Translate this page into:
Hyper-ovulation, endometriosis, and hyperplasia associated with tamoxifen exposure in Swiss albino mice
⁎Corresponding author. fmuhammad@ksu.edu.sa (Muhammad Farooq Khan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The available literature on the safety profile of tamoxifen in human patients and in experimental animals is mostly contradictory. The present study was therefore, designed to understand the safety profile of tamoxifen using Swiss albino mice. Swiss albino mice of age 13 weeks were orally administered tamoxifen (35 mg/kg/day) for 80 days. The breast, liver, and ovaries were used to evaluate the toxicity of tamoxifen in these organs using histopathology by H& E staining, the organ indices, estimation of liver enzymes, sex hormones, and antioxidant enzymes were compared with control mice group. The histopathological profiles indicated that breasts of the treated mice were not affected; however, it caused major damage to the liver, uterus, and ovaries. Tamoxifen also induced a moderate level of apoptosis by elevating the level of caspase-3 in the liver, uterus, and ovaries of the treated mice. The estrogen and progesterone levels were also significantly reduced. Tamoxifen induced hyper ovulation in treated mice group which has never been reported before. These data suggest that hepatotoxicity and hyper ovulation associated with tamoxifen could not be overruled and hence an alternate hormone disruptors other than tamoxifen should be investigated.
Keywords
Tamoxifen
Hormone disruptors
Liver enzymes
Caspase-3
1 Introduction
Breast cancer is a diverse type of disease affecting thousands of women every year. Variety of factors have been reported which are responsible for causing breast cancer and at the same time number of treatment options are also available for the disease. Breast tumors often require hormones for growth.
Around 1.67 million new cases of breast cancer are diagnosed each year and 66% of breast cancer includes hormone receptor-positive breast cancer worldwide (Wood et al., 2017). The proportion of patients with each subtype ranged from 12.6 to 53.5% of the overall population (HR-/HER2 + least common and HR + /HER2-most common) (Gao et al., 2012)
Hormone therapy benefit the patients who are diagnosed with hormone receptor positive breast cancer. The hormone therapy slow down or block binding of the hormone(s) with receptor, resulting reduced proliferation of tumor cells. Tamoxifen (triphenylethylene antiestrogen), is drug of choice for hormone therapy and used as an adjuvant therapy in breast cancer treatment. The available literature about the safety of tamoxifen in human breast cancer patients and experimental animals is quite controversy. Several studies have reported tamoxifen with minimal or no side effects in human patients (Guerrieri-Gonzaga et al., 2016; Thomson et al., 2017; DeCensi et al., 2019). Tamoxifen was also reported as a safe and effective drug in premenopausal patients as a preoperative therapy in a clinical study involving 53 patients with estrogen receptor (ER)-positive and human epidermal growth factor receptor 2 (HER2)-negative invasive breast cancer (Shimizu et al., 2014).
However, quite recently many adverse side effects have been reported in breast cancer patients with tamoxifen use. The development of resistance to tamoxifen in human patients is a major hurdle for effective therapy. The patients initially respond to treatment, but relapse of disease occur by the development of resistance against tamoxifen (Viedma-Rodriguez et al., 2014; Ojo et al., 2015). All the studies mentioned above have reported the toxicities of tamoxifen at various levels but very little information are available regarding the extent of tamoxifen toxicity in various organs especially those in ovaries and uterus.
Therefore this study was designed to evaluate the damage induced to various vital organs (liver, ovaries, and uterus) by tamoxifen by detail histopathological analysis. Swiss albino mice of age 13 weeks were administered tamoxifen (35 mg/kg) orally daily for 80 days. The indices of the breast, ovaries, and liver, as well as liver enzymes, sex hormones, and antioxidant enzymes were analyzed to assess the toxicity of tamoxifen. The breast, ovaries, liver, and uterus were examined to evaluate any alteration in the structure of these tissues.
