluble sugars
Cultivars (Cvs)
1
70806.1***
76.68***
6.319***
23.45***
Drought (D)
2
26.586 ns
12.21***
6.353***
10.81***
Ascorbic Acid (AsA)
3
8620.7***
1.777***
0.891 ns
0.006 ns
Cvs x D
2
5604.5**
0.396 ns
0.116 ns
0.291*
Cvs x AsA
3
1376.1 ns
0.083 ns
0.046 ns
0.108 ns
D x AsA
6
742.9 ns
0.074 ns
0.126 ns
0.082 ns
Cvs x D x AsA
6
699.9 ns
0.198 ns
0.08 ns
0.04 ns
Error
72
1103.9
0.209
2.461
0.059
Total amino acids Total soluble proteins Peroxidase Catalase
Cultivars (Cvs)
1
0.066*
642.78***
8392.56***
44.700***
Drought (D)
2
0.013ns
493.05***
1715.01***
55.742***
Ascorbic Acid (AsA)
3
0.117***
20.440**
955.76***
2.644***
Cvs x D
2
0.021ns
83.140***
116.68ns
9.881***
Cvs x AsA
3
0.006ns
0.798ns
2.075ns
0.011ns
D x AsA
6
0.003ns
4.495ns
135.88ns
0.090ns
Cvs x D x AsA
6
0.007ns
1.389ns
113.19ns
0.030ns
Error
72
0.009
4.333
133.65
0.294
Superoxide dismutase
Cultivars (Cvs)
1
2.961***
Drought (D)
2
2.816***
Ascorbic Acid (AsA)
3
0.082*
Cvs x D
2
0.005ns
Cvs x AsA
3
0.010ns
D x AsA
6
0.009ns
Cvs x D x AsA
6
0.007ns
Error
72
0.025
Varying water deficit regimes significantly reduced number of capsules on each plant, capsule (okra fruit) weights. Different foliar applications such as AsA, LJ and OJ markedly enhanced the capsule (dry and fresh) weights plus capsules/plant at varying irrigations. Foliar application of AsA caused a maximal improvement in all weights of capsules/plant, while LJ and OJ showed maximum improvements in number of capsules per plant in cv. Bhindi Sanwali under control. Cv. Bhindi Sanwali was good than cv. Sabz Pari in terms of yield attributes under control and stress levels (Fig. 2).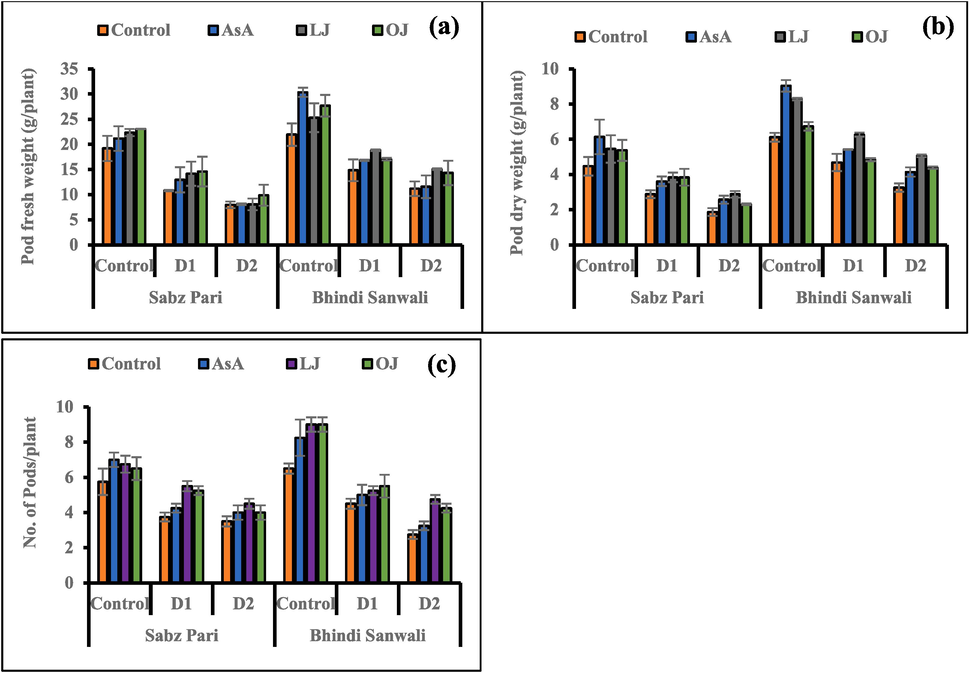
Yield attributes including pods fresh weight (a), pods dry weight (b) and number of pods per plant (c) of 60 day-old plants of two cultivars of okra (Abelmoschus esculentus L.) subjected to foliar-applied different sources of ascorbic acid under varying irrigation regimes (Mean ± S.E.; n = 4). Control (received 12 irrigations); D1 (8 irrigations) and D2 (6 irrigations); AsA, Ascorbic Acid; LJ, Lemon Juice; OJ, Orange Juice.
