Translate this page into:
Immunoprophylactic potential of recombinant outer membrane protein of Salmonella enterica serovar Typhi against enteric fever in BALB/c mice
⁎Corresponding author at: Department of Biotechnology Lahore College for Women University, Lahore, Pakistan. Shagufta.Naz@lcwu.edu.pk (Shagufta Naz),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Objectives
This study was design to explore the immunogenic behavior of LamB and SpaO outer membrane protein of Salmonella serovar Typhi against enteric fever in BALB/c mice.
Methods
SpaO or LamB genes of locally isolated strain of S.Typhi was amplified, cloned and expressed. Recombinant protein SpaO and LamB were purified and lyophilized to prepare aluminum hydroxide Gel precipitated vaccine. Clinical trials were performed in BALB/c mice. The Immunoprophylactic potential of recombinant SpaO or LamB alone and in combination at different concentrations were determined using Complement fixation test along with whole culture attenuated and commercial vaccine.
Results
The immune response of rSpaO and rLamB proteins in combination at 500 µg/ml was significantly high at 48th day and maintained until 60thday. Protective immune potential of recombinant monovalent and multivalent vaccine assessed upon challenge in mice with 109 cells of S.Typhi virulent culture. Out of five, no mice could survive in control group although rSpaO and rLamB in combination at 500 µg/ml conferred 80 % protection. Whereas the monovalent rSpaO and rLamB vaccine elucidate protection 60 % or 40 % respectively.
Conclusion
From this study, it is conclude that combine vaccine rSpaO and rLamB effectively enhanced the immunological response and withstand the challenge of the causative agent more effectively as compared to the monovalent vaccines.
Keywords
Outer membrane protein
Immunological response
Recombinant vaccine
Salmonella Typhi
Complement fixation test
1 Introduction
Enteric fever is one of common infectious diseases of South East Asian countries like Pakistan (Yasin et al., 2018) and cause high rate of mortality among children and adults. For several years, the phenotypic trait of multidrug resistance (MDR) extensively distributed among S. Typhi (Eng et al., 2015). Multi drug resistance strains are present all over the world particularly in India, Vietnam or Pakistan instead of European countries (Ochiai et al., 2008). In 2015 approximately 17 million cases of paratyphoid and typhoid fever reported globally in Southeast Asia, South Asia or sub-Saharan African region and cause 2,50000 deaths reported each year in Pakistan (Mogasale et al., 2014). Vaccine is effective way to control the occurrence of diseases, Salmonella whole cell, live attenuated and recombinant vaccine are effective approaches to control the disease (Klemm et al., 2018). Salmonella outer membrane proteins are good candidate for vaccine preparation as it act as potential Immunoprophylactic agent and it induce good humoral and cellular response (Saxenan et al., 2017). First line for the treatment of enteric fever is ampicillin, trimethoprim-sulfamethoxazole and chloramphenicol have been used from many years (Crump et al., 2015). Currently limited vaccines for enteric fever are commercially available, nevertheless some limitations including short-term immunity and high cost have been reported (Singh et al., 2017).
OMPs: OmpF, OmpC, OmpL and OmpA that exhibit high immune response against typhoid (Yang, 2013). However, when in combination one or more OMP used for immunization a strong protein immunity can be achieved (Muthiadin et al., 2018). OMP 28 could be use in animal study for better protection (Saxena et al., 2012). OMP 28 comprises eight variable regions, which are found on the membrane outer side or having highest probability presented for recognition of B-cell and provoke immune response. For eliciting immune response, these variable regions highly predicted to act as possible B-cell epitopes (Arockiasamy et al., 2004). In human response, it was observe that after vaccination, activation of B or T cells were observed which showed, significantly, circulatory antibody- secreting cells or highest Omp-specific serum IgG titers. Likewise highest expansion of CD4+ T cells of OMP- specific in peripheral blood along substantial increase in production of TNFα or IFN-γ were also observe (Carreno et al., 2017). Immunization with 30 µg of OMPs showed 100 % protection when mice were challenge with two strains of S.Typhi (9,12,d,Vi and Ty2) upto 1,000 50 % lethal doses (LD50). Moreover, protection rate of 30 % have been attain when challenged with Salmonella Typhimurium up to 500 LD50 (Isibasi et al., 1988).
