Translate this page into:
Hydrogeochemical evaluation of shallow alluvial aquifer of Wadi Marwani, western Saudi Arabia
*Corresponding author malahmadi@kau.edu.sa (M.E. Al-ahmadi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the light of progressive depletion of groundwater reservoir and water quality deterioration of the Wadi Marwani alluvial aquifer, an investigation on chemical data of dissolved major and minor constituents in 16 recent groundwater samples was performed. The main objective was the detection of processes responsible for the geochemical evolution and mineralization throughout the area. Wadi Marwani is one of the most important wadis in the central western part of Suadi Arabia to the northeast of Jeddah City. It is intensively inhabited during the last decenniums, leading to expansion of the residential and agricultural areas. The recharge rate of the shallow unconfined aquifer of Wadi Marwani was estimated to be 18% of the annual precipitation using the chloride mass-balance method. Groundwaters of the study area are characterized by the dominance of Ca + Mg over Na + K. HCO3 was found to be the most dominant anion in the upstream and replaced by Cl towards downstream reflecting geochemical evolution along flow path and the influence of agricultural activities and residential areas. TDS is positively correlated with most of major ions, which suggested the impact of agricultural activities on groundwater chemistry through leaching of readily soluble salts from the soil zone. NO3 and TDS showed similar trend which suggested leaching of nitrate fertilizers applied in the agricultural areas. Anomalously high nitrate concentration in few water samples is mainly attributed to leakage of domestic wastewater from the residential area that is very close to these wells. Fluoride concentration showed uniform spatial distribution, which revealed that it is mainly derived from dissolution of silicate minerals forming the aquifer matrix. Most of the groundwater samples are undersaturated with respect to the common carbonate and sulfate minerals, whereas oversaturated with respect to quartz and chalcedony. Groundwater quality assessment revealed that the groundwater can be safely used for drinking. However, further microbiological examination should be carried out where signs of sewage contamination were detected.
Keywords
Alluvial aquifer
Water hardness
Geochemical modeling
Anthropogenic activities
Contamination
Saudi Arabia
1 Introduction
As groundwater is often the only reliable source of fresh water and it is the only renewable water resource in arid regions, this is due to sporadic rainfall; groundwater recharge is limited to infiltration during the intermittent flood in dry wadi channels (wadi is a dry stream bed with surface runoff). In such regions, groundwater is commonly the only water resource. Saudi Arabia lies within one of the driest areas in the world representing the eastern continuation of the Sahara Desert in North Africa. Water resources in such areas are mainly derived from groundwater and infrequent surface runoff, where water conservation projects are applied. In Saudi Arabia, groundwater is considered as a major source of fresh water that is used in different purposes. It is currently exploited from two main sources: (1) deep aquifer systems with nonrenewable groundwater in sedimentary rocks to the east of the Arabian Shield, and (2) shallow alluvial aquifers and fractured Precambrian basement rocks in the mountainous western and southwestern parts. The latter represents the most important renewable water resource in west Saudi Arabia, which is used for irrigation and domestic purposes, and for drinking in many areas. These shallow alluvial aquifers are mainly located in the major wadis draining the Arabian Shield area. The increasing exploitation due to farming frequently causes deterioration in water quality. Therefore, variations in natural and human activities reflect spatial variations in the hydrochemical parameters of the groundwater. The difference of dissolved ions concentration in groundwater are generally, governed by lithology, velocity and quantity of groundwater flow, nature of geochemical reactions, solubility of salts and human activities (Karanth, 1997; Bhatt and Salakani, 1996). Wadi Marwani is one of the most important wadis in the western part of Saudi Arabia to the northeast of Jeddah City. It is intensively inhabited during the last decenniums, leading to expansion of the residential and agricultural areas. An important high potential shallow alluvial aquifer underlies this wadi, where groundwater is pumped from several hand dug wells to use for different purposes. Suitable quantity and quality of groundwater become a more crucial alternative resource to meet the drastic increase in social and agricultural development and to avoid the expected deterioration of groundwater quality due to heavy abstraction for miscellaneous uses. Recently, several agricultural and settlement activities have been developed in this area. These activities pose a major threat to groundwater storage and quality through the impact of agricultural practices and domestic waste disposal on the shallow groundwater. The main objective of this study was the evaluation of natural and human-induced chemical processes in the shallow alluvial aquifer of Wadi Marwani. The paper intends to find an appropriate combination of methods for a qualitative and quantitative description of aqueous geochemical processes. Assessment of groundwater quality for irrigation and drinking purposes were also carried out. This kind of investigation, when carried out extensively, would enable planners and policymakers to evolve a strategy to solve similar problems elsewhere.
