Translate this page into:
Immune response in the larva of the dung beetle Phyllognathus excavatus against human blood cells as foreign bodies
⁎Corresponding authors. uesmif6@gmail.com (Fatma Guesmi), amsallagui@yahoo.fr (Mohamed Salah Allagui)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The dung beetle Phyllognathus excavatus (Coleoptera: Scarabaeidae) is a widespread and common species throughout its distribution (North Africa, southern Europe, and Asia). The larvae remain most of their life in compost-rich soil feeding on organic matter. This report aimed to identify and describe the larval immune effector cells (hemocytes) types and to investigate their immunological responses. For this, hemolymph was collected to investigate the different circulating cells using both light and scanning electron microscopy. The Larval hemolymph cells were identified and hemocytes immune responses under-reacting with human blood cells was investigated. The hemogram parameters were analyzed in the larval hemolymph, mixture of larval hemolymph and human blood, and healthy adult man blood (as a reference). Experiments showed that granulocytes, plasmatocytes, and coagulocytes were involved in the first line in the lysis of human red blood cells. Results showed that the larvae present also a great number of platelets-like and lymphocytes-like. Besides, the majority of the human red blood cells were killed under the larval immune defense. Thus, the content of human red blood cells was reduced by 75 % when mixed with the larval hemolymph whose student test showed a significant decrease (α < 0.01). Our findings, validated through immunological responses induced by larvae hemocytes towards human bood cells, show that hemocytes from scarab larvae, involved in immune defense mechanisms, can defend against infections by foreign pathogens and offer an insight into the novel application for mammal diseases.
Keywords
Phyllognathus excavatus larvae
Hemolymph cells
Immune defense
Coagulocytes
Granulocytes
Plasmatocytes
Human blood cells lysis
1 Introduction
Insects have a complex and powerful innate immune system composed of humoral and cellular defense responses. Humoral defenses consist of a cascade of enzymes that regulates hemolymph coagulation, production of reactive intermediates and antimicrobial peptides (Lavine and Strand, 2002; Pereira et al., 2018). While, cellular defense refers to phagocytosis, nodule formations, apoptosis, and encapsulation mediated by hémocytes (Sheehan et al., 2018). The principal function of the hemocytes is to ensure a protection against the infectious agents. Indeed these active cells of the immune system are able of recognition and to eliminate microbes and other foreign bodies through several cellular activities. They act in coordination with the humoral factors to answer the attacks of the invaders. Hemocytes recognize pathogenic microorganisms through the interaction of their pathogen recognition proteins (PRRs) with pathogen-associated molecular patterns (PAMPs) of the foreign body or, by detecting humoral immune effectors. In the event that a body is recognized as foreign, the defense responses promoted by hemocytes are controlled by signaling factors and effector molecules that mediate cytotoxicity and cell adhesion, with phagocytosis being the center of the innate immune response (Lavine and Strand, 2002). The main types of hemocytes are, plasmatocytes, granulocytes, spherule cells, prohemocytes and oenocytoides (Ribeiro and Brehélin, 2006; Strand, 2008). But, the immune roles of hemocytes vary among species, infection threats, and life cycle stadium (Negri et al., 2014). The hemocytes responsible for phagocytosis vary between insects. Several studies have shown that plasmatocytes and granulocytes are involved in the phagocytosis process (Amaral et al., 2010; Giglio et al., 2015). Other hemocyte types are respnsables for this ocucupation, such as oenocytoides and prohemocytes (Giglio et al., 2008; Giulianini et al., 2003). Oenocytoids play also an important role with melanizing capsules in clotting as injuries become melanized (Lavine and Strand, 2002). Prohemocytes are considered as stem cells that can differentiate into different other types of hemocyte types (Lanot et al., 2001).
In insects, hemocytes are well known by their role in innate immune system but they are also responsible for the aggregation and the coagulation of the hemolymphe. They are intimately linked with wound healing (Götz and Boman, 1985; Nappi and Vass, 1993) and they have the ability to product several proteins at tissue damage that regulate the clot formation. Clotting occurs in three steps: Adhesion of hemocytes to form cellular plags, hemocyte agglutination, and the coagulation of hemolymphe (Hose et al., 1990). These characteristics suggested that during evolution, at some stage, hemocytes diverged into more specialized cells such as phagocytes and platelets. This seems to be the reason why platelets have numerous functions related to immunity and why these functions are always active (Semple and Freedman, 2010). Both insects and mammals share an ancestral innate immune system that includes humoral and cellular responses (Krautz et al., 2014). In mammals, the fundamental role of platelets is well known in both hemostasis and thrombosis. While the importance of platelets in hemostasis and thrombosis has been well known for decades, there is a wide range of new reports showing the involvement of platelets in immune responses (Jenne et al., 2013; Leslie, 2010). Platelets are small in size (about 4 µm) and enucleated cytoplasmic bodies present in the circulation (about 200 000/µL bloods in humans). One platelet has around 60 granules that store many inflammatory molecules and mediators with immune functions (Rumbaut and Thiagarajan, 2010). Four types of granules are known yet: α-granule, dense granules, lysomal granule and T-granules. Granules stored a wide array of mediators include adhesion molecules, cytokines, chemokines, histamines, defensins. Due to these molecules, platelets have the ability to stick to leukocytes and facilitate their mobilization to the sites of injury. They play a central role in pathogens capture, sequestration, encasing and internalization (Elzey et al., 2005; Klinger and Jelkmann, 2002; Yeaman and Bayer, 2006). Importantly, recent work has identified the mechanism by which platelets contribute the destruction of pathogens through soluble mediators (Love et al., 2012; McMorran et al., 2012; McMorran et al., 2009). In insects, coagulocytes were the well-known type of cell-based coagulation. They easily discharge their cytoplasm and extrude cytoplasmic granules on contact with foreign surfaces (Giulianini et al., 2003; Grégoire, 1984).