Water deficit stress induced suppression in leaf chlorophyll (a, b) pigments of okra (Table 1). The 50 % irrigation level was more effective in reducing these pigments. Although, chlorophyll a/b ratio unchanged under water stress. Moreover, foliar-applied different sources of AsA improved the photosynthetic contents (a, b and total chlorophyll) subjected to both water regimes. However, no significant effects were noticed in the ratio of chlorophyll a/b in this study (Fig. 3). Overall, cv. Bhindi Sanwali was better in chlorophyll pigments than the other cultivar.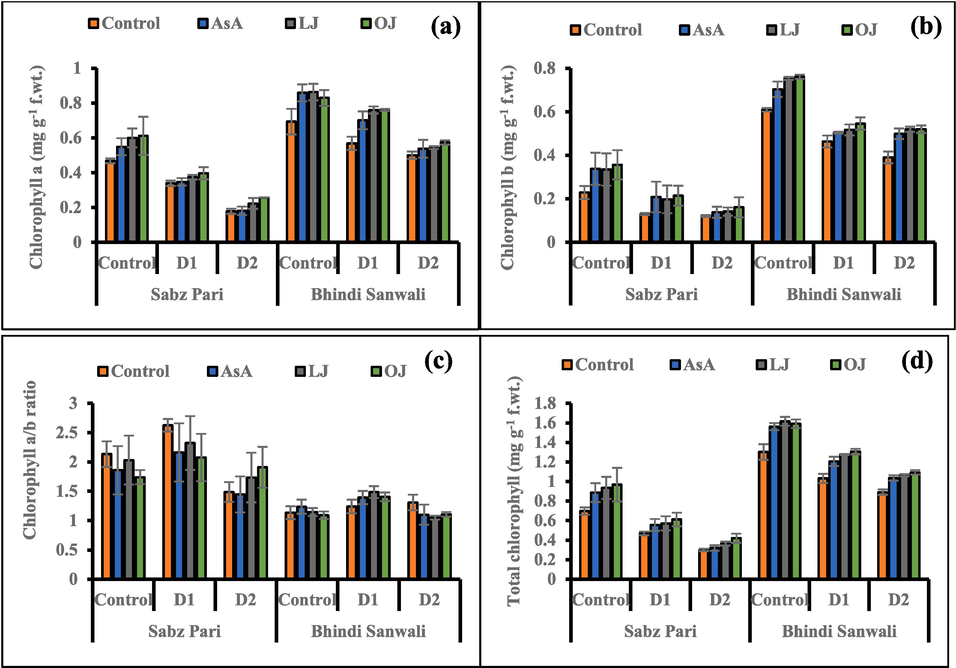
Chlorophyll a (a), chlorophyll b (b), chlorophyll a/b ratio (c) and total chlorophyll contents (d) of 60 day-old plants of two cultivars of okra (Abelmoschus esculentus L.) subjected to foliar-applied different sources of ascorbic acid under varying irrigation regimes (Mean ± S.E.; n = 4). Control (received 12 irrigations); D1 (8 irrigations) and D2 (6 irrigations); AsA, Ascorbic Acid; LJ, Lemon Juice; OJ, Orange Juice.
Accumulation of H2O2 amplified substantially (P ≤ 0.001) in the leaves of both okra cultivars under varied regimes. However, a considerable suppression in this metabolite was determined in okra plants due to supplementation of different AsA sources, particularly LJ and OJ (Table 1). Accumulation of H2O2 in cv. Bhindi Sanwali was more obvious as compared to that in cv. Sabz Pari, particularly under non-stress conditions (Fig. 4).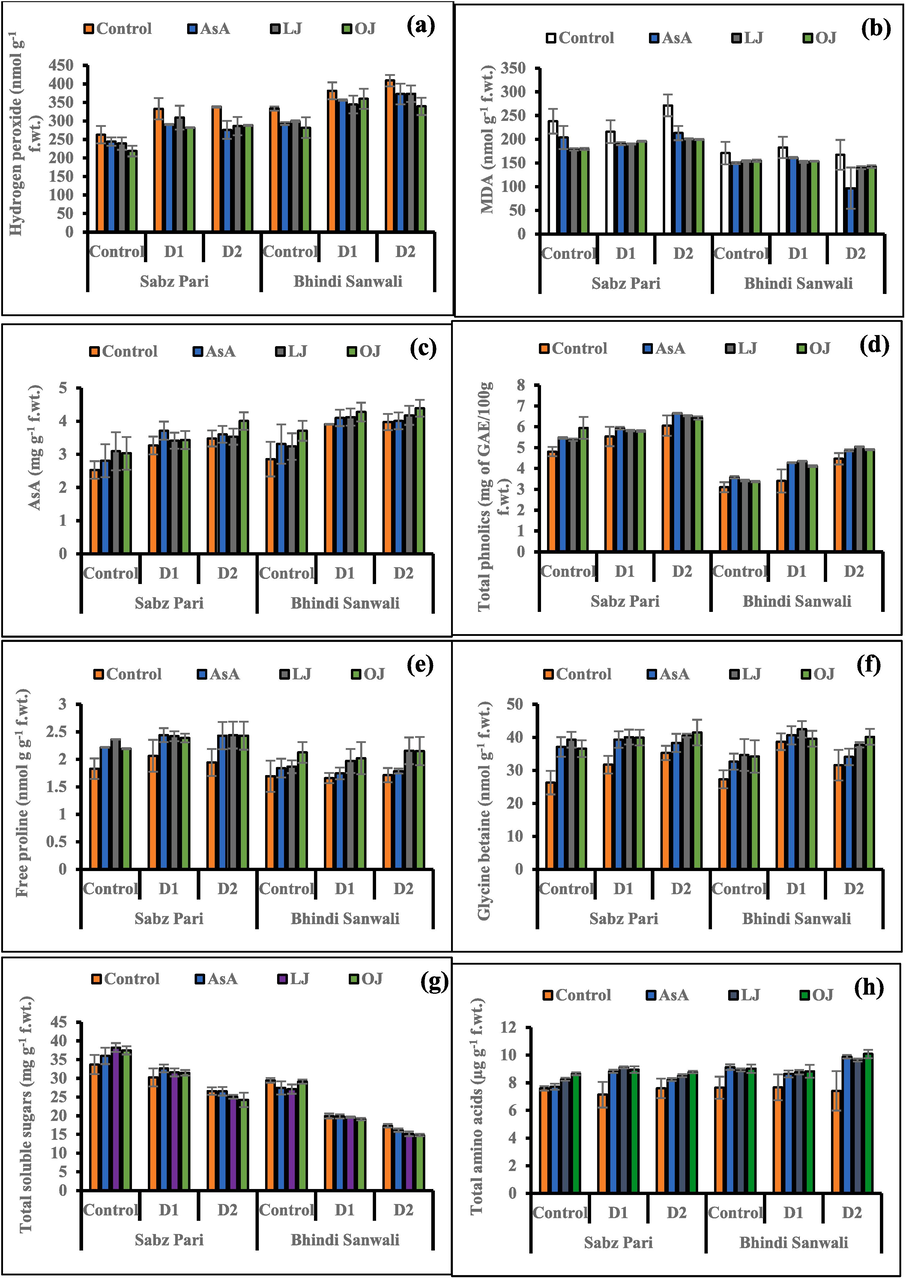
Hydrogen peroxide (a), malondialdehyde (b), AsA (c), total phenolics (d), free proline (e), GB (f), total soluble sugars (g) and total amino acids (h) of 60 day-old plants of two cultivars of okra (Abelmoschus esculentus L.) subjected to foliar-applied different sources of ascorbic acid under varying irrigation regimes (Mean ± S.E.; n = 4). Control (received 12 irrigations); D1 (8 irrigations) and D2 (6 irrigations); AsA, Ascorbic Acid; LJ, Lemon Juice; OJ, Orange Juice.