2 Study area
Wadi Marwani lies in the Rabigh region of the central western Saudi Arabia to the northeast of Jeddah (about 90 km) and is located approximately between longitudes 39°22′ and 39°55′E and latitudes 22°05′ and 22°19′N (Fig. 1). It is considered as the most important tributary of Wadi Khulais, which supplies Jeddah city with an appreciable amount of domestic water. Moreover, this area is an important agricultural area in the coastal region, which supplies Jeddah with its requirements of vegetables and fruits. The Wadi Marwani area is characterized by an elevation of about 350 m above mean sea level (amsl) in the mountainous part that is totally made up of Precambrian basement rocks, whereas the wadi floor is a plain with an elevation varying between 90 and 240 m amsl. The catchment area of Wadi Marwani is 2347 km2, a total length of 70 km, maximum width of 2 km and minimum width of 150 m (Al-Nujaidi, 1978). Wadi Marwani flows through a stream of an irregular shape and direction and it is generally from east to west. The wadi floor is covered by alluvial deposits of variable grain sizes ranging from sand to gravel and boulder. The area is of arid to semi-arid climate, which is greatly modified by the Red Sea in the west and the Harrat Rahat in the east. Summer season is characterized by a humid hot climate, where maximum temperature ranges between 33 and 43 °C. Winter is characterized by mild climate with maximum temperature varies between 22 and 30 °C, generally with low relative humidity. The study area receives sporadic and scanty rainfall, which is extremely variable both spatially and temporally. Rainfall generally increases from west to east, where relatively high rates are usually recorded at the eastern boundaries of the catchment areas. Rains occur in fall, winter and spring, where the maximum total annual rainfall reached 135 and 155 mm as recorded in Khulais and Al-Kamil meteorological stations, respectively. Runoff occurs as flash floods associated with heavy short-duration storms. Most of this runoff water is