SpaO is a surface presentation antigen or performed invasion or adhesion of pathogen such as Salmonella Typhi, Paratyphi or Typhimurium. Foremost components of the sorting platform for Typhimurium SPI-1 injectsome: the AAA + ATPase InvC, its regulator SpaO, OrgB or OrgA protein identified by proteomic analyses (Hueck, 1998). Although for formation of the sorting platform SpaO have shown to be essential about its molecular structure (Bzymek et al., 2012). Whearse outer membrane protein such as maltoporin β -barrel play a vigorous role in adhesion, virulence or act as enzymes responsible for the uptake and metabolism of glucose polymers (maltodextrins) (Gromiha and Suwa, 2007). One of a porin named LamB that encodes an outer membrane protein had play their vital role in transport of maltodextrins maltose also in phage adsorption. It usually active in trimer form (Boulain et al., 1986). A huge assortment of LamB mutants have been utilize in order to probe roles of different parts of protein. It has shown that outer membrane protein of similar size of LamB (49 kDa) successfully expressed and purified (Kaur and Jain, 2013).
The current study was undertaken to determine the immune response of rLamB or rSpaO of outer membrane protein alone or in combination from local S.Typhi were tested in vivo at different concentration to draw a clear picture of their immunopotential so that data can be used in future for the clinical.
2 Materials and Methods
2.1 Ethical approval
Animal Right Committee of University of Veterinary and Animal Sciences, Lahore, Pakistan rendered the ethical approval for this work.
2.2 Collection of samples
Total fifty blood samples of patient affected with enteric fever were collected from tertiary care hospital of Sialkot, Punjab, Pakistan. Thirty samples isolated as positive for S. Typhi were confirm by commercial available kit Analytical Profile Index (API 20-E kit, BiOMerieux, France). For further confirmation of the strains 16S rRNA were perform. The sequence of primer listed in Table 1. After the amplification of the genes DNA extracted by using GeneJET Gel Extraction Kit (Thermo Fisher Scientific). Afterwards Gel electrophoresis excised the bands. In equal amount GB buffer was added in gel slice. About 5–10 min gel slice was melt at 55 °C. After that, samples were transfer to extraction column or at 10,000 rpm centrifuged for 1 min. Discard the flow throw. Wash buffer 750 µl was use for washing the column. Transfer the column to new centrifuge tube or in 50 µl distill water eluted the DNA.
Primers
Sequences
Length
Tm(Co)
GC
SpaO-F
5 ‘CGCCATATGTCATTGCGTGTGAGACAG-3
27
48
50
SpaO-R
5 ‘CTCGAGTTCCCCATTACCAGACTC-3
24
47
50
LamB-F
5 ‘CATATGATGATTACTCTGCGCAA-3
23
42
41
LamB-R
5 CTCGAGTTACCACCAGATTTCCA3
23
43
41
Hundred male mice BALB/c (weight 18–20 g) recruited from National Institute of Health (NIH), Islamabad Pakistan. The mice were kept at the animal house of University of Veterinary and Animal Sciences, Lahore, Pakistan (UVAS) and provided the recommended feed or water under control conditions.
2.3 Amplification, expression and purification of outer membrane protein
Target genes SpaO and LamB (APV96323, AYT88671) amplified and cloned into pTZ57R/T cloning vector (Thermo Fisher USA) and expressed in pET28a expression vector (Takara/Clontech). Prokaryotic expression system pET28a-SpaO-E.coli BL21 (DE3) and pET28a-LamB-E.coli BL21 (DE3) were induced with 0.5 mM IPTG in LB medium to express the SpaO and LamB proteins. Prior to immunization, prokaryotic expression system pET28a-SpaO-E.coli BL21 (DE3) and pET28a-LamB-E.coli BL21 (DE3) were induced with 0.5 mM IPTG in LB medium to express the SpaO and LamB proteins.