Malondialdehyde (MDA) concentration enhanced in both okra cultivars subjected to water-deficit watering regimes. While different sources of ascorbic acid considerably (P ≤ 0.05) decreased the MDA contents under water deficit regimes. However, more significant decrease in MDA contents were noticed in cv. Sabz Pari than the other ones (Fig. 4).
Drought stress improved AsA and total phenolics in both okra cultivars. However, foliar-applied AsA, LJ and OJ substantially enhanced the total phenolics and AsA in okra plants under varied irrigations. Of both okra cultivars, cv. Sabz Pari was best in these biochemical attributes to cv. Bhindi Sanwali under water deficit environment (Fig. 4).
Water deficit stress showed no significant effects on leaf proline contents while GB concentration enhanced markedly in okra plants subjected to water deficit stress. Applications of AsA, LJ and OJ significantly improved leaf proline contents of both okra cultivars. No significant differences were noticed in response to drought stress as well as external applications of different ascorbic acid sources in both okra cultivars under varying irrigations (Fig. 4).
Under drought stress a prominent increase (P ≤ 0.01) was observed in leaf total soluble sugars of okra. Moreover, different ascorbic acid sources also improved the concentration of total soluble sugars in both okra cultivars (Fig. 4). Of all exogenous applications, 50 % LJ and 50 % OJ increased more significantly total soluble sugars in cv. Sabz Pari than in the other cultivar under control conditions.
Total free amino acid (TAA) concentration considerably (P ≤ 0.001) increased in the leaves of both okra cultivars with frequently increasing water deficit stress from D1 to D2 (Fig. 4). Moreover, external applications of different ascorbic acid sources increased TAA in the leaves of two okra cultivars. The maximum increase in TAA was noticed in 50 % OJ treatment, especially D2 irrigation. However, both okra cultivars showed no significant difference in response to dwater stress and foliar applied varying AsA sources.