lost by infiltration through highly permeable alluvial deposits filling the wadi channel, where possible recharge of the underlying shallow aquifer can occur. The available runoff records at Wadi Marwani gauge station during the period 1966–1976 shows that the maximum amount was 223.9 × 106 m3 recorded in 1974/1975.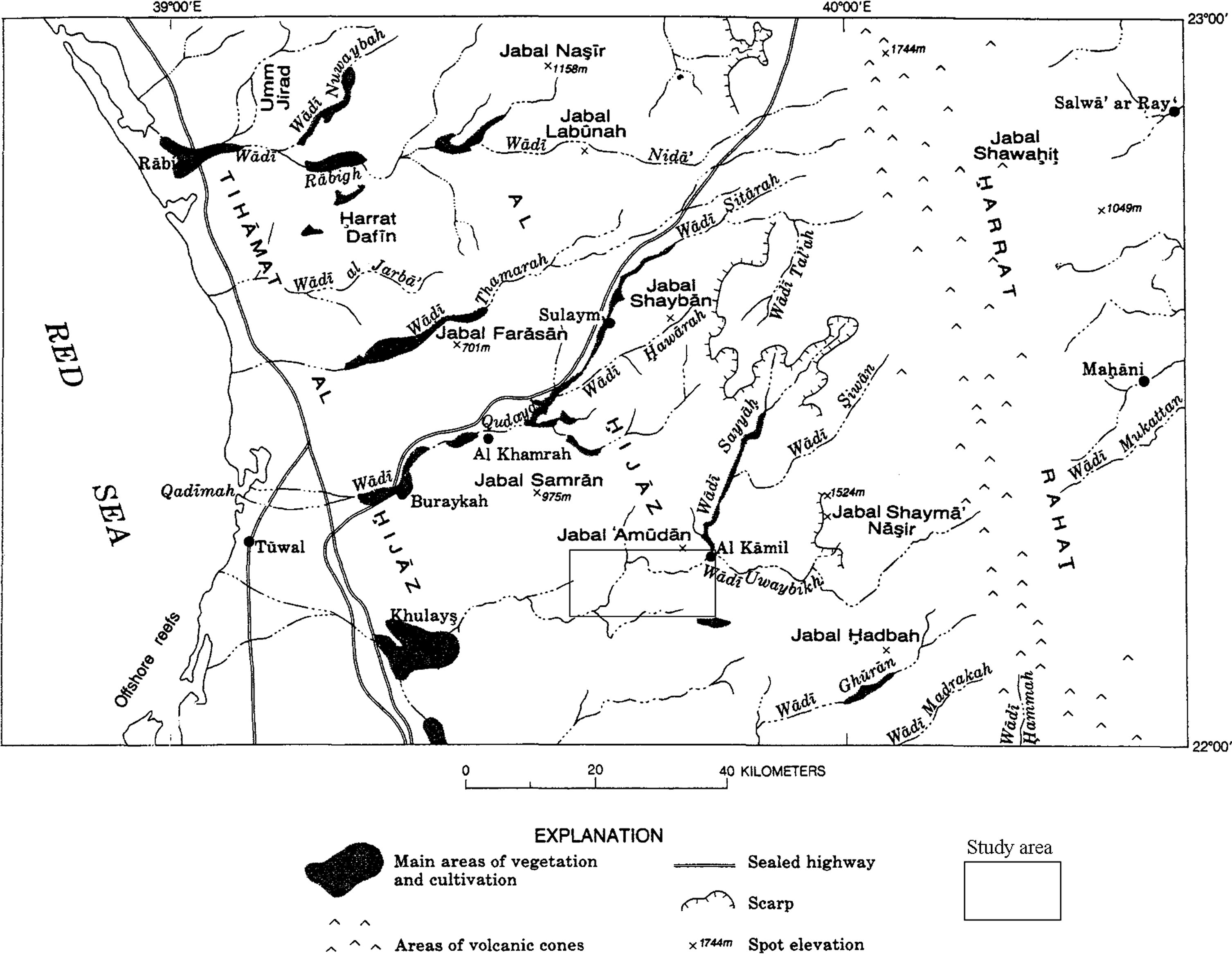
Location map of the study area.
3 Geologic setting
Wadi Marwani is located to the southeast of Rabigh where the entire area represents an important part of the southern Hijaz region in the western part of Saudi Arabia. The area occupies a sector of the Arabian Shield, which is composed of Precambrian igneous and metamorphic rocks (Fig. 2). The geologic setting of this region was described by many authors (Skiba and Gilboy, 1975; Al-Nujaidi, 1978; Skiba, 1980). The dominant rock unit in the study area is the alkali olivine basalt of the Rahat Group (Hammah Formation) ranging in age from Paleocene to Miocene with subordinate alkalic intermediate lava, some minor feldspathoidal lava and some minor pyroclastics. The upstream part of Wadi Marwani drains this dominant rock unit. Sericite–chlorite schist of the Samran Group (Late Proterozoic) represents the oldest rock unit encountered in the central part of Wadi Marwani. Major part of the wadi drains an area comprises different rock units of the Kamil Suite, which is made up of slightly metamorphosed hornblende–biotite tonalite and granodiorite with minor granitic and dioritic rocks. Moreover, some outcrops of post-tectonic unmetamorphosed plutonic rocks are encountered in the study area including granite, quartz diorite, quartz monzodiorite, syenite and gabbro. The floor of Wadi Marwani is filled with Quaternary alluvial deposits being the weathering products of the varying rock units drained by the wadi. This alluvial cover is mainly composed of medium to coarse grained gravel along the wadi channels and fine sands and clay in the flat area.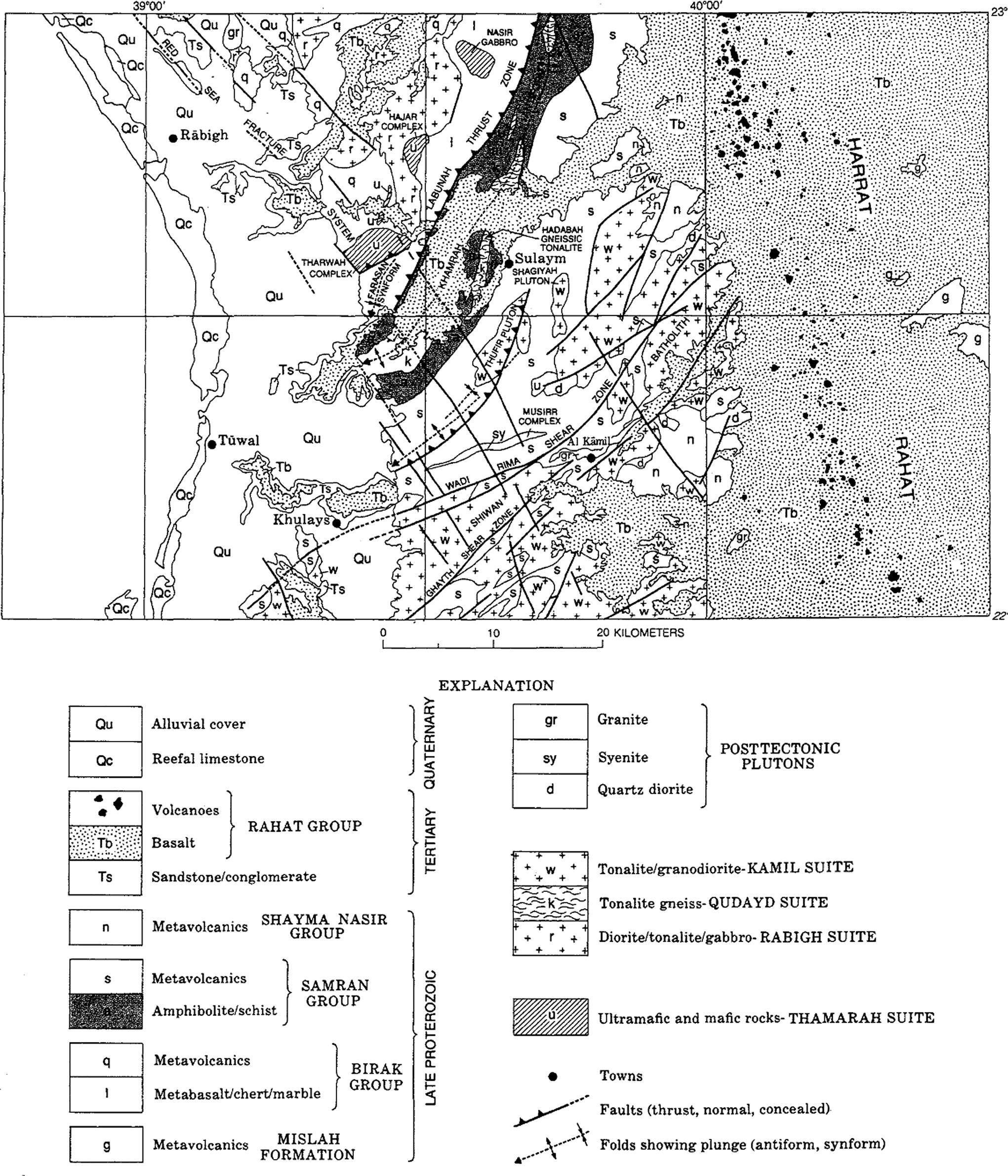
Geologic map of the Rabigh area (Ramsay, 1986).