2 Material and methods
2.1 Animals
Virgin female mice (Swiss albino strain) weighing 25 ± 5 g and of 13 weeks age were used in these experiment. All experiments were carried out in accord with the guide lines for the care and use of laboratory animals.
2.2 Breast, liver, and ovary indices
Mouse were weighed and their left breast, liver, and ovary were removed and weighed. Breast, liver, and ovary indices were calculated
2.3 Biochemical analysis
2.3.1 Estimation of Aspartate Aminotransferase (AST) activity
Aspartate Aminotransferase (AST) activity in treated mice were calculated using Aspartate Aminotransferase (AST) Activity Assay kit cat# MAK055 from Sigma-Aldrich (St. Louis, MO 63103, USA) following methods described by (Huang et al., 2006).
2.3.2 Estimation of Alanine Aminotransferase (ALT) activity
Alanine Aminotransferase (ALT) activity in treated mice were calculated using Alanine Aminotransferase (ALT) activity Assay kit cat# MAK052 Sigma (Sigma-Aldrich St. Louis, MO 63103, USA) following instruction of manufacturer.
2.3.3 Estrogen
To estimate the level of estrogen, the competitive inhibition enzyme immunoassay technique was utilized using Mouse Estrogen (E) ELISA kit (Cat # CSB-E07280m CUSABIO TECHNOLOGY LLC, 6161 Savoy Dr. Suite 1018 Houston, TX 77036, USA) following protocol as described by manufacturer.
2.3.4 Estimation of progesterone
Progesterone ELISA was used for the quantitative measurement of progesterone in mouse serum using Mouse/Rat Progesterone ELISA kit (Catalog Number SE120087 Sigma- Aldrich St. Louis, MO 63103, US) following the manufacturer’s instructions.
2.3.5 Estimation of antioxidant enzymes.
2.3.5.1 Malondialdhyde (MDA)
Lipid peroxidation was estimated according to the method established by (Ohkawa et al., 1979)
2.3.5.2 Glutathione (GSH)
GSH was determined chemically in the liver homogenate using Ellman’s reagent (Ellman, 1959).
2.3.5.3 Catalase (CAT)
The catalase activity in the plasma was assayed by the method established by Aebi (1984).
2.3.5.4 Nitric oxide
The assays of nitrite in the serum and liver homogenate were done according to the method established by Berkels et al. (2004).
2.4 Histological examination
The tissue sample from breast liver, ovary, and uterus of control and treated group were collected and fixed in 10% neutral buffered formalin. Following fixation, the specimens were dehydrated in an ascending series of alcohol, inserted in wax, and sectioned. Hematoxylin and eosin (H&E), and Masson’s trichrome (M. Tr) stains were used to stain the section.
2.5 Immunohistochemistry
Immunohistochemistry staining was performed using the avidin–biotin complex (ABC) following methods described by Aldahmash et al. (2016). The primary antibodies used were anti-ki67 for the breast section and anti-caspase-3 for the breast, liver, ovary, and uterus sections.
2.6 Statistical analysis
One-way analysis ANOVA and the statistical comparisons among the groups were completed with Duncan’s test using a statistical package program (SPSS version 16.0) to determine the data mean ± St Deviation. P values ≤ 0.05 was considered significant.
3 Results
3.1 Tamoxifen treatment did not modified the organ index in treated mice
Tamoxifen treatment (35 mg/kg) altered the tested organ indexes; however, these differences were statistically insignificant when compared to the control group. There were insignificant increases in the breast, liver, and ovary indexes of the tamoxifen-treated mouse group compared to the control group (Table 1).
Groups
Breast Index*
Liver Index*
Ovary Index*
Control
0.29 ± 0.02n = 5
6.4 ± 0.3n = 5
0.17 ± 0.04
n = 5
Tamoxifen
0.33 ± 0.02
n = 57.0 ± 0.1
n = 50.19 ± 0.02
n = 5
P values¥
0.07
0.08
0.08
3.2 Tamoxifen induced liver damage in treated mice via altering the liver enzymes
The biochemical analysis results of liver enzymes of the tamoxifen-treated mouse group and the control group are shown in Table 2. The tamoxifen treatment significantly altered the liver enzymes. Alanine Aminotransferase (ALT) and Aspartate Aminotransferase (AST) levels were increased significantly in treated mice group as compared to the control group.