Resuspended BL21 (DE3) with recombinant protein for lysis of bacteria using binding buffer (20 mM Tris-Cl, 20 mM Imidazole, 250 mM NaCl) of pH 8.0 or exposed to sonication for 3 min with 5 sec on and 8 sec pulse off. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS- PAGE) 12 % was use for visualization of samples. The cell pellets were washed at 4 °C for overnight. Resuspend the IB pellet of SpaO using 30 ml of buffer with 8 M urea pH 8.0 (10 mM Tris-Hcl, 100 mM NaH2PO4, 100 mM NaCl, 8 M urea) on the other hand for LamB used solubilization buffer with 6M, 8M or 10 M urea after that stirred for overnight at 37 °C on magnetic stirrer and next day centrifuged at 4 °C at 15000 rpm for 30 min. Supernatant was collected. 3 M urea was used for dialysis the samples for overnight. Ni- NTA chelating Sapharose column were used for purification of rLamB and rSpaO (Hochuli, 1990). After that equilibrated the column for 2–3 h with 20 ml of an IB solubilization buffer. The supernatant were bind with the column. Then using the wash buffer (20 mM Tris-Hcl, 250 mM NaCl, and 50 mM imidazole) the column was washed. SpaO and LamB was eluted by the use of elution buffer (20 mM Tris-Hcl, 250 mM NaCl, 250 mM imidazole), (20 mM Tris-Hcl, 250 mM NaCl, 150 mM imidazole). Collected 1 ml fractions or examined with 12 % SDS-PAGE. Ni-NTA affinity chromatography was use for purification of recombinant proteins. Purified protein was lyophilize for vaccine formulation. Protein concentration was measurement by taking absorbance at 280 nm on spectrophotometer and OD at 280 considered 1 mg/ml. Spectrum was very clear and only one peak at 280 nm that shows the purity of protein.
2.4 Formulation of aluminum hydroxide gel recombinant vaccine
Recombinant vaccines based of Aluminum Hydroxide Gel (AHG) were prepare (Peng, 2015). The adjuvant used for human that approved worldwide was alum (Harandi et al., 2010). To separate the water from the gel 15 ml of alum gel were centrifuge for 5 min then mixed with recombinant protein. The Aluminum Hydroxide Gel (AHG) was prepared with the addition of Aluminum Ammonium Sulphate 800 g in 1000 ml carbonated water comprising Sodium Carbonate (Na2CO310H2O) of 1000 g. Using daily manual of 2 h stirring all gases produced were remove. The suspension was washed using cold distilled water 6 time by centrifugation. Mixed the collected pellet with vaccine suspension with rate of 2 %. In order to obtain homogenized suspension stirred the suspension for 15 min at 300 rpm.
2.5 Commercial vaccine formulation
The commercial vaccine (Vi Capsular Polysaccharide Typhoid Vaccine, AMSON PHARMA (PVT) LTD) injected in the mice at the rate of 0.1 ml per mice. Mixed with phosphate buffer saline of 0.5 ml Typbar vaccine or 0.1 ml to each 5 mice were inject.
2.6 Preparation of whole cell culture vaccine
Total viable bacterial count estimated in the purified suspension as described by Miles and Misra, 1938. Briefly, sterilized 10 ml nutrient broth in test tubes. Prepare tenfold dilutions of the stock culture (10-1 to 10-10). Finally, poured one ml of each concentration on Salmonella Shigella agar on petri plates. Then incubated the plates at 37 °C for 24 h.
Determined the bacterial count in the culture with the following formula.
Added 2.5 ml of formaldehyde (0.5 %) in culture flask for inactivation of bacteria or incubate at 37 °C for 24 h on shaking. After 24 h inactivation of cells confirmed on SS agar plates.
2.7 Vaccination trial in BALB/c mice
Five different groups were design to investigate the immunogenic potential of the vaccine. Each experimental group comprises of five mice. The groups were; Group 1 (rSpaO), Group 2 (rLamB), Group 3 (Combine rSpaO + rLamB), Group 4 (Typbar Commercial vaccine) and Group 5 (Whole culture vaccine S.Typhi). Five different concentration of recombinant outer membrane protein for each group were used (500, 250, 125, 60 and 30 µg/ml) respectively. One group was taken as control and adjuvant phosphate buffer saline (PBS) was injected. While group 1, 2 and 3 subcutaneously injected with recombinant proteins of different concentrations along with adjuvant aluminum hydroxide gel (AHG) (final concentration of 2 %) followed by booster at 14th day after the primary dose.
2.8 Collection of blood samples
Blood samples was collected and serum was obtained by centrifugation on 0, 14, 28, 48, 60 and 90 days post primary dose and stored at −80 °C for further analysis. Evaluation of antibody titer performed using Complement fixation test (CFT) (Boulain et al., 1986).
2.9 Challenge study
For challenge study, used 24 h broth bacterial culture. The viable bacterial were count by dilution pour plate. Bacterial suspension (S.Typhi) with 2 x109 CFU/ml was used (Adone et al., 2016). 2.5 ml of formaldehyde (0.5 %) was use for the inactivation of bacteria and incubated on shaking at 37 °C for 24 h. 1 ml inactivation culture poured on plates and incubated at 37 °C for 24 h. The control was inject with 0.2 ml of culture as well as in the experimental groups of mice. For seven days, post challenge mice observed.