The scarab beetle Phyllognathus excavatus (Coleoptera: Scarabaeidae) is widespread and very common species throughout its distribution range. It is known from over Palearctic region (Ghannem et al., 2016; Joaquín, 2016). In Tunisia the species is abundant in forests and in rural zones observed mostly in summer. Its larvae remain most of their life (fall, winter and spring), in soil feeding on organic matter. In South Tunisia, they are often evident in rural zones nearby livestock dung (compost-rich soil). The larva is white bluish, curved, “C” shaped slow moving and rather large.
In this regard, the report evaluated herein is designed to illustrate some of the different hemolymph cells circulating in the larva of P. excavatus and then to determine among them the various cell types involved in the immune defense against foreign materials by a mixture with human blood (human red blood cells as a target). The aim of the present paper is to demonstrate the ability of the scarab larval immune to defend themselves against foreign bodies through their behavior towards the cellular elements figured in human blood (erythrocytes, leukocytes, platelets), and to estimate the content of certain hemolymph cells types using light and scanner microscopy, and automated analyzer.
2 Materiel and methods
2.1 Identification
The identification of the dung beetle was conducted based on characteristics and diagnostic keys available in the literature (original descriptions): (Forster, 1771; Janssens, 1942; Paulian, 1941), and in comparison with the Phyllognathus species depicted in the following papers (Al-Ali et al., 2015; Errouissi et al., 2009; Márquez-Rodríguez, 2016).
2.2 Sampling
Numerous larvae of the scarab beetle Phyllognatus excavatus (Coleoptera: Scarabaeidae) were collected from rural zone (west southern of Tunisia) and reared in moistened soil in 10 L plastic tanks at ambient temperature. Larvae reaching the last instar (L3) were selected for the assays. The hemolymph was obtained by pricking the larvae with needles.
2.3 Light and scanning electron microscopy analysis in larval hemocytes
For light microscopy, pooled of hemolymph was collected by syringe after previously perfusing the thorax of larva with heparin as anticoagulant (Grégoire, 1953). Hemolymph dropped on glass slides and allowed to settle for some minutes in natural air condition. Cells were fixed in methanol for 10 min. Hemocytes were then stained with May-Grunewald-Giemsa stain and slides were quickly washed with demineralized water. After room temperature, the slides were dehydrated with ethanol.
To support the light microscopy and to know the exact sizes and shapes of certain cells, scanning electron microscopy was used. For SEM, hemolymph was obtained by picking the larvae with needles and placed directly on fragments of slides (Richardson et al., 2018). These were then allowed to dry in room temperature for some minutes and carried out under SEM.
To investigate the reaction of hemocytes on human blood, a drop of blood was added to the hemolymph placed on slides and the same steps described above were followed.
2.4 Hematology analysis
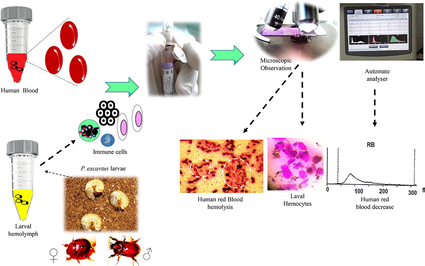
So that, to more improve results obtained under microscopy, automated hematology analyzes were carried out. Samples were processed in the Mindray BC-3600 analyzer. Thus, to investigate whether larval hemolymph contains small cells (lymphocytes-like) and platelets-like, hemolymph sample was collected in EDTA tube and analyzed using the automated analyzer. To estimate the rate and to ensure the type of eliminated cells after mixing larval hemolymph and human blood, a mixture of 2 mL of hemolymph with 2 mL of blood was softly agitated in an EDTA tube for some seconds and then brought to the analysis using the automated. A complete blood count of a healthy adult man was performed using the automated as a reference to compare the results. Proposed in vitro screening of insect immune pathway and mechanistic testing is shown in Fig. 1.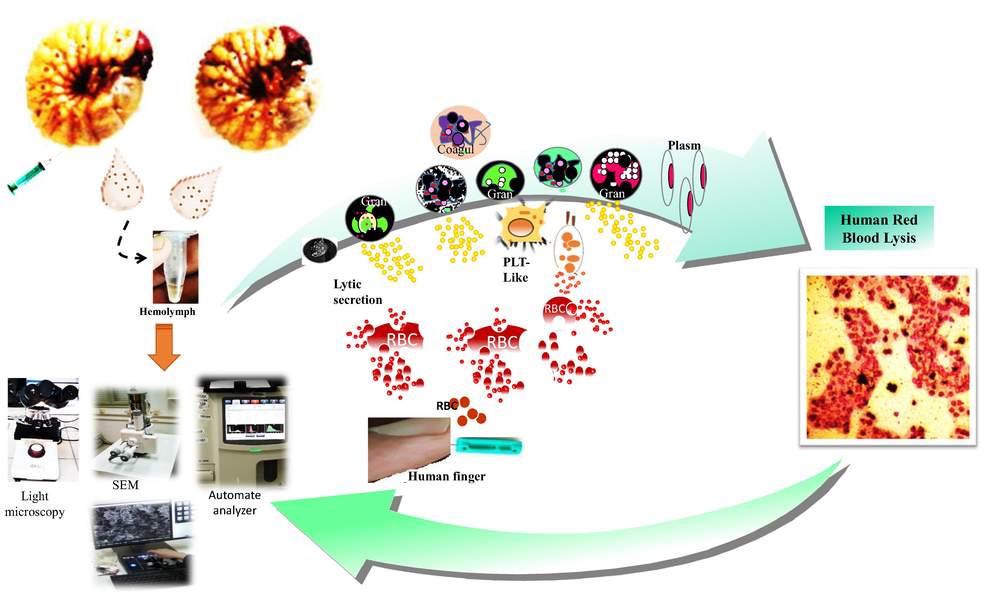