Groups
ALT (u/l)*
AST (u/l)*
Control
37 ± 0.1
110 ± 0.3
Tamoxifen
40 ± 0.6
112 ± 1.6
P values ¥
0.03
0.04
3.3 Tamoxifen lowered estrogen and progesterone level in treated mice
The changes in hormone (estrogen and progesterone) levels upon exposure to tamoxifen in control and treated mice group are presented in Table 3. Tamoxifen treatment lowered the estrogen level significantly but a non-significant raise in progesterone levels was observed in treated mice group versus control.
Groups
Estrogen (ng/ml)*
Progesterone(ng/ml)*
Control
63 ± 0.2
0.2 ± 0.07
Tamoxifen
43 ± 0.2
0.3 ± 0.16
P values ¥
0.03
0.08
3.4 Tamoxifen treatment induced oxidative stress in treated mice
As shown in Table 4, the tamoxifen-treated mouse group had significantly higher MDA levels than did the control group The GSH level was significantly lower in the tamoxifen-treated mouse group than in the control group. An insignificant decrease was seen between the treatments in the CAT levels. Nitric oxide (NO) levels increased significantly in the tamoxifen-treated mice group.
Groups
MDA*
GSH*
CAT*
NO*
Control
31 ± 0.1
52 ± 0.2
6 ± 0.4
70 ± 0.8
Tamoxifen
48 ± 0.2
31 ± 0.3
4 ± 0.6
98 ± 0.6
P values ¥
0.02
0.03
0.07
0.02
3.5 The tamoxifen treatment induced histopathological changes in breast, ovary and liver of treated mice
Samples of the control group breast tissue showed normal histology and revealed few inactive glands with small ducts. The breast was composed largely of adipose connective tissue, without any depositions of fibers (Fig. 1: A, C, and E). Microscopic examination of breast tissue from mice treated with tamoxifen also revealed healthy breast sections with mammary glands and ducts in adipose tissue (Fig. 1: B, D, and F).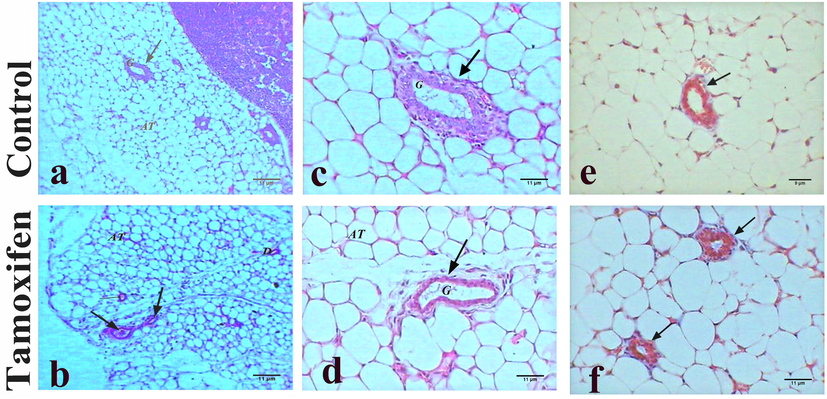
Histopathology of breast tissues. Representative photomicrograph showing the histopathology of breast tissue of control mice (A, C, and E: upper panel) and tamoxifen-exposed breast tissue (B, D, and F: lower panel). A) Control mice breast showed normal mammary gland (G) in adipose tissue (AT), H&E, 100×; B) Tamoxifen treated mice showed normal breast with mammary gland (arrows) and adipose tissue (AT), H&E, 100×; C) Normal mammary gland (G) of control mice, H&E, 400×; D) Breast of mice treated with tamoxifen showed normal mammary gland (G) and adipose tissue (AT), H&E, 400×; E) Normal structure of breast of control mice without depositions of fibers, M. Tr, 400×; F) Tamoxifen treated mice showed normal mammary gland and adipose tissue (AT), M. Tr, 400×.