2.10 Complement fixation test (CFT)
Briefly, poured 50 ml of PBS in all wells of the round bottom 96 well plate. 50 µl of the respective sample added in the first wells of first column and sample were diluted upto 10th wells. 50 µl of 96 well plate (row wise 1–12) serum samples was poured in first well and diluted in 2–4 pattern upto 9th well. Next 50 µl of the antigen added in all wells 1-10th. Incubated the plate for 15 min at 37 °C for antigen antibody reaction. After incubation, 50 µl of Guinea pig complement added from 1 to 12th well. Lastly 50 µl sensitized RBCs (RBCs react with amboceptor’s) was poured in each well plate and incubated for 2 h at 37 °C. The formation of RBCs in the bottom of the well was representative of positive sample (positive for antibody). Whereas the hemolysis was the indication of negative result.
2.11 Statistical analysis
The statistical significance of data was find out using One- way and two way analysis of variance (ANOVA) by means of SPSS program version 20 (International Business Machines Corporation) and at p ≤ 0.05 significance was tested. Presented the results as mean values ± standard error, and selected for each condition three independent replicates.
3 Results
The sample streaked on Salmonella-shigella agar (SSagar) which was positive for Salmonella. Blackish center colonies were observe on SSagar specifying that the sample was positive for Salmonella. Bacilli gram-negative appearance was indicate the confirmation of Salmonella. Further confirmation of suspected Salmonella enterica were carried out with biochemical test using API (E-20) Kit. Positive samples were further confirm by 16SrRNA PCR for molecular characterization.
3.1 In vivo immune response assay
Complement fixation test (CFT) used to determine the serum antibody titer. Different vaccine groups antibody titer rSpaO, rLamB, combine (rSpaO and rLamB), commercial vaccine or whole culture at all concentrations shown in Fig. 1. After booster dose on 14th day immune response was highly significant (P < 0.01). Whereas it declined on 90th day post vaccination. At concentration 500 µg/ml, the serological comparison of commercial Typbar and combine (rSpaO and rLamB) vaccine concluded that vaccine in combination (rSpaO and rLamB) is relatively effective same as commercial Typbar vaccine. When the result was statistically analyzed particularly with in the groups it was concluded that antibody titer of whole culture vaccine was significantly higher (P < 0.01) at all concentrations as compared to monovalent rSpaO, rLamB, combine (rSpaO and rLamB) or commercial Typbar vaccine on 48th and 90th day post priming. Whereas, immune response of combine vaccine with respect to monovalent vaccine is significantly better. Antigens when used in combination synergize each other by producing better immune response on 48th and 60th day (Fig. 1). Moreover, when statistically analyzed vaccine in combination of 500 and 250 µg/ml concentration it was concluded that both were immunogenically producing same antibody titer with no significant difference. However, upon 125, 60 and 30 µg/ml concentrations, the combine vaccine (rSpaO and rLamB) produced better efficacy on 48th and 60th day than respective monovalent rSpaO and rLamB vaccine.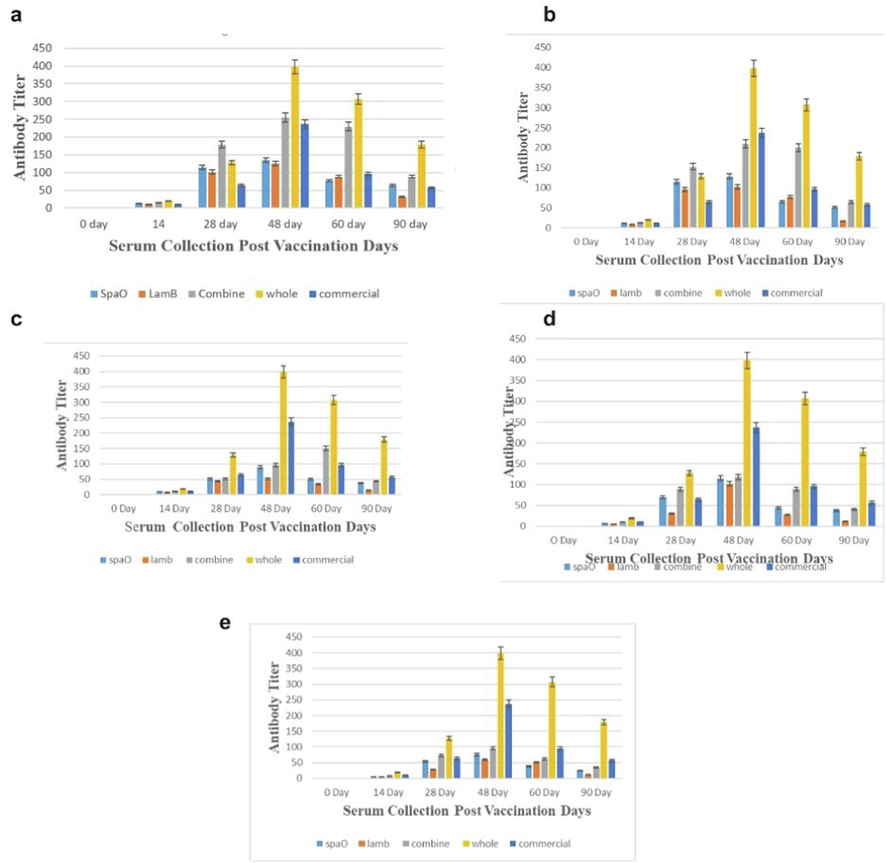
Immune response of different vaccines at concentration 500, 250, 125, 60 and 30 µg/ml: a) rSpaO, b) rlamB, c) combine (rSpaO and rlamB), d) whole culture vaccine and e) commercial vaccine against S.Typhi.