Proposed experimental set-up indicates the immune pathway induced by the scarabid beetle larvae hemocytes. HRBC: human red blood cells; Gran: Granulocytes; PLT-Like: Platelet-Like; Plasm: plasmatocytes; Cogul: coagulocytes..
3 Results and discussion
The principal results obtained in this study are classified in two categories: The main circulating hemocytes in larval hemolymph and their immune response against human blood cells as foreign bodies.
3.1 Circulating cells in P. excavatus larval hemolymph
In this study, different circulating cells in the hemolymph of the P. excavatus larva were observed under both light microscopy and scanning electronic microscopy (SEM).
Insect hemolymph presents numerous hémocytes types that are mainly identified using their functional, morphological, and histochemical characteristics. The characterization of the hemocytes drew the attention of the researchers for a long time and made the object of various classifications. The classification of insect hemocytes varies among species and differs from author to another. While hemocyte types described in literature do not occur in all insects, the five most recognized and common types are plasmatocytes, granulocytes, oenocytoids, prohemocytes, and spherulocytes (spherule cells) (EL Sadawy et al., 2020; Lavine and Strand, 2002; Ling and Yu, 2005; Strand, 2008). In mosquitoes, Hillyer and Christensen (2002) classified circulating hemocytes of adult Aedes aegypti (Diptera, Culicidae) into granulocytes, oenocytoids, adipohemocytes and thrombocytoids. Other studies based on previous investigations classified hemocytes from A. aegypti into only granulocytes, oenocytoids and prohemocytes (Strand, 2008). Culex pipiens quinquefasciatus transfers many important diseases, such as West Nile virus, lymphatic filariasis and Japanese encephalitis (Da Silva et al., 2000). Despite its epidemiological importance, authors contradict each other in the classification of hemocytes. Brayner et al. (2005), Brayner et al. (2007) divided circulating hemocytes of this vector into six different types. In contrast, in a more recent review, hemocytes in this mosquito are conclusively thought as are four types: prohemocytes, oenocytoids, plasmatocytes and granulocytes (Wang et al., 2011). In hymenoptera, two type of circulating hemocytes were identified in the hemolymph of the worker honeybee larva (Apis mellifera L.) by phase contrast and transmission electron microscopies. These were the plasmatocytes and granulocytes. The ultrastructural investigation of the bee hemocytes revealed the possible presence of a third type of cells, which has been called “macrophage” by analogy with vertebrate macrophages (Belaid et al., 2013). Galleria mellonella larva (Lepidoptera, Pyralidae) has been widely used as a model to investigate the virulence of several human pathogens. Recent study undertaken by Wu et al. (2016) allowed the recognition of four types of hemocytes (plasmatocytes, granular cells, spherule cells and oenocytoides) in the hemolyph of the wax moth. In comparative studies on the immunity defense mechanisms in larvae and adults of Cetonischema aeruginosa (Coleoptera, Scarabaeidae), the ultrastructure of the circulating cells in the third instar larval stage has been explored using both light and transmission electron microscopy. The different hémocytes found in the larval hemolymph were identified as granulocytes prohemocytes, plasmatocytes, coagulocytes, oenocytoids and spherulocytes (Giulianini et al., 2003).