The control group liver sections showed a normal structure of liver consisting mainly of a center vein surrounded by an anastomose network of hepatocytes strands, separated from each other by blood sinusoids with abundant Kupffer cells (Fig. 2: A and C). The liver section from mice treated with tamoxifen showed pathological changes represented by dilatation of the central vein that filled with edema and was surrounded by activated Kupffer cells, hepatocytic degeneration, and steatosis manifested by microvesicles (Fig. 2: B). The section stained with Masson’s trichrome revealed depositions of collagenous fibers that surrounded the congested central vein (Fig. 2); whereas another section displayed accumulation of collagenous fibers in necrotic area besides inflammatory cells (Fig. 2: D).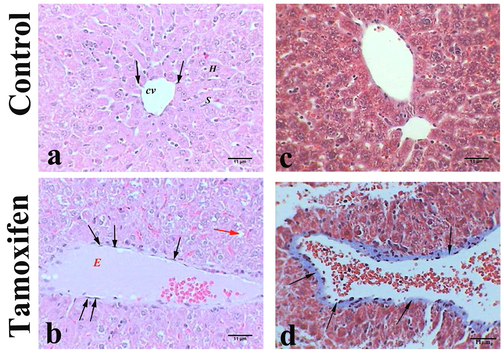
Histopathology of liver tissues. Representative photomicrograph showing the histopathology of liver tissue of control mice (A and C: upper panel) and tamoxifen-exposed liver tissue (B and D: lower panel). A) Control mice had normal liver with central vein (CV), sinusoid (S), hepatocytes (H), and Kupffer cells (arrows), H&E, 400×; B) Mice liver treated with tamoxifen showed dilated vein congested with edema (E) and Kupffer cell (arrows) micro vesicles (red arrows), H&E, 400×; C) Control mice liver showed normal structure, M. Tr, 400×; D) Mice liver treated with tamoxifen showed dilated vein congested with erythrocytes and surrounded by collagenous fibers (arrows), M. Tr, 400×.
The ovaries of un-exposed (control) mice showed small almond shaped structure of a normal ovary, cortex and ovarian follicles with highly vascular medulla and helicine arteries (Fig. 3: A). The ovary cortex contained primordial follicles around the edge (Fig. 3: C), and there were fewer follicles in different stages of development including the primary follicle, antral follicle, and corpus luteum (Fig. 3: C, E, and G). The ovaries of mice treated with tamoxifen revealed an enlarged ovary filled with numerous primordial and primary follicles (Fig. 3: B and D). In addition, the appearance of a large number of corpus lutea implied hyper-ovulation (Fig. 3: F), resulting in cyst formation (Fig. 3: H).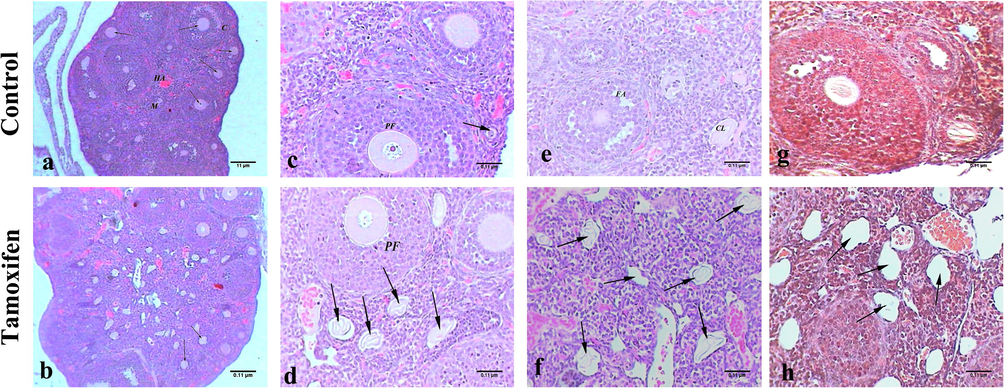