3.2 Challenged study against S. Typhi
Protective immune potential of different recombinant vaccine in BALB/c mice evaluated by challenge study for which challenged the vaccinated groups of mice with pathogenic field isolate of Salmonella Typhi (2x109) subcutaneously. Immune response of combine (rSpaO and rLamB) and monovalent vaccine evaluated against challenge of S. Typhi. Results shown in Table 2. In all vaccinated group no mice could survive at 14th day. At 500 µg/ml percentage of protection on 60th day was 60 %, 60 %, 100 %, 100 % and 80 % of rSpaO, rLamB, combine (rSpaO and rLamB) for whole culture vaccine or commercial Typbar vaccine. Whereas the protection rate was 60 %, 40 %, 80 %, 100 % and 80 % on 90th day at rSpaO, rLamB, combine (rSpaO and rLamB) for whole culture vaccine and commercial vaccine Typbar. Although the percentage protection of recombinant combine vaccine at 500 µg/ml was high (80 %) with respect to others (Table 2).
Sr. No
Vaccine type (APV)
Concentration
Death
14th day protection
% age Protection
Death
60th day protection
% age Protection
Death
90th day protection
% age Protection
1
rSpaO
500 µg/ml
5
0
0
1
3
60
2
3
60
2
rLamB
500 µg/ml
5
0
0
2
3
60
2
3
60
3
Combine (rSpaO and rLamB)
500: 500 µg/ml
5
0
0
0
5
100
1
4
80
4
Whole culture vaccine
109 CFU/ml
5
0
0
0
5
100
0
5
100
5
Commercial vaccine(reference vaccine)
0.025 mg
5
0
0
1
4
80
1
4
80
6
rSpaO
250 µg/ml
5
0
0
2
3
60
2
3
60
7
rLamB
250 µg/ml
5
0
0
2
3
60
2
3
60
8
Combine (rSpaO and rLamB)
250: 250 µg/ml
5
0
0
1
4
80
2
3
60
9
rSpaO
125 µg/ml
5
0
2
2
40
4
1
20
10
rLamB
125 µg/ml
5
0
0
2
2
40
4
1
20
11
Combine (rSpaO and rLamB)
125: 125 µg/ml
5
0
0
1
3
60
3
2
40
12
rSpaO
60 µg/ml
5
0
0
2
1
20
4
1
20
13
rLamB
60 µg/ml
5
0
0
3
1
20
4
1
20
14
Combine (rSpaO rLamB)
60:60 µg/ml
5
0
0
1
2
40
3
2
40
15
rSpaO
30 µg/ml
5
0
0
3
1
20
5
0
0
16
rLamB
30 µg/ml
5
0
0
2
1
20
5
0
0
17
Combine (rSpaO rLamB)
30: 30 µg/ml
5
0
0
3
2
40
4
1
20
3.3 Immuno comparison of different vaccine between the groups
To access the appropriate concentration of rSpaO, rLamB, combine (rSpaO and rLamB). They were compared with each other in kill cross manner means rSpaO × rLamB, rSpaO × combine and rLamB × combine. Selected the concentration in order to comparison at which percentage protection was above 50 % and there were 500 and 250 ug/ml per dose. Vaccine in combination at 250 ug/ml each showed protection as compared to rSpaO 250 ug/ml or LamB 250 ug/ml per dose vaccine. In challenged study percentage, protection was 80 % and 60 %. Vaccine at concentration of 500 ug rSpaO is highly protected than 250 rSpaO that showed rSpaO or rLamB in combination at 250 ug/ml result equal or better to the rSpaO vaccine 500 ug/ml per dose.