In our study, four different hemocytes where observed by the microscopy in the hemolymph of the third instar larval Phyllognathus excavatus. They were identified as plasmatocytes, granulocytes, oenocytoides, and coagulocytes. Granulocytes appear oval in shape with short pseudopodia (∼15 µm in diameter) (Fig. 2A, E). Oenocytoids present a round shape containing dense granules (Fig. 2B). Plasmatocytes were the most observed type of hemocytes. They appear to be elongate, plate, and irregular in shape (∼15 µm in lentgh) (Fig. 2C, D). Coagulocytes (Fig. 2E, F) are oval in shape and very small (3–5 µm) comparing with the other hemocyte types.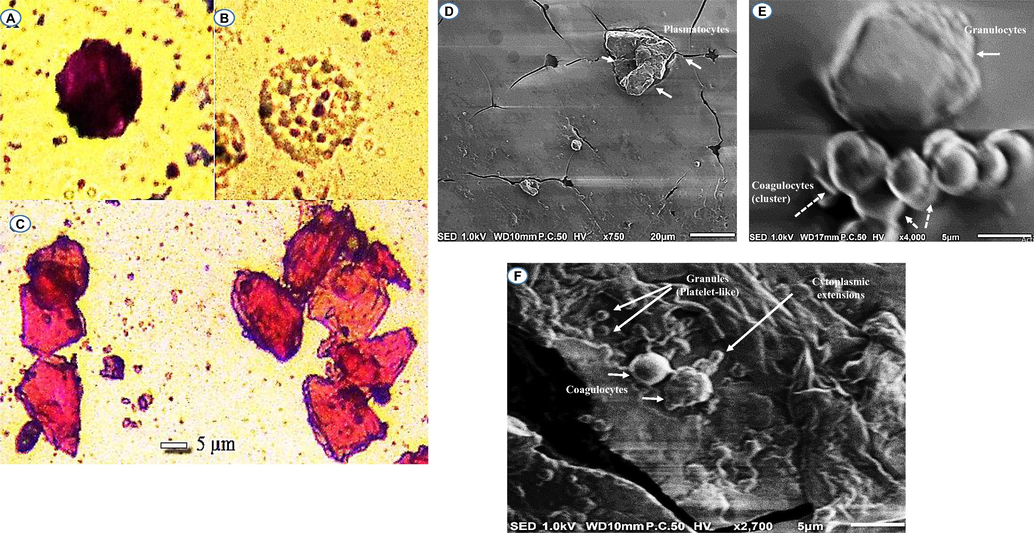
Circulating cells in the hemolymph of P. excavatus. Granulocytes observed by LM (A) and SEM (E) (scale bar = 5 µm); Oenocytoides observed by LM (B); Plasmatocytes observed by LM (C) (scale bar = 5 µm) and SEM (D) (scale bar = 20 µm); Coagulocytes detected by SEM (E, F) (scale bar = 5 µm).
3.2 Immune defense in P. excavatus larva
In our study, it seems that larval hemolymph contain a large number of small cells (coagulocytes). As seen in Fig. 2F, the SEM suggested the involvement of these cell type circulating in the scarab larval into the hemolymph coagulation (promote clot formation by extruding threadlike, cytoplasmic extension, and granules). It seems that coagulocytes circulating in the hemolymph of the larva of P. excavatus as autonomous drones for hemostatic and immune surveillance share remarkable functional similarities with mammalian blood platelets. In mammals, platelets are the smallest cell type in circulating blood, averaging only from 2.0 to 5.0 μm in diameter, 0.5 μm in thickness, and having a mean cell volumes between 6 and 10 femtoliters. They are subcellular fragments released from megakaryocytes that circulate in blood as anucleate and discoid cells. The average human body has in the order one trillion circulating blood platelets (200 000/µL), each platelet has an average lifespan of 8–10 days. One hundred billion new platelets must be produced daily from bone marrow megakaryocytes to maintain platelet counts between 150 × 109 and 400 × 109 platelets per liter of whole blood (Thon and Italiano, 2012). Their small size and discoid shape result in their being pushed to the vessel edge by blood flow (Hartwig, 2007), allowing them to flatten over the damaged surface rapidly. Despite their small size and anucleate structure, platelets play diverse roles in many physiological processes. Platelets also have an important impact on immune cell extravasation due to their adhesive receptors and their high abundance in the blood (Boilard, 2017). They are also known as the cellular mediator of thrombosis (Morrell et al., 2014). Among their main functions, platelets respond to blood vessel damage by secreting the contents of their granules and aggregating to form a platelet clot (pand-aids) (Thon and Italiano, 2012). They are healers that release growth factors and other soothing molecules that help damaged tissue to rebuild (Leslie, 2010). As evident from the studies of many researchers, platelets are also soldiers that trigger the protective response known as inflammation, alert immune cells, and even attack microbial invaders (Avecilla et al., 2004; Larson and Watson, 2006; Thon and Italiano, 2012).