Histopathology of ovary tissue. Representative photomicrograph showing the histopathology of ovary tissue of control mice (A, C, E, and G: upper panel) and tamoxifen-exposed ovary tissue (B, D, F and H: lower panel). A) The ovary of control mice showed normal structure, helicine arteries (HA), cortex (C), medulla (M), and follicles (arrows), H&E, 100×; B) Mice ovary treated with tamoxifen showed hyperovulation with different stages of follicles (arrows), H&E, 100×; C) Normal primary follicle (PF) and primordial follicle (arrow) of control mice, H&E, 400×; D) Mice ovary treated with tamoxifen showed numerous corpus lutea (arrows) and primary follicles (PF), H&E, 400×; E) The ovary of control mice had normal corpus luteum (CL) and follicular antrum (FA), H&E, 400×; F) Mice ovary treated with tamoxifen numerous corpus lutea (arrows) referred to hyper ovulation, H&E, 400×; G) Control mice ovary showed normal ovarian structure, M. Tr, 400×; H) Mice ovary treated with tamoxifen multiple corpus lutea (arrows), M. Tr, 400×.
The control group uterus section showed normal architecture, consisting mainly of endometrium and myometrium. The endometrium was lined with columnar ciliated and secretory epithelia. Stroma of the endometrium contained primarily non-bundled collagen fibers, and uterine glands penetrated the full thickness of endometrium. The myometrium displayed the thickest tunic of the uterus and contained interwoven layers of smooth muscle fibers separated by connective tissue containing venous plexus and lymphatics (Fig. 4: A, C, and E). The uterus section from the mouse group treated with tamoxifen had wide dilatation of uterine glands filled with secretions that appeared outside the endometrium to press on myometrium (endometriosis) (Fig. 4: B and D). The section stained with Masson’s trichrome showed hyperplasia of ciliated columnar epithelium (Fig. 4: F)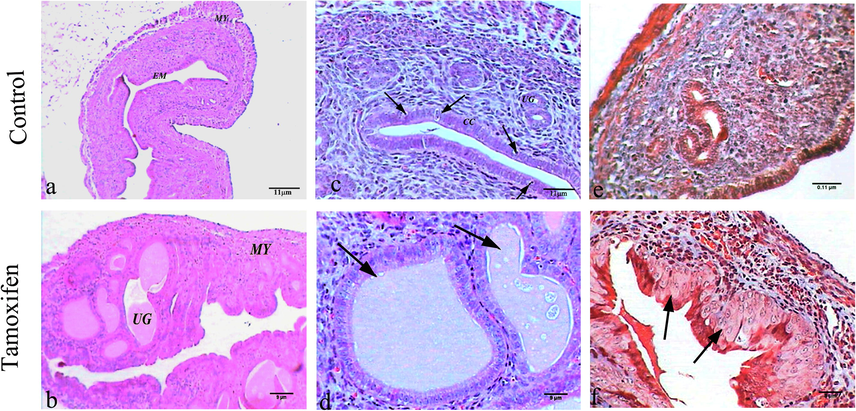
Histopathology of uterus tissues. Representative Photomicrograph showing the histopathology of Uterus tissue of control mice (A, C, and E: upper panel) and tamoxifen-exposed uterus tissue (B, D, and F: lower panel). A) The uterus of control mice showed normal endometrium (EM) and myometrium (MY), H&E, 100×; B) Mice uterus treated with tamoxifen showed wide dilated uterine gland (UG) filled with secretions and myometrium (MY) (endometriosis), H&E, 100×; C) Control mice uterus showed uterine gland (UG), columnar cell (CC) and secretory epithelia (arrows), H&E, 400×; E) Tamoxifen treated mice showed hyperplasia of ciliated columnar epithelium in uterus (arrows), M. Tr, 400×; F) Control mice uterus showed normal structure, M. Tr, 400×.