4 Hydrogeologic setting
The western part of Saudi Arabia is considered as an important region from the hydrogeological point of view, where it has very important water resources, on which many development projects have been recently established. Abdulrazzak et al. (1992), Bazuhair and Wood (1996) and Al-Shaibani (2008) estimated groundwater recharge rates for some selected wadis in western Saudi Arabia using the chloride mass-balance method. Hydrogeological and geophysical studies of the Wadi Marwani area have been carried out by several investigators. Italoconsult (1967, 1976), Al-Nujaidi (1978) and Bazuhair et al. (1992) studied the hydrogeology of the water resources of Wadi Marwani–Khulais area using electrical geophysical methods. In the present study, field surveys were carried out to investigate geology and to measure groundwater levels in 16 hand dug wells scattered along Wadi Marwani. Groundwater exists under unconfined conditions in the alluvial deposits. Groundwater is exploited from a shallow alluvial unconfined aquifer where the depth to water table varies from 5 to 15.3 m below the ground surface. Shallow depth of groundwater and the occurrence of highly porous and permeable overlying alluvial deposits increase the aquifer vulnerability to contamination from human activities in this area. Therefore, recirculation of groundwater is common when the water is used for irrigation. Groundwater levels vary between 386 m in the upstream portion and 238 m near the downstream. The groundwater flows from east towards west with an average value of hydraulic gradient of 0.004. Al-Nujaidi (1978) revealed that the depth to basement rocks in Wadi Marwani varies from 25 to 100 m. This variation of the depth to basement rocks was interpreted as a result of faulting structures. The saturated thickness of the aquifer varies due to variation in depth to basement rocks as well as the lateral extent of the aquifer. The aquifer transmissivity varies between 144 and 5760 m2/day and its specific yield ranges between 0.001 and 0.006 (Al-Nujaidi, 1978).