Leaf total soluble protein concentration improved considerably under varying water deficit levels as well by different foliar applications of AsA (P ≤ 0.01; Fig. 5). Maximum total soluble protein accumulation was noticed by foliage spray of synthetic AsA and 50 % LJ treatments. However, of both okra cultivars, maximum accumulation of total soluble proteins was observed in okra cv. Sabz Pari under both regimes.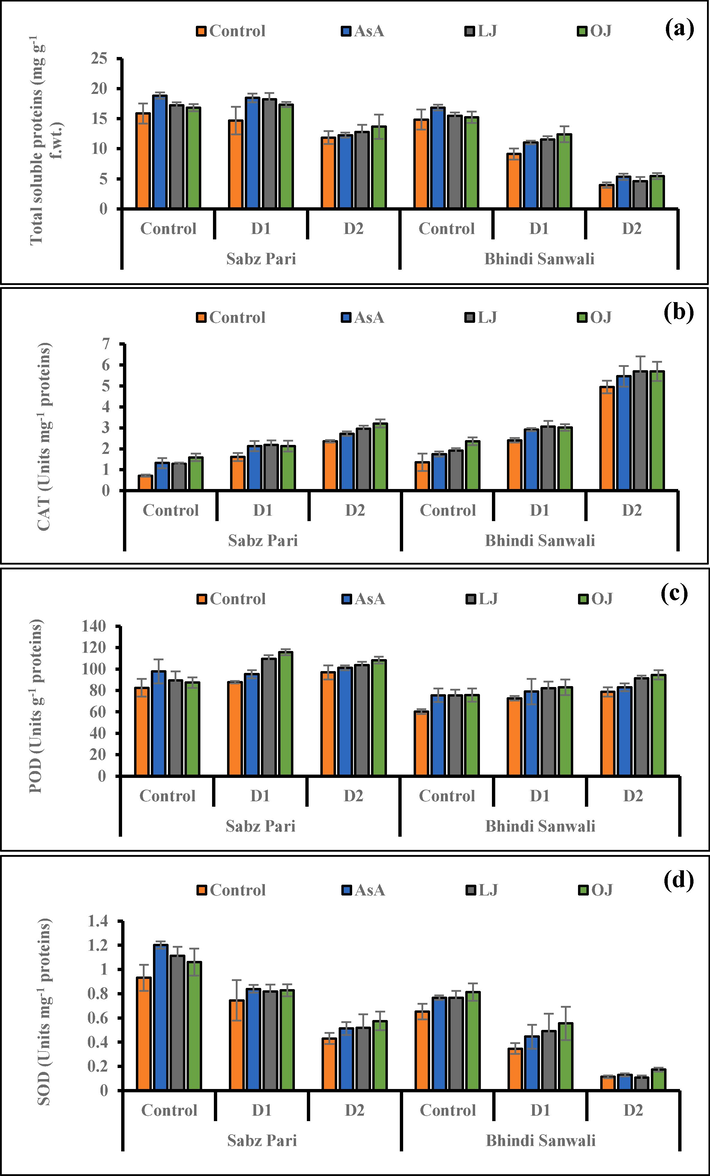
Total soluble proteins (a), activities of enzymatic antioxidants including catalase (b), peroxidase (c) and superoxide dismutase (d) of 60 day-old plants of two cultivars of okra (Abelmoschus esculentus L.) subjected to foliar-applied different sources of ascorbic acid under varying irrigation regimes (Mean ± S.E.; n = 4). Control (received 12 irrigations); D1 (8 irrigations) and D2 (6 irrigations); AsA, Ascorbic Acid; LJ, Lemon Juice; OJ, Orange Juice.
Activities of antioxidants (CAT, SOD, POD) enhanced under dry-arid conditions. However, foliar applied different ascorbic acid sources enhanced the activities of CAT (P ≤ 0.05), POD and SOD in the water deficit okra plants (Fig. 5). Maximum CAT activity was noticed by foliar applied 50 % LJ and 50 % OJ, especially at D2 irrigation regime. While a maximal increase was observed in SOD enzyme activity in cv. Sabz Pari, particularly under control conditions and D1 watering regime by the external application of AsA and 50 % OJ (Fig. 5). Of both okra cultivars, cv. Bhindi Sanwali was better in CAT activity, while cv. Sabz Pari was better in SOD activity.
4 Discussion
Water stress causes adverse effects on plants like imbalance in plant growth, delay in cell elongation, low availability of nutrients, cell/tissue damages, etc.; all these are considered as an obstacle to crop productivity (Mubarak et al., 2021). Growth and productivity of plants can be improved by supplementation of varied chemical compounds under non-stress and stress conditions (El-Metwally et al., 2022). In this investigation the influence of supplementation with synthetic AsA (150 mg L -1) and phytoextracts enriched with natural AsA on growth, yield as well as biochemical attributes of two okra cultivars under stress regimes was assessed. The results showed that withholding of irrigations such as D1 (eight irrigations) and D2 (six irrigations) decreased weights/lengths (root and shoot), fruit yield and number of capsules/plant of two okra cultivars. These results correspond with earlier studies that showed that growth and yield of many crops decreased due to arid conditions (Tesfamarium et al., 2010; Javed et al., 2011; Aziz et al., 2018). This decrease in growth and yield attributes may be associated to water stress induced stomatal closure, reduction in photosynthesis, inhibition in enzyme activities, membrane leakage, impairment in nutritional and hormonal metabolisms, and oxidative stress (Shafiq et al., 2021). However, foliar applications of AsA-enriched phytoextracts [LJ (50 %) and OJ (50 %)] and synthetic AsA improved biomass and yield production of stressed okra cultivars. Especially, 50 % each of LJ and OJ enhanced the fruit numbers and their weights more efficiently as compared to the other treatment. Previously, foliar spray of AsA eliminates the harmful effects of drought stress by promoting rate of photosynthesis and photoreceptors, water relations and oxidative defense (Akram et al., 2018).