3.6 Tamoxifen treatment induced moderate level of apoptosis in the liver and ovaries of treated mice
The control group mice breast showed negative immunoreactivity against anti Ki-67 that referred to no proliferation of the tumor in the breast and no reaction against anti caspase-3 in the breast, liver, ovary, and uterus tissues, which represented no apoptosis (Fig. 5: A–D). The breast section showed no immunoreactivity to caspase-3 antibody in the mouse group treated with tamoxifen (Fig. 5: E). The liver showed slight immune-response against caspase-3 antibody represented by faint brown spots in hepatocytes implying slight apoptosis (Fig. 5: F). The ovary displayed a few brown patches in the ovarian follicles due to weak immune response to caspase-3 antibody implying slight apoptosis (Fig. 5: G). The uterus showed faint brown spots due to slight immunoreactivity against the caspase-3 antibody (Fig. 5: H).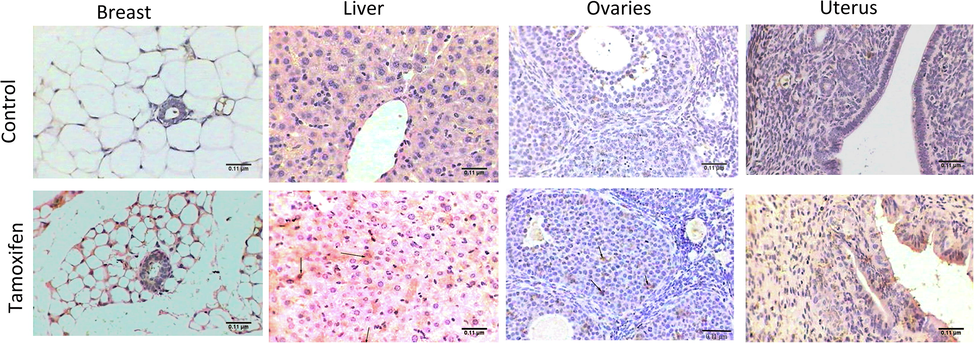
Immunohistochemistry against caspase-3 antibody. A) Photomicrograph of control mice breast showed negative immunoreactivity against caspase-3 antibody (ABC, 400×). B) Mice breast treated with tamoxifen showed negative immunoreactivity to caspase-3 antibody (ABC, 400×). C) Control mice liver showed negative immunoreactivity against caspase-3 antibody (ABC, 400×). D) Tamoxifen treated mice liver showed moderate immune response against caspase-3 antibody (arrows) (ABC, 400×). E) Control mice ovary showed negative immunoreactivity against caspase-3 antibody (ABC, 400×). F) Mice ovary treated with tamoxifen showed weak immune response to caspase-3 antibody (arrows) (ABC, 400×). G) Control mice uterus showed negative immunoreactivity against caspase-3 antibody (ABC, 400×). H) Tamoxifen treated mice uterus treated with showed brown spots due to weak immunoreactivity against caspase-3 antibody (ABC, 400×).
4 Discussion
The present study was designed to determine whether tamoxifen use is safe or not by exploring the experimental animal toxicity model. An elevated levels of ALT and AST has been previously reported by some studies (Gao et al., 2016). However, the focus of those studies seemed to be limited to just estimate the tamoxifen related hepatotoxicity, and the extent of damage which tamoxifen induced in other organs such as breast, ovaries and uterus were completely ignored. The expansion of portal tract, the proliferation in bile duct and dilated central veins with regenerated liver nodule has been previously reported as toxic effect of tamoxifen in liver of rats (El-Beshbishy et al., 2010; Gao et al., 2016). The dilated central vein and hepatocyte degeneration upon treatment of tamoxifen in mice was also observed in this study. However the observation that the livers of tamoxifen treated mice had central veins filled with edema and surrounded by activated Kupffer cells, and especially the steatosis manifested by macrovesicles has been reported only in this study.