5 Sampling and analytical procedures
Sixteen groundwater samples were collected from private supply wells at depths of 5.00–15.30 m for hydrochemical investigation (Fig. 3). All the samples were analyzed for major ions (calcium Ca2+, magnesium Mg2+, sodium Na+, potassium K+, bicarbonate
, sulfate
, and chloride Cl−) and some minor ions (nitrate
, nitrite
, fluoride F− and silica SiO2) using the standard methods (American Public Health Association, APHA, 1998). Electrical conductivity (EC) and hydrogen ion concentration (pH) were measured at the wellhead using portable EC- and pH-meter. Chemical analyses for major and some minor ions were performed at the Laboratory of Faculty of Earth Sciences, King Abdulaziz University, Saudi Arabia. Total dissolved solids (TDS) were measured by sample evaporation techniques.
was determined by titration against standard HCl. Cl− was estimated by titration against standard solution of AgNO3.
was determined gravimetrically. Ca2+ and Mg2+ were analyzed by compleximetric titration against standard EDTA solution. Na+ and K+ were analyzed by flame photometry.
,
, F− and SiO2 were determined colorimetrically using spectrophotometric technique (APHA, 1998). The analytical precision for the measurements of cations and anions is indicated by the ionic balance error, which was computed on the basis of ions expressed in milliequivalent per liter. The values were observed to be within a standard limit of ±5% (Domenico and Schwartz, 1998). All concentration values were expressed in milligram per liter (mg/l) unless otherwise indicated.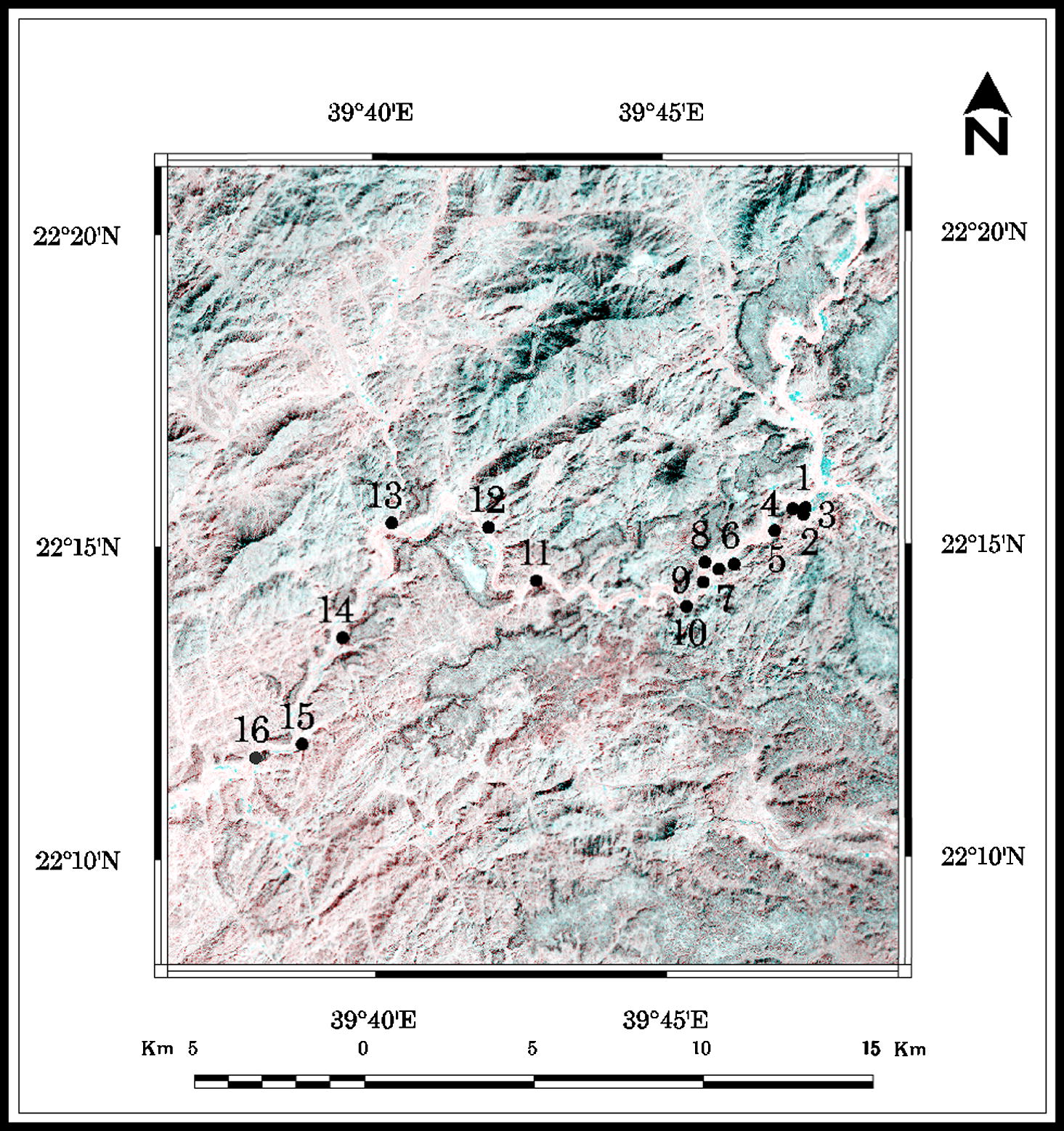
Well location map of Wadi Marwani.