4 Discussion
Enteric fever, caused by Salmonella enterica serovars, is a foremost public health challenge for the developing nations (Das et al., 2015). Although in Pakistan with respect of Salmonella multidrug-resistant strains occurrence its management become more complex due to increased survivability, communicability as well as virulence as a result there is an increased ratio of mortality and morbidity (Qamar et al., 2014). Presently there is no local vaccine available, which could protect against S. Typhi infection in Pakistan. Therefore, it is essential that from the local isolate the effective vaccine formulated. In current study, two recombinant outer membrane proteins of Salmonella expressed and formulated to check their immune response in vivo.
In the present study, it were concluded that antibody titer at concentration of 500 and 250 µg/ml of rSpaO reached to peak on 48thday and remained significantly higher until 60thday, was decline on 90th day. It is reveal that vaccine antibody titer at different phases because of the reason that an extra follicular response had been observe with the stimulation of initial exposure of vaccine antigen. Due this response, there is a quick appearance of immunoglobulins or formerly immune response starts to build up (Mendes et al., 2018). When there is a proliferation of B cells occurs and differentiate into plasma cells, as a result there is an increase of immunoglobulins titers up to a peak value. Due to these plasma cells there are fast decline in antibody titers. Though, when secondary immune response happen by means of booster exposure to antigen, reactivation of immune cells had been observed, so there is a rapidly raise in immunoglobulins level (Clem, 2011). During a few weeks, peak antibody levels maintain by means of short-lived plasma cells. Subsequently, initially there is a decline in antibody titer with an equal quick kinetics as following primary immunization (Margolick et al., 2006). With long-lived plasma cells, antigen specific antibodies produced continuously which extended survival niches in the bone marrow that eventually decline by slower kinetics. These explanations are in line with Ruan et al. (2008) they used rSpaO vaccine doses of 500 or 250 µg/ml and injected into rats subcutaneously. While compared the antibody titer of monovalent vaccine it was conclude that rLamB vaccine showed less efficacy as compared to rSpaO at same concentration. Through, in zebra fish vaccination or challenge with heterogeneous virulent Vibrio strain Lun et al. (2014) evaluated cross protective property of rLamB. Afterwards, immunization with rLamB protein, it revealed that from Vibrio infection zebra fish showed protected. From this study it was concluded that LamB was a conserved antigen between the tested Vibrio species and it might be used as a better potentially vaccine candidate for Vibrio’s. These results are in line with Hamid and Jain, (2008) 49 kDa OMP of S.Typhimurium is highly immunogenic and evoked both humoral and cell mediated response while upon challenge with high doses (upto 100 times the 50 % lethal dose) to immunized rates (upto 100 times the 50 % lethal dose) then the protection percentage was 100 %. In combination, (rSpaO and rLamB) antibody titer was high as related with particular monovalent vaccines. Our findings are in line with Jiang et al. (2012) when mice were challenge with S. Paratyphi A, 66.7 % and 83.3 % percentage protection have been showed by 100 μg or 200 μg of rSpaO-OmpA. Immune protective rates produced were suggestively higher as compared of equal rSpaO or rOmpA (P < 0.05). Agglutination titers 1:5–1:40 presented from sera of rSpaO-OmpA immunized mice to the different S. paratyphi species H antigens while for proteins rSpaO, rOmpA or rSpaO-OmpA immunodiffusion titers were 1:1–1:16 respectively.
Immune stimulatory is Salmonella OmpA as exposed with IFN-g production stimulation or in Salmonellosis have a vital role in modulation of the immune response on the other hand MHC expression enhanced as well as co– stimulatory molecules in T cells or dendritic cells (Jang et al., 2013). Protection level enhanced when the mice were immunize with two antigens as compared to monovalent rSpaO or rH1a against subsequent infection (Ruan et al., 2008). rOmp and rOmF of S.Typhi are more immunogenic protein in murine models and enhanced the cell-mediated immunity (Verma et al., 2010). On the basis of findings of earlier workers we can conclude that recombinant combine outer membrane protein are conserved and it can be used as a strong candidate for vaccine development against S.Typhi.