Water deficit stress causes oxidative stress which results increased ROS production that disturbs chlorophyll pigments and rate of photosynthesis (Gubrelay et al., 2013; Akram et al., 2018). In the current study, reduced irrigations suppressed chlorophyll contents (a and b). The findings are analogous to a previous study performed by Sadiq et al. (2018) on mungbean plants that indicated that withholding of irrigations considerably declined the photosynthetic pigments. Moreover, exogenous application of different AsA sources enhanced photosynthetic pigments of okra plants. Such findings were found in quinoa plants (Aziz et al., 2018) showing a considerable increase in chlorophyll levels under foliar applied AsA (150 mg L-1) and OJ (25 %) subjected to arid conditions.
Under stress, the concentrations of H2O2 generally increase in plants which may act as a signaling molecule at low level and plays a significant role in several biotic and abiotic stress tolerance mechanisms of plants, while at higher concentration it is harmful to several metabolic processes (Bhattacharjee, 2012; Ju et al., 2018). Moreover, malondialdehyde (MDA) also plays a role as a signaling molecule to cause oxidative stress-induced membrane injury under water limited stress (Shafiq et al., 2015). In the current investigation, water deficit stress raised the contents of MDA and H2O2 levels in the leaves of okra. Similar results were also observed by Sarker and Oba (2018) in Amaranthus plants and Aziz et al. (2018) in drought stressed quinoa plants. In addition, exogenously applied 150 mg L-1 of AsA, 50 % LJ or 50 % OJ significantly decreased the over-accumulation of H2O2 and MDA level in the leaves of okra cultivars. These results showed similarity with the earlier studies performed by Nie et al. (2018) in cucumber and Deeba et al. (2012) in G. herbaceum.
As an antioxidant AsA plays a prominent role in enzymatic detoxification of hydrogen peroxide and promotes the plant defensive mechanism against water deficiency (Hemavathy et al., 2011). Ascorbic acid is known to directly involved in the scavenging mechanism of ROS (Lushchak and Semchuk, 2012). In this experiment, the concentration of leaf AsA and total phenolics increased under water deficit stress applied through withholding of irrigations. In a previous study, Aziz et al. (2018) documented that the concentrations of endogenous AsA increased in quinoa plants under water deficit stress. Moreover, phenolic contents also generally increase in plants exposed to water deficit environment (Al-Hassan et al., 2015; Sadiq et al., 2018). However, we observed that external applied AsA, OJ and LJ markedly enhanced total phenolics and AsA in okra plants under no stress and stress regimes. Several previous studies exhibited that endogenous AsA concentration increases in different plants by the foliar applications of AsA under water deficit stress (Ghorbanli et al., 2013; Aziz et al., 2018).
Plants under abiotic stress conditions tend to produce a myriad of organic substances such as proline and glycine betaine that play a marked role as osmoprotectants (Akram et al., 2016; Shafiq et al., 2021). We found water shortage showed no influence on leaf proline contents, while the concentration of GB increased markedly under varying withholding irrigations. While exogenous applications of AsA, LJ and OJ significantly enhanced leaf proline contents of both okra cultivars. Previously, Aziz et al. (2018) reported that in quinoa plants the concentration of proline was not affected by water deficit stress, while the contents of GB increased when plants were exposed to varying water deficit regimes. By contrast, Sadiq et al. (2018) showed that water deficiency increased the contents of proline as well as GB in mungbean plants. Moreover, exogenously applied foliar applications of AsA either natural or synthetic improved the concentrations of proline in rapeseed (Razaji et al., 2014), cucumber (Naz et al., 2016) as well as soybean (Amira and Qados, 2014).
Under drought a high concentration of several important metabolites such as soluble sugars, TAA and soluble proteins were determined in the okra leaves. Furthermore, foliar applications of different sources of AsA also improved the concentrations of these metabolites in the leaves of both okra cultivars. Previous studies performed on okra by Amin et al. (2009), soybean by Amira and Qados (2014) and quinoa by Aziz et al. (2018) reported that the contents of sugars increased by the application of AsA under water deficit stress. Additionally, the contents of total free amino acids increased in different plants under stress regimes (Zonouri et al., 2014). In the present study, all sources of AsA either synthetic or natural increased the concentrations of TAA and total soluble proteins in okra leaves. These findings correspond to the observations in okra by Amin et al. (2009), grapes by Zonouri et al. (2014) and quinoa by Aziz et al. (2018). These researches suggest that the accumulation of soluble proteins and TAA is linked with drought stress tolerance mechanisms of plants.