In order to determine the different hemolymph cells involved in the immune defense against foreign bodies, a mixture of larval and human blood in vitro shows the hemolysis of red blood cells by coagulocytes. As detected in Fig. 3A, a trigger of coagulocytes having almost the same dimension with blood cells (5 µm) in the hemolysis of the surrounding foreign human red blood cells. It is suggested that this cell type are also able to kill invaders (not only involved in the clot formation). Moreover, plasmatocytes (in cluster and individual) and granulocytes (Fig. 3B–E) were revealed to be professional foreign bodies’ killer in Phyllognathus excavatus larvae. Thus, upon their exposure to human blood cells, they are able to recognize foreign surfaces and secrete soluble mediators in the vicinity that serve to pathogens phagocytosis and lysis. Additionally, results in our study showed that platelets-like figured in Fig. 2F, 3C and counted in Table 1, may well play a great role in distinguishing foreign crops and immune system defense. On this basis (the larval hemocytes attack human red blood cells as foreign bodies), it is suggested that beetle larval hemolymph can also be used to combat unwanted bodies in humans, such as cancer cells, and in other therapeutic applications. But this needs further studies.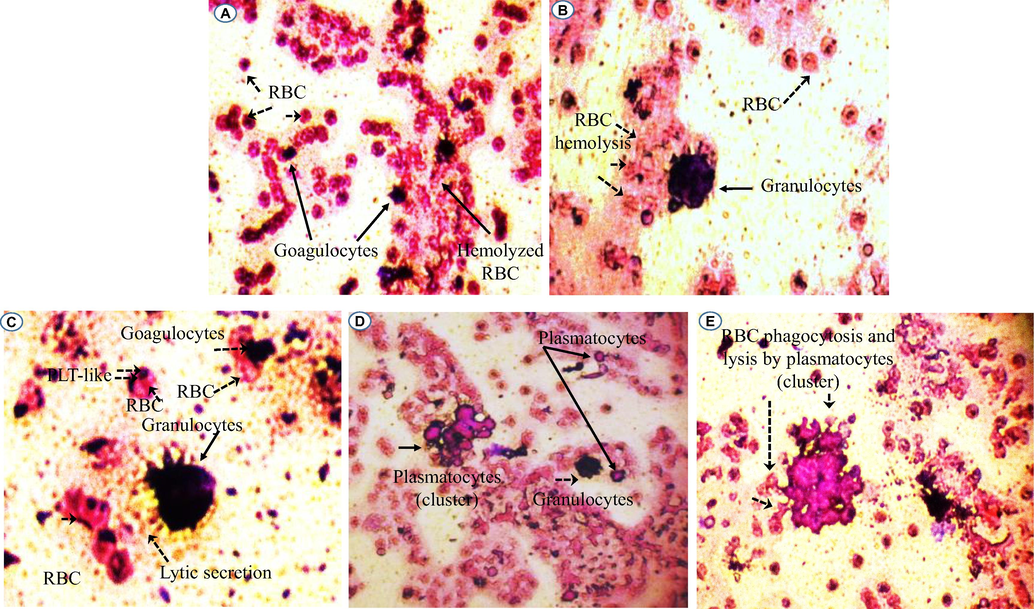
Hemolysis of red blood cells by larval small cells (A). Granulocyte, platelets-like and coagulocytes (B, C) and plasmatocytes (D, E) detected in the Phyllognathus excavatus hemolymph involved in the cellular immune reaction. PLT-Like: Platelet-Like; RBC: red blood cells.
Element analysis
Result
Unit
Element analysis
Result
Unit
WB
R
10.0
103/µL
MCH
*****
pg
Lymph#
RH
8.0
103/µL
MCHC
*****
g/dL
Mid#
*****
103/µL
RDW-CV
*****
Gran#
*****
103/µL
RDW-SD
*****
fL
Lymph#
RH
0.801
PLT
L
26
103/µL
Mid#
*****
MPV
9.0
fL
Gran#
*****
PDI
H
18.5
RB
L
0.0
106/µL
PCT
L
0.23
mL/L
HGB
L
0.0
g/dL
P-LCC
L
8
109/L
HCT
L
0.0
%
P-LCR
0.302
MCV
*****
fL
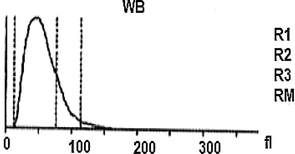
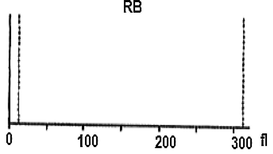
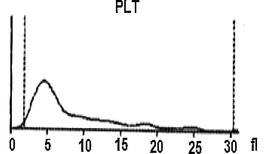
On the viewpoint of immune events, more investigation under the automated hematology analyzer confirms that the hemolymph of the study larva contain a large number of lymphocytes-like and platelets-like. It seems that the larval coagulocytes were considered as lymphocytes by the automated analyzer, as they share almost the same characters. As showed in Table 1 the content of the larval hemolymph in coagulocytes (lymphocytes-like) reach 8 × 106/µL. The hemogram showed also the presence of platelets-like (∼26 × 103/µL) circulating in the larval hemolymph. It is assumed that among the different hemolymph cells, vast number of platelets-like derived from coagulocytes (Fig. 2f) was present, which readily fragmentize playing practically the same role (clot formation and immune defense). As well as, their contribution in invaders attack was shown (Fig. 3C). Notably, the notion of platelets-like in invertebrates is not new. When studying the hemolymph cells of the mollusk, Incilaria fruhstorferi (Gasteropoda: Pulmonata), Furuta et al., 1990; Furuta and Yamaguchi, 2011) found that the largest slug native of Japan possesses three types of hemolymph cells: macrophage-like, lymphocyte-like and fibroblast-like.