Subsequently, this study suggests that against typhoid fever recombinant combine vaccine (rSpaO and rLamB) formulation with alum based as an adjuvant provoked long lasting immunity. Live vaccines induced strong immune protection attributed to the probability that natural infection may imitate by live vaccine with secreted proteins or therefore naturally evoke the immune response of the host (Jang et al., 2013). Salmonella is widely use in live attenuated vaccine and its protection can be improved by using engineered strains of Salmonella (Periaswamy, et al., 2012). Nevertheless, numerous live attenuated vaccines are limited by their essential ability to access systemic organs in many of the vaccinated hosts, particularly those which are immunocompromised (Vishwakarma et al., 2012). Whole cell vaccine found to be short lived usually of two or three years. In small percentage of infants the encephalitis-type reaction occurred receiving the whole-organism pertussis vaccine, these vaccines for pertussis have led to development of a new cellular vaccine (Zaitsev, 2013). Furthermore, certain vaccinated individuals developed a hypersensitivity reaction (Taylor and Francis, 2008). To overcome these disadvantages, as an alternative a non– toxic recombinant vaccine has developed.
Most Whole culture vaccines attenuated which are using today with adapting the wild type virus to a new environment (e.g. replication in a novel cell line or low temperature) attained their reduced virulence along with replication rate reduced in humans. For pathogens such as HTV the usage of attenuated vaccines is hazardous so there is a need to develop a recombinant vaccine with better efficacy where pathogen genes are successfully expressed from a benign virus vector along with immunogenic activity (Bull et al., 2019).
It is suggested from this study that whole cell vaccine is LPS based and short lived and need repeated booster doses, to overcome this problem recombinant combine vaccine containing rSpaO and rLamB can be produced an ideal solution for a long-term immunity.
5 Conclusion
The findings from the study suggested that outer membrane protein could be protective vaccine candidate when used in combination. In order to explore different aspects of enteric fever. This study can be broaden with different assessments that may comprise exploration of immunological behavior of combine and conjugated vaccine of outer membrane protein. A new effective vaccine with better efficacy could be launched if the study extended to the phase 2, 3 and 4 in biological developments.
Acknowledgments
All the authors acknowledge and thank their respective Institutes and Universities.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Development of a sterne-based complement fixation test to monitor the humoral response induced by anthrax vaccines. Front. Microbiol.. 2016;7:19.
- [Google Scholar]
- Conformational epitope mapping of Omp C, a major cell surface antigen from Salmonella typhi. J. S. Biol. 2004;148:22-33.
- [Google Scholar]
- Mutagenesis by random linker insertion into the LamB gene of Escherichia coli K12. M.G.G.. 1986;205:339-348.
- [Google Scholar]
- Two translation products of Yersinia yscQ assemble to form a complex essential to type III secretion. Biochem.. 2012;51:1669-1677.
- [Google Scholar]
- Evolution of Salmonella Typhi outer membrane protein-specific T and B cell responses in humans following oral Ty21a vaccination: A randomized clinical trial. PLoSONE. 2017;12:0178669.
- [Google Scholar]
- Crump, J. A., Sjolund-Karlsson, M., Gordon, M.A., Parry, C.M. 2015. Epidemiology.
- Salmonella: A review on pathogenesis, epidemiology and antibiotic resistance. Front. Biol.. 2015;8:284-293.
- [Google Scholar]
- Gromiha, M.M., Suwa, M. 2007. Current developments on β-barrel membrane proteins. Curr. Protein P. Sci. 8, 580-599.
- Characterization of an outer membrane protein of Salmonella enterica Serovar Typhimurium that confers protection against Typhoid. Clin. Vaccine Immunol.. 2008;15:1461-1471.
- [Google Scholar]
- Purification of recombinant proteins with metal chelate adsorbent. J. Genet Eng.. 1990;12:87-98.
- [Google Scholar]
- Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol. Mol. Biol. Rev.. 1998;62:379-433.
- [Google Scholar]
- Protection against Salmonella typhi infection in mice after immunization with outer membrane proteins isolated from Salmonella typhi 9, 12, d, Vi. Infect. Immun.. 1988;56:2953-2959.
- [Google Scholar]
- Dendritic cells stimulated with outer membrane protein A (OmpA) of Salmonella typhimurium generate effective anti- tumor immunity. Vaccine. 2013;29:2400.
- [Google Scholar]
- Generation of SpaO-OmpA fusion gene of Salmonella paratyphi A and the immunoprotection of expression product of the fusion gene. J. Microbiol. Immun. Infect.. 2012;32:152-156.