To minimize ROS generated under stress conditions, plants tend to produce key antioxidant enzymes in their cells/tissues (Sadiq et al., 2018). We found that water stress upregulated the activities of antioxidant enzymes studied in okra leaves. These findings are similar to Cao et al. (2017) who showed increased activities of antioxidant enzymes under water deficit stress. In addition, foliar applications of different AsA sources also improved the activities of CAT, POD and SOD enzymes. Particularly, the activity of CAT was considerably enhanced by the foliar application of 50 % each of LJ and OJ at D2 irrigation regime. Same readings were noticed by Aziz et al. (2018) that exogenous application of both synthetic and natural AsA increased the activities of enzymes in quinoa plants under water limited stress. In other studies, performed by Athar et al. (2009) and Malik et al. (2015) in wheat and Dolatbadian et al. (2010) in maize it was shown that the stress tolerance mechanism of plants can be enhanced by the upregulation of antioxidant enzymes.
Overall, withholding of irrigations decreased plant growth and yield attributes, and photosynthetic pigments of okra. However, secondary metabolites and activities of CAT, POD and SOD enzymes increased under water stress. Exogenous application of AsA (natural and synthetic) enhanced growth and yield attributes, photosynthetic pigments, plant metabolites and activities of enzymatic antioxidants in okra plants. Application of natural sources of AsA such as 50 % LJ and 50 % OJ showed better improvement in okra plants under varying water deficit regimes. So, further biochemical research may need to be done on this phytoextracts to know the better effectivity of LJ and OJ than the synthetic AsA.
6 Author’s contributions
The research conceptualization was a collaborative effort involving all authors. MY, NAA, and MA were primarily responsible for drafting the experimental design, while NA conducted the experiments. MAES and ZUK were involved in data analysis and validation. NAA, MAES and MA contributed to the language editing of the manuscript. All authors participated in reviewing and approving the final version of the manuscript before its submission.
Conflict of interest
Authors declare there is no potential of conflict in this research article.
CRediT authorship contribution statement
Muhammad Younis: Conceptualization, Investigation, Methodology. Nudrat Aisha Akram: Conceptualization, Project administration, Supervision, Writing – original draft. Muhammad Ashraf: Conceptualization, Investigation, Methodology. Mohamed A. El-Sheikh: Formal analysis, Funding acquisition, Software, Validation, Writing – original draft. Zakir Ullah Khan: Resources, Writing – review & editing.
Acknowledgements
The authors would like to extend their sincere appreciation to the Researchers Supporting Project Number (RSP2024R182), King Saud University, Riyadh, Saudi Arabia for supporting this work.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Trehalose pretreatment induces drought tolerance in radish (Raphanus sativus L.) plants: some key physio-biochemical traits. Acta Physiol. Plant.. 2016;38:3.
- [Google Scholar]
- Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci.. 2017;8:613.
- [Google Scholar]
- Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma. 2018;255:163-174.
- [Google Scholar]
- Effects of salt and water stress on plant growth and on accumulation of osmolytes and antioxidant compounds in cherry tomato. Not. Bot. Hort. Agrobot. Cluj-Napoca. 2015;43(1):1-11.
- [Google Scholar]
- Influence of Ascophyllum nodosum extract foliar spray on the physiological and biochemical attributes of okra under drought stress. Plants. 2022;11:790.
- [Google Scholar]
- Evaluation of interaction effect of drought stress with ascorbate and salicylic acid on some of physiological and biochemical parameters in okra (Hibiscus esculentus L.) Res. J. Biol. Sci.. 2009;4:380-387.
- [Google Scholar]
- Effect of ascorbic acid antioxidant on soybean (Glycine max L.) plants grown under water stress conditions. Int. J. Adv. Res. Biol. Sci.. 2014;1:189-205.
- [Google Scholar]
- Drought induced changes in growth, osmolyte accumulation and antioxidant metabolism of three maize hybrids. Front. Plant Sci.. 2017;8(69):1-12.
- [Google Scholar]
- Copper enzymes in isolated chloroplasts polyphenol-oxidase in Beta vulgaris. J. Plant Physiol.. 1949;24:1-15.
- [Google Scholar]
- Photosynthesis under stressful environments: An overview. Photosynthetica. 2013;51:163-190.
- [Google Scholar]
- Inducing salt tolerance in wheat by exogenously applied ascorbic acid through different modes. J. Plant Nutr.. 2009;32:1799-1817.
- [Google Scholar]
- Influence of natural and synthetic vitamin C (ascorbic acid) on primary and secondary metabolites and associated metabolism in quinoa (Chenopodium quinoa Willd.) plants under water deficit regimes. Plant Physiol. Biochem.. 2018;12:192-203.
- [Google Scholar]
- Rapid determination of free proline for water-stress studies. Plant Soil. 1973;39:205-207.
- [Google Scholar]
- Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ.. 2021;777:146204
- [Google Scholar]
- An inductive pulse of hydrogen peroxide pretreatment restores redox-homeostasis and oxidative membrane damage under extremes of temperature in two rice cultivars. Plant Growth Regul.. 2012;68:395-410.
- [Google Scholar]
- Responses to drought stress in Prunus sargentii and Larix kaempferi seedlings using morphological and physiological parameters. Forest Ecol. Manag.. 2020;465:118099
- [Google Scholar]
- Ascorbic acid - The little-known antioxidant in woody plants. Antioxidants. 2019;8:645.