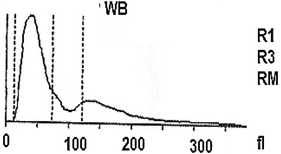
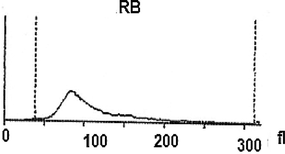
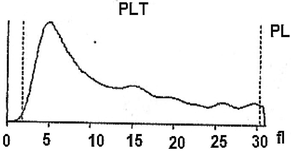
Further, a mixture of larval hemolymph with human blood showed the destruction of approximately 3/4 of the human red blood cells as showed in Table 2 under the larval immune system compared with a complete red blood count of a healthy adult man (Table 3). Fig. 4 depicts the results. Fig. 4A shows that the larval hemolymph contains lymphocytes-like but with higher content than that of human blood. The mixture increased the content of this type of cell. Fig. 4C shows that the larval hemolymph presents platelets-like but with lower content than that of human blood. Fig. 4B revealed two main notes: (i) the larval hemolymph is devoid of red blood cells. (ii) the content of human red blood cells was reduced by 75 % when mixed with larval hemolymph (∼4.06 × 106/µL vs. ∼ 0.58 × 106/µL) with a significant difference (p < 0.01). This can be explained in fact only red blood cells were considered as foreign crops by the larval immune system (coagulocytes, plasmatocytes, granulocytes, and platelets-like) and then the majority was killed. Thus, insect hemocytes recognize foreign bodies and phagocytose pathogens in a manner similar to that of mammalian leukocytes (Browne et al., 2013), just as insects and maamals share innate immune responses (Krautz et al., 2014).
Element analysis
Result
Unit
Element analysis
Result
Unit
WB
RH
12.7
103/µL
MCH
RH
181.0
pg
Lymph#
RH
7.3
103/µL
MCHC
RH
142.9
g/dL
Mid#
RH
1.8
103/µL
RDW-CV
RH
0.194
Gran#
R
3.6
103/µL
RDW-SD
RH
120.5
fL
Lymph#
RH
0.576
PLT
L
78
103/µL
Mid#
R
0.141
MPV
H
12.6
fL
Gran#
RL
0.283
PDI
H
18.8
RB
RL
0.58
106/µL
PCT
L
0.98
mL/L
HGB
L
10.5
g/dL
P-LCC
39
109/L
HCT
RL
7.3
%
P-LCR
H
0.500
MCV
RH
126.7
fL
Element analysis
Result
Unit
Element analysis
Result
Unit
WB
6.2
103/µl
MCH
30.0
pg
Lymph#
2.0
103/µl
MCHC
31.6
g/dL
Mid#
0.6
103/µl
RDW-CV
L
0.170
Gran#
3.6
103/µL
RDW-SD
H
51.2
fL
Lymph#
0.328
PLT
L
134
103/µL
Mid#
0.090
MPV
H
8.5
fL
Gran#
0.582
PDI
H
15.9
RB
4.06
106/µL
PCT
L
1.14
mL/L
HGB
12.2
g/dL
P-LCC
35
109/L
HCT
38.7
%
P-LCR
H
0.260
MCV
95.2
fL
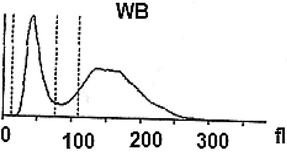
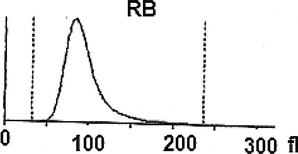
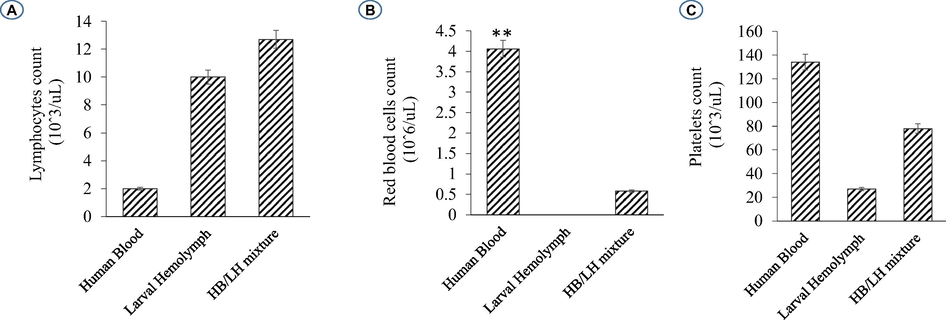
Histograms showing the variation of some elements figured in the larval hemolymph and human blood compared to a mixture of human blood with larval hemolymph. Lymphocytes (A); Red blood cells (B); Platelets (C).
4 Conclusion
This report highlights Phyllognathus excavatus larva hemocyte ontogeny and provides new insight into the immune responses induced upon exposure to human blood cells. Among invertebrates, the underlying mechanism of the scarabid beetle immune systems should be investigated by its application in clinical responses to severe viral, parasitic and bacterial infections.
Acknowledgements
The authors acknowledge the support of the “Ministry of High Education, Scientific Research and Technology, Tunisia” for this study. The authors extend their appreciation to the deanship of scientific research for funding this article by Taif University Research Supporting Project number (TURSP-2020/301), Taif University, Taif, Saudi Arabia.