- [Google Scholar]
- High level expression of 49kDa outer membrane protein of Salmonella enterica serovar typhi. Ann. Biol. Res.. 2013;4:107-117.
- [Google Scholar]
- Emergence of an extensively drug-resistant Salmonella enterica Serovar typhi clone harboring a promiscuous plasmid encoding resistance to fluoroquinolones and third- generation cephalosporins. ASM. 2018;9:105-118.
- [Google Scholar]
- The outer membrane protein, lamB (maltoporin), is a versatile vaccine candidate among the Vibrio species. Vaccine. 2014;32:809-815.
- [Google Scholar]
- Margolick, J.B., Markham, R.B., Scott, A.L. 2006. Infectious Disease Epidemiology: Theory and Practice. Chapter 10. In: Nelson KE, Masters CF, editors. The immune system and host defense against infections. Boston: Jones and Bartlett: Pp. 317-43.
- Pattern recognition receptors and the host cell death molecular machinery. Front. Immunol.. 2018;9:2379.
- [Google Scholar]
- The estimation of the bactericidal power of the blood. Am. J. Hyg.. 1938;38:732-749.
- [Google Scholar]
- Mogasale, V., Maskery, B., Ochiai, R. L., Lee, J. S., Mogasale,V.V., Ramani, E., Kim,Y. E., Park, J. K., Wierzba, T. F. 2014. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment.
- Muthiadin, C., Aziz, I.R., Hatta, M., Nasrum, M. 2018. Immunoreactivity of 36kDa Outer Membrane Proteins (OMP) Salmonella enterica serovar Typhi as Candidate Immunodiagnostic for Typhoid Fever. Int J. Pharm. Sci Res. 10.
- A study of typhoid fever in five Asian countries: disease burden and implications for controls. Bull. Health Organ.. 2008;86:260-268.
- [Google Scholar]
- A simple method to prepare super amphiphobic aluminum surface with excellent stability. Colloids Surf. A Physicochem. Eng. Asp.. 2015;481:143-150.
- [Google Scholar]
- Periaswamy, B., Maier, L., Vishwakarma, V., Slack, E., Kremer, Michael, M., Andrew, J. G., Suar, M., Hardt, W.D. 2012. Live Attenuated S. Typhimurium Vaccine with Improved Safety in Immuno Compromised Mice. PLoS ONE.7, 45433.
- A three-year review of antimicrobial resistance of Salmonella enterica serovars Typhi and Paratyphi A in Pakistan. J. Inf. Dev. Ctries.. 2014;8:981-986.
- [Google Scholar]
- Recombinant SpaO and H1a as immunogens for protection of mice from lethal infection with Salmonella paratyphi A: Implications for rational design of typhoid fever vaccines. Vaccine. 2008;26:6639-6644.
- [Google Scholar]
- Cloning, sequencing, and in silico characterization of Omp 28 of Salmonella Typhi (strain MTCC 733) to develop r-DNA vaccine for typhoid fever. J. Nat. Sci. Biol.. 2012;3:133-138.
- [Google Scholar]
- Vaccination with Salmonella typhi recombinant outer membrane protein 28 induces humoral but non-protective immune response in rabbit. Vet. World. 2017;10:946-949.
- [Google Scholar]
- Immunogenic outer membrane proteins (Omps) of Salmonella: Potential candidate for sub-unit vaccine. Virol. Immunol. J.. 2017;1:2.
- [Google Scholar]
- Taylor., Francis. 2008. Use of Madin Darby canine kidney cells for the manufacture of live, attenuated influenza vaccines Briefing document, MedImmune, Vaccines and Related Biologicals Advisory Committee (VRBPAC). CBER.
- Verma, S., Thakur, S., Kanga, A., Singh, G., Gupta, P. 2010. Emerging Salmonella ParatyphiA enteric fever and changing trends in antimicrobial resistance pattern of salmonella in Shimla. IJMM.28, 51-53.
- Identification and characterization of Omp L as a potential vaccine candidate for immune protection against salmonellosis in mice. Vaccine. 2013;31:2930-2936.
- [Google Scholar]
- Epidemic process and vaccine prophylaxis of pertussis. Zhurnal Mikrobiol. Epidemiol. Immunobiol.. 2013;3:103-110.
- [Google Scholar]
Further reading
- Salmonella typhi outer membrane protein STIV is a potential candidate for vaccine development against typhoid and paratyphoid fever. Immunobiol.. 2019;224:371-382.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jksus.2023.102983.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