- [Google Scholar]
- A rapid and sensitive method for microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem.. 1976;72:248-254.
- [Google Scholar]
- Physiological and proteomic analyses of the drought stress response in Amygdalus mira (Koehne) roots. BMC Plant Biol.. 2017;17:53.
- [Google Scholar]
- Effect of drought stress on gas exchange characteristics of four soybean genotypes. Bangladesh J. Agric. Res.. 2016;41(2):195-205.
- [Google Scholar]
- Physiological and proteomic responses of cotton (Gossypium herbaceum L.) to drought stress. Plant Physiol. Biochem.. 2012;53:6-18.
- [Google Scholar]
- Climate resilient crops for improving global food security and safety. Plant Cell Environ.. 2018;41:877-884.
- [Google Scholar]
- Stimulation effects of glutamic and 5-Aminolevulinic acids on photosynthetic pigments, physio-biochemical constituents, antioxidant activity, and yield of peanut. Gesunde Pflanz. 2022;74:915-924.
- [Google Scholar]
- Biological activity of vegetal extracts containing phenols on plant metabolism. Molecules. 2016;21:205.
- [Google Scholar]
- Ascorbic acid and thiols as potential biomarkers of ozone tolerance in tropical wheat cultivars. Ecotoxicol. Environ. Safety. 2019;171:701-708.
- [Google Scholar]
- Ecotypic morphological and physio-biochemical responses of two differentially adapted forage grasses, Cenchrus ciliaris L. and Cyperus arenarius Retz. to drought stress. Sustainability. 2021;13:8069.
- [Google Scholar]
- Investigation of proline, total protein, chlorophyll, ascorbate and dehydroascorbate changes under drought stress in akria and mobile tomato cultivars. Iranian J. Plant Physiol.. 2013;3:651-658.
- [Google Scholar]
- Potential applications of cyanobacteria: Spirulina platensis filtrates and homogenates in agriculture. World J. Microbiol. Biotechnol.. 2019;35:80.
- [Google Scholar]
- Rapid assay for determination of water-soluble quaternary ammonium compounds. Plant Soil. 1983;70:303-307.
- [Google Scholar]
- Effect of heavy metal Cd on some physiological and biochemical parameters of barley (Hordeum vulgare L.) Int. J. Agric. Crop Sci.. 2013;5(22):2743.
- [Google Scholar]
- The gasometric determination of free amino acids in blood filtrates by the ninhydrin-carbon dioxide method. J. Biol. Chem.. 1943;150:231-250.
- [Google Scholar]
- Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophy.. 1968;125:189-198.
- [Google Scholar]
- The role of phytohormones in alleviating salt stress in crop plants. Aust. J. Crop Sci.. 2011;5:726-734.
- [Google Scholar]
- Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem.. 2018;130:501-510.
- [Google Scholar]
- Phenolic constituents in the leaves of northern willows: methods for the analysis of certain phenolics. J. Agric. Food Chem.. 1985;33(2):213-217.
- [Google Scholar]
- Sulfur-enriched leonardite and humic acid soil amendments enhance tolerance to drought and phosphorus deficiency stress in maize (Zea mays L.) Sci. Rep.. 2020;10:6432.
- [Google Scholar]
- Khalid, M.F., Hussain, S., Ahmad, S., Ejaz, S., Zakir, I., Ali, M.A., Ahmed, N., Anjum, M.A. 2019. Impacts of Abiotic Stresses on Growth and Development of Plants. Plant Tolerance to Environmental Stress: Role of Phytoprotectants. CRC Press, USA pp. 1-8.
- A review of ascorbic acid potentialities against oxidative stress induced in plants. J. Agrobiol.. 2011;28:97-111.
- [Google Scholar]
- Can biochar improve pumpkin productivity and its physiological characteristics under reduced irrigation regimes? Sci. Hort.. 2018;247:195-204.
- [Google Scholar]
- Tocopherol biosynthesis: chemistry, regulation and effects of environmental factors. Acta Physiol. Plant.. 2012;34:1607-1628.
- [Google Scholar]
- Impact of foliar application of syringic acid on tomato (Solanum lycopersicum L.) under heavy metal stress-insights into nutrient uptake, redox homeostasis, oxidative stress, and antioxidant defense. Front. Plant Sci.. 2022;13:950120
- [Google Scholar]
- Drought-enhanced xylem sap sulfate closes stomata by affecting ALMT12 and guard cell ABA synthesis. Plant Physiol.. 2017;174:798-814.
- [Google Scholar]
- Effect of ascorbic acid application on physiology of wheat under drought stress. Pak. J. Agric. Sci.. 2015;52:209-217.
- [Google Scholar]
- Methionine-induced regulation of growth, secondary metabolites and oxidative defense system in sunflower (Helianthus annuus L.) plants subjected to water deficit stress. PloS One. 2021;16(12):0259585.
- [Google Scholar]
- Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot.. 2007;99(6):1161-1173.
- [Google Scholar]
- Changes in calcareous soil activity, nutrient availability, and corn productivity due to the integrated effect of straw mulch and irrigation regimes. J. Soil Sci. Plant Nutr.. 2021;21:2020-2031.