Author’s contributions
RA, FG and MSA contributed to the design of the study. RA developed the initial draft of the manuscript; RA and FG performed the statistical analyses and experiments. All authors analyzed and interpreted the data, critically revised the manuscript, and approved the final draft
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ecological and Biological Studies of Some Species of Scarabaeidae and Life Cycle of Phyllognathus excavatus Forster in Damascus Countryside, Syria. Jordan. J. Agric. Sci.. 2015;11(4)
- [Google Scholar]
- Circulating hemocytes from larvae of Melipona scutellaris (Hymenoptera, Apidae, Meliponini): Cell types and their role in phagocytosis. Micron. 2010;41(2):123-129.
- [Google Scholar]
- Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat. Med.. 2004;10(1):64-71.
- [Google Scholar]
- Ultrastructure des hémocytes de l’abeille ouvrière Apis mellifera L. (Hymenoptera: Apidae) au cinquième stade larvaire. Bulll. Soc. Zool. Fr.. 2013;138(1–4):5-15.
- [Google Scholar]
- Boilard, E.P.A., 2017. Nigrovic, in Kelley and Firestein's Textbook of Rheumatology (Tenth Edition).
- Ultrastructural characterization of the hemocytes of Culex quinquefasciatus (DIPTERA: Culicidae) Micron. 2005;36(4):359-367.
- [CrossRef] [Google Scholar]
- Haemocyte population and ultrastructural changes during the immune response of the mosquito Culex quinquefasciatus to microfilariae of Wuchereria bancrofti. Med. Vet. Entomol.. 2007;21(1):112-120.
- [CrossRef] [Google Scholar]
- An analysis of the structural and functional similarities of insect hemocytes and mammalian phagocytes. Virulence. 2013;4(7):597-603.
- [CrossRef] [Google Scholar]
- Immune Defense Mechanisms of Culex quinquefasciatus (Diptera: Culicidae) against Candida albicans Infection. J. Invertebr. Pathol.. 2000;76(4):257-262.
- [CrossRef] [Google Scholar]
- Susceptibility of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) to entomopathogenic nematodes with regard to its immune response. Biol. Control. 2020;148:104308.
- [CrossRef] [Google Scholar]
- The emerging role of platelets in adaptive immunity. Cell. Immunol.. 2005;238(1):1-9.
- [CrossRef] [Google Scholar]
- Seasonal occurrence and local coexistence within scarabaeid dung beetle guilds (Coleoptera: Scarabaeoidea) in Tunisian pasture. Europ. J. Entomol.. 2009;106:85-94.
- [CrossRef] [Google Scholar]
- Forster, J. Re., 1771. Novae species Insectorum, Centuria I. veneunt apud T. Davies. White, London, [viii +] 100 pp. https://doi.org/10.5962/bhl.title.152194
- Hemolymph cells and the platelet-like structure of the land slug, Incilaria bilineata (Gasteropodae: Pulmonatae) Anat. Anz.. 1990;170:99-109.
- [Google Scholar]
- Transplant rejection in terrestrial molluscs. Invertebrate Survival J. 2011:2333-9721.
- [Google Scholar]
- Taxonomic notes on the ground beetles (Coleoptera: Carabidae) of Tunisia. Arquivos Entomolóxicos. 2016;15:65-82.
- [Google Scholar]
- Circulating hemocytes from larvae and adults of Carabus (Chaetocarabus) lefebvrei Dejean 1826 (Coleoptera, Carabidae): Cell types and their role in phagocytosis after in vivo artificial non-self-challenge. Micron. 2008;39(5):552-558.
- [CrossRef] [Google Scholar]
- Immune challenges trigger cellular and humoral responses in adults of Pterostichus melas italicus (Coleoptera, Carabidae) Arthropod Struct. Dev.. 2015;44(3):209-217.
- [CrossRef] [Google Scholar]
- Ultrastructure of the hemocytes of Cetonischema aeruginosa larvae (Coleoptera, Scarabaeidae): involvement of both granulocytes and oenocytoids in in vivo phagocytosis. Tissue Cell.. 2003;35(4):243-251.
- [CrossRef] [Google Scholar]
- Blood coagulation in arthropods. iii. Reactions of insect hemolymph to coagulation inhibitors of vertebrate blood. Biol. Bull.. 1953;104(3):372-393.
- [CrossRef] [Google Scholar]
- Haemolymph coagulation in insects and taxonomy. Bull. KBelg. Inst. Nat. Wet.. 1984;55:3-48.
- [Google Scholar]
- Platelets. Elsevier; 2007. p. :75-97.
- Characterization of hemocytes from the yellow fever mosquito, Aedes aegypti. Histochem. Cell Biol.. 2002;117(5):431-440.
- [CrossRef] [Google Scholar]
- A Decapod Hemocyte Classification Scheme Integrating Morphology, Cytochemistry, and Function. Biol. Bull.. 1990;178(1):33-45.