In the present study we have observed that breast tissue from mice treated with tamoxifen revealed healthy breast sections with normal mammary glands and ducts in adipose tissue, which mean that tamoxifen does not induced toxicity in breast tissues and also it help to reduce the tumor volume. Tamoxifen administration resulted in significant reduction to estrogen levels and an insignificant increase in progesterone levels as compared to the control group, which is in agreement with several previous studies (Shehata et al., 2014). Previous studies have shown that exposure to tamoxifen resulted in the depletion of reduced GSH and the accumulation of oxidized GSH and lipid peroxidation and also induced the inhibition of hepatic activity of GSH reductase, GSH peroxidase, superoxide dismutase, and CAT (Rabinowich and Shibolet, 2015). The present study also revealed elevated levels of MDA and NO, whereas decreased levels of GSH and CAT associated with increased oxidative stress due to the tamoxifen administration was seen.
The most important observation which was observed in this study is that administration of tamoxifen induced hyper ovulation in treated mice. The tamoxifen related hyper ovulation has never been reported previously. However, a group in Weill Medical College of Cornell University NY, USA has tried tamoxifen as a stimulator to get higher number of embryos to perform in vitro fertilization (IVF) in breast cancer patients, and suggested tamoxifen stimulation as a safe method of IVF and fertility preservation in breast cancer patients (Oktay et al., 2003, 2005). However, careful studies on large scale are needed to confirm the ovarian hyperplasia upon tamoxifen treatment. The tamoxifen induced mild to severe level of apoptosis in liver, ovaries and uterus which was and level of caspase-3 was elevated in these organs. The apoptosis response to tamoxifen in liver and ovaries has been reported in previously (Gao et al., 2016) but the induction of apoptosis in uterus has never been reported before.
All this data suggested that tamoxifen even though a very useful drug to cure and prevent the reoccurrence of breast cancer, it should be used with much care and researchers and clinicians should search for the alternative hormone receptors modulators which could have minimum of no side effects.
Over the years, number of selective hormone disruptors as an alternate to tamoxifen have been tested in clinical trials in order to see the efficacy and safety of these alternate as compared to tamoxifen.. One of such alternative is “Toremifene “. Toremifene which is also an estrogen receptor modulator (Roelfsema et al., 2018) has been suggested as a valid and safe alternative to tamoxifen in breast cancer patients (Lan et al., 2018). Endoxifen, is the active tamoxifen metabolite, also has been suggested as preferred methodology for or ER-positive breast cancer patients (de Vries Schultink et al., 2018). The result of a randomized controlled trials which have compared the efficacy and safety of Anastrozole as an alternative hormone disruptor to tamoxifen, suggested that patients with breast cancer had benefited from the anastrozole treatment (Yang et al., 2017).
5 Conclusion
Prolonged exposure resulted in damage to the liver, uterus, and ovaries in mice whereas the breast tissue was unaffected. The hyper ovulation could be beneficial outcome of tamoxifen as stimulating agent for in vitro fertilization. The results of this and other related studies recommends that alternative hormone disruptors other than tamoxifen should be investigated for effective treatment in hormone positive breast cancers patients.
Funding
King Saud University, Riyadh, Saudi Arabia, Researchers Support Project (RSP-2020/214).
Acknowledgement
Authors are grateful to King Saud University, Riyadh, Saudi Arabia for funding the work through Researchers Support Project (RSP-2020/214).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Reno-protective effects of propolis on gentamicin-induced acute renal toxicity in swiss albino mice. Nefrología. 2016;36(6):643-652.
- [CrossRef] [Google Scholar]
- Measurement of nitric oxide by reconversion of nitrate/nitrite to no. Methods Mol. Biol.. 2004;279:1-8.
- [CrossRef] [Google Scholar]
- Therapeutic Drug Monitoring of endoxifen as an alternative for CYP2D6 genotyping in individualizing tamoxifen therapy. The Breast. 2018;42:38-40.