- [Google Scholar]
- Implications of water stress-induced changes in the levels of endogenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol. Plant.. 1983;58:166-170.
- [Google Scholar]
- Research progress and perspective on drought stress in legumes: A review. Int. J. Mol. Sci.. 2019;20(10):2541.
- [Google Scholar]
- Variations in quality constituents of green tea leaves in response to drought stress under south Indian condition. Sci. Hort.. 2018;233:359-369.
- [Google Scholar]
- Impact of ascorbic acid on growth and some physiological attributes of cucumber (Cucumis sativus) plants under water-deficit conditions. Pak. J. Bot.. 2016;48:877-883.
- [Google Scholar]
- Leaf extract of neem (Azadirachta indica) alleviates adverse effects of drought in quinoa (Chenopodium quinoa Willd.) plants through alterations in biochemical attributes and antioxidants. Saudi. J. Biol. Sci.. 2022;29(3):1367-1374.
- [Google Scholar]
- Photosynthetic capacity, ion homeostasis and reactive oxygen metabolism were involved in exogenous salicylic acid increasing cucumber seedlings tolerance to alkaline stress. Sci. Hort.. 2018;235:413-423.
- [Google Scholar]
- Extra-cellular but extra-ordinarily important for cells: apoplastic reactive oxygen species metabolism. Front. Plant Sci.. 2017;8:1353.
- [Google Scholar]
- The effects of seed priming by ascorbic acid on some morphological and biochemical aspects of rapeseed (Brassica napus L.) under drought stress condition. Int. J. Biosci.. 2014;4:432-442.
- [Google Scholar]
- Impact of exogenously applied tocopherol on some key physio-biochemical and yield attributes in mungbean [Vigna radiata L.) Wilczek] under limited irrigation regimes. Acta Physiol. Plant.. 2018;40:131.
- [Google Scholar]
- Drought stress effects on growth, ROS markers, compatible solutes, phenolics, flavonoids, and antioxidant activity in Amaranthus tricolor. Appl. Biochem. Biotechnol.. 2018;186:999-1016.
- [Google Scholar]
- Spatial variations in the biochemical potential of okra [Abelmoschus esculentus L. (Moench)] leaf and fruit under field conditions. PloS One. 2022;17:0259520.
- [Google Scholar]
- Does exogenously-applied trehalose alter oxidative defense system in the edible part of radish (Raphanus sativus L.) under water-deficit conditions? Sci. Hort.. 2015;185:68-75.
- [Google Scholar]
- Assessment of physio-biochemical indicators for drought tolerance in different cultivars of maize (Zea mays L.) Pak. J. Bot.. 2019;51:1241-1247.
- [Google Scholar]
- Influence of glycine betaine (natural and synthetic) on growth, metabolism and yield production of drought-stressed maize (Zea mays L.) plants. Plants. 2021;10:2540.
- [Google Scholar]
- Effect of abiotic stress under light and dark conditions on carotenoid content in pumpkin (Cucurbita moshata) calluses. J. Fundamental Appl. Sci.. 2018;9:861.
- [Google Scholar]
- Oxidative stress mitigation and initiation of antioxidant and osmoprotectant responses mediated by ascorbic acid in Brassica juncea L. subjected to copper (II) stress. Ecotoxicol. Environ. Safety. 2019;182:109436
- [Google Scholar]
- Variability assessment of okra (Abelmoschus esculentus (L.) Moench) genotypes based on their qualitative traits. Int. J. Agron.. 2021;6678561
- [Google Scholar]
- Temporal and spatial changes in gene expression, metabolite accumulation and phytohormone content in rice seedlings grown under drought stress conditions. Plants. 2017;90(1):61-78.
- [Google Scholar]
- Growth behavior of tomato (Solanum lycopersicum L.) under drought stress in the presence of silicon and plant growth promoting rhizobacteria. Soil Environ.. 2016;35(1):65-75.
- [Google Scholar]
- Role of oxidative damage in tulip bulb scale micropropagation. Plant Sci.. 1997;130:207-216.
- [Google Scholar]
- Oxidative stress and some antioxi-dant systems in acid rain treated bean plants: protective role of exogenous polyamines. Plant Sci.. 2000;151:59-66.
- [Google Scholar]
- Tolerance of photosynthesis to photoinhibition, high temperature and drought stress in flag leaves of wheat: A comparison between a hybridization line and its parents grown under field conditions. Plant Sci.. 2006;171(3):389-397.
- [Google Scholar]
- The estimation of carbohydrates in plant extract by anthrone. Biochem. J.. 1954;5:508-514.
- [Google Scholar]
- Influence of drought hardening on the resistance physiology of potato seedlings under drought stress. J. Integr. Agric.. 2018;17:336-347.
- [Google Scholar]
- Effect of foliar spraying of ascorbic acid on chlorophyll a, chlorophyll b, total chlorophyll, carotenoids, hydrogen peroxide, leaf temperature and leaf relative water content under drought stress in grapes. Bull. Environ. Pharmacol. Life Sci.. 2014;3:178-184.
- [Google Scholar]