- [CrossRef] [Google Scholar]
- Dynastinae (Coleoptera Lamellicornia) Fam. Scarabaeidae. Exploration du Parc National Albert. Mission G.F. de Witte (1933–1935) Bruxelles. Fasc.. 1942;38:25-26.
- [Google Scholar]
- Platelets: bridging hemostasis, inflammation, and immunity. Int. J. Lab. Hematol.. 2013;35(3):254-261.
- [CrossRef] [Google Scholar]
- Nueva cita de Phyllognathus excavatus Forster, 1771 (Coleoptera: Scarabaeidae: Dynastinae) en el norte de Túnez. Arquivos Entomolóxicos. 2016;15:337-338.
- [Google Scholar]
- Review: Role of Blood Platelets in Infection and Inflammation. J. Interferon Cytokine Res.. 2002;22(9):913-922.
- [CrossRef] [Google Scholar]
- Postembryonic Hematopoiesis in Drosophila. Dev. Biol.. 2001;230(2):243-257.
- [CrossRef] [Google Scholar]
- A product of their environment: Do megakaryocytes rely on extracellular cues for proplatelet formation? Platelets. 2006;17(7):435-440.
- [CrossRef] [Google Scholar]
- Insect hemocytes and their role in immunity. Insect Biochem. Mol. Biol.. 2002;32(10):1295-1309.
- [CrossRef] [Google Scholar]
- Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328(5978):562-564.
- [CrossRef] [Google Scholar]
- Prophenoloxidase binds to the surface of hemocytes and is involved in hemocyte melanization in Manduca sexta. Insect Biochem. Mol. Biol.. 2005;35(12):1356-1366.
- [CrossRef] [Google Scholar]
- Platelet Factor 4 Activity against P. falciparum and Its Translation to Nonpeptidic Mimics as Antimalarials. Cell Host Microbe. 2012;12(6):815-823.
- [Google Scholar]
- Nueva cita de Phyllognathus excavatus Forster, 1771 (Coleoptera: Scarabaeidae: Dynastinae) en el norte de Túnez. Arquivos Entomolóxicos. 2016;15:337-338.
- [Google Scholar]
- Platelets Kill Intraerythrocytic Malarial Parasites and Mediate Survival to Infection. Science. 2009;323(5915):797-800.
- [Google Scholar]
- Platelet Factor 4 and Duffy Antigen Required for Platelet Killing of Plasmodium falciparum. Science. 2012;338(6112):1348-1351.
- [Google Scholar]
- Emerging roles for platelets as immune and inflammatory cells. Blood. 2014;123(18):2759-2767.
- [CrossRef] [Google Scholar]
- Melanogenesis and the Generation of Cytotoxic Molecules During Insect Cellular Immune Reactions. Pigment Cell Res.. 1993;6(3):117-126.
- [CrossRef] [Google Scholar]
- Apis mellifera haemocytes in-vitro, What type of cells are they? Functional analysis before and after pupal metamorphosis. J. Apic. Res.. 2014;53(5):576-589.
- [CrossRef] [Google Scholar]
- Pereira, T.C., Pimentel de Barros, P., Fugisaki, L.R.O., Rossoni, R.D., Ribeiro, F.C., Teles de Menezes, R., Junqueira, J.C., Scorzoni, L., 2018. Recent Advances in the Use of Galleria mellonella Model to Study Immune Responses against Human Pathogens. J. Fungi (Basel), 4(4), 128. https://doi.org/10.3390/jof4040128
- Paulian, R., 1941. Faune de France. 38. Coleopteres Scarabeides. Paris, Lechevalier ed., 240 p., 445 figs.
- Insect haemocytes: What type of cell is that? J. Insect Physiol.. 2006;52(5):417-429.
- [CrossRef] [Google Scholar]
- Morphological and functional characterization of honey bee, Apis mellifera, hemocyte cell communities. Apidologie. 2018;49(3):397-410.
- [CrossRef] [Google Scholar]
- Platelet-Vessel Wall Interactions in Hemostasis and Thrombosis. Colloquium Series on Integrated Systems Physiology: From Molecule to Function. 2010;2(1):1-75.
- [CrossRef] [Google Scholar]
- Platelets and innate immunity. Cell. Mol. Life Sci.. 2010;67(4):499-511.
- [CrossRef] [Google Scholar]
- Innate humoral immune defences in mammals and insects: The same, with differences? Virulence. 2018;9(1):1625-1639.
- [CrossRef] [Google Scholar]
- Platelets: Production, Morphology and Ultrastructure. Handb. Exp. Pharmacol.. 2012;210:3-22.
- [CrossRef] [Google Scholar]
- A systematic study on hemocyte identification and plasma prophenoloxidase from Culex pipiens quinquefasciatus at different developmental stages. Exp. Parasitol.. 2011;127(1):135-141.
- [Google Scholar]
- Ultrastructural and functional characterization of circulating hemocytes from Galleria mellonella larva: Cell types and their role in the innate immunity. Tissue Cell. 2016;48(4):297-304.
- [CrossRef] [Google Scholar]
- Platelets in antimicrobial host defense. In: Michelson A., ed. Platelets (2nd ed.). New York: Academic; 2006. p. :727-755.
- [CrossRef] [Google Scholar]