- [CrossRef] [Google Scholar]
- Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial Neoplasia. J. Clin. Oncol.. 2019;37(19):1629-1637.
- [CrossRef] [Google Scholar]
- Amelioration of tamoxifen-induced liver injury in rats by grape seed extract, black seed extract and curcumin. Indian J. Exp. Biol.. 2010;48(3):280-288.
- [Google Scholar]
- Tumor hormone/HER2 receptor status and pharmacologic treatment of metastatic breast cancer in Western Europe. Curr. Med. Res. Opin.. 2012;28(7):1111-1118.
- [CrossRef] [Google Scholar]
- Tamoxifen induces hepatotoxicity and changes to hepatocyte morphology at the early stage of endocrinotherapy in mice. Biomed. Rep.. 2016;4(1):102-106.
- [CrossRef] [Google Scholar]
- Benefit of low-dose tamoxifen in a large observational cohort of high risk ER positive breast DCIS: low dose tamoxifen and breast DCIS. Int. J. Cancer. 2016;139(9):2127-2134.
- [CrossRef] [Google Scholar]
- Aspartate aminotransferase (ast/got) and alanine aminotransferase (alt/gpt) detection techniques. Sens. Basel. 2006;6(7):756-782.
- [CrossRef] [Google Scholar]
- Toremifene, rather than tamoxifen, might be a better option for the adjuvant endocrine therapy in CYP2D6*10T/T genotype breast cancer patients in China. Int. J. Cancer. 2018;143(10):2499-2504.
- [CrossRef] [Google Scholar]
- Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem.. 1979;95(2):351-358.
- [CrossRef] [Google Scholar]
- Factors promoting tamoxifen resistance in breast cancer via stimulating breast cancer stem cell expansion. Curr. Med. Chem.. 2015;22(19):2360-2374.
- [Google Scholar]
- Fertility preservation in breast cancer patients: IVF and embryo cryopreservation after ovarian stimulation with tamoxifen. Hum. Reprod.. 2003;18(1):90-95.
- [Google Scholar]
- Fertility preservation in breast cancer patients: a prospective controlled comparison of ovarian stimulation with tamoxifen and letrozole for embryo cryopreservation. J. Clin. Oncol.. 2005;23(19):4347-4353.
- [CrossRef] [Google Scholar]
- Drug Induced steatohepatitis: an uncommon culprit of a common disease. Biomed Res. Int.. 2015;2015:168905.
- [CrossRef] [Google Scholar]
- Effects of toremifene, a selective estrogen receptor modulator, on spontaneous and stimulated gh secretion, igf-i, and igf-binding proteins in healthy elderly subjects. J Endocr Soc. 2018;2(2):154-165.
- [CrossRef] [Google Scholar]
- The influence of tamoxifen on normal mouse mammary gland homeostasis. Breast Cancer Res.. 2014;16(4):411.
- [CrossRef] [Google Scholar]
- Preoperative endocrine therapy with goserelin acetate and tamoxifen in hormone receptor-positive premenopausal breast cancer patients. Breast Cancer. 2014;21(5):557-562.
- [CrossRef] [Google Scholar]
- A randomized, placebo-controlled trial of diindolylmethane for breast cancer biomarker modulation in patients taking tamoxifen. Breast Cancer Res. Treat.. 2017;165(1):97-107.
- [CrossRef] [Google Scholar]
- Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review) Oncol. Rep.. 2014;32(1):3-15.
- [CrossRef] [Google Scholar]
- Patient-reported quality of life and treatment satisfaction in patients With HR+/HER2– advanced/metastatic breast cancer. Clin. Ther.. 2017;39(8):1719-1728.
- [CrossRef] [Google Scholar]
- A meta-analysis of randomized controlled trials comparing the efficacy and safety of anastrozole versus tamoxifen for breast cancer. Oncotarget. 2017;8(29):48362-48374.
- [CrossRef] [Google Scholar]