6 Results and discussion
Hydrochemical characteristics of groundwater in the study area are summarized in Table 1. The pH of groundwater varies within small range (7.2–7.4), which elaborates a slight trend of alkaline chemical reaction within the groundwater system. The electrical conductivity (EC) varies from 490 to 2295 μS/cm. Table 1 shows that only one water sample is fresh water (<500 μS/cm), while most of the samples are marginal waters (500–1500 μS/cm) and only one sample shows brackish water type (1500–5000 μS/cm) in the study area. TDS values range between 372 mg/l in the upstream part of Wadi Marwani to 1568 mg/l in the downstream part and it appears that about 15 samples have TDS less than 760 mg/l while only one sample (no. 13) reached to 1568 mg/l. The average TDS for the study area is 630 mg/l. It is clear that there is a gradual increase in groundwater salinity from upstream to downstream which coincides with the groundwater flow direction. Extraordinary salinity values of groundwater sample no. 2 in the upstream part, sample no. 7 in the middle part, and sample no. 13 near the downstream may be attributed to the influence of residential and farming activities at these locations. This can be caused by leakage of domestic wastewater from improperly designed septic tanks, which are usually unlined, and by return flow from irrigation practices in the farm areas. These two influences are reflected in the high salinity values. Such a wide range of EC and TDS values indicates that hydrochemistry of groundwaters in Wadi Marwani is controlled by various hydrochemical processes such as anthropogenic pollution and water–rock interaction. As shown in Table 1, the concentrations of major dissolved constituents were also quite variable. Such wide ranges of ionic concentrations also indicate the involvement of several hydrochemical processes influencing the water quality. EC: electrical conductivity, TDS: total dissolved solids, TH: total hardness, Temp. H: temporary hardness.
Well no.
pH
EC (μS/cm)
TDS
Ca
Mg
Na
K
HCO3
SO4
Cl
SiO2
NO3
NO2
F
TH
Temp. H
1
7.30
490
372
47.2
15.3
45.6
3.9
177.8
66
52.7
18.97
12.90
0.037
0.24
180.7
145.7
2
7.29
1040
754
118.2
39.6
74.8
4.8
273.5
103
155.2
22.70
86.00
0.035
0.52
457.8
224.2
3
7.30
510
400
47.4
17.8
51.5
4.2
236.2
61
43.4
21.10
11.70
0.047
0.75
191.5
191.5
4
7.30
525
413
48.8
18.5
53.1
3.6
218.7
70
52.8
19.80
13.10
0.037
0.72
197.9
179.2
5
7.30
760
590
63.3
25.9
95.5
4.8
209.8
111
114.8
20.20
32.80
0.047
0.41
264.5
171.9
6
7.30
830
602
72.0
27.6
78.2
4.6
184.5
125
127.5
17.90
28.10
0.039
0.69
293.2
151.2
7
7.20
1042
741
98.4
30.4
109.3
5.4
217.5
184
162.9
21.04
27.50
0.034
0.49
370.6
178.3
8
7.32
910
636
77.1
27.5
88.6
4.9
156.9
172
144.9
16.44
23.40
0.057
0.41
305.5
128.6
9
7.22
580
591
76.2
26.8
81.7
4.5
180.8
147
124.8
16.66
27.50
0.058
0.53
300.4
148.2
10
7.30
890
619
71.3
27.4
82.3
5.2
185.4
137
132.2
18.88
25.00
0.056
0.36
290.6
151.9
11
7.40
785
538
62.2
24.7
69.2
4.4
132.7
136
116.5
18.96
21.20
0.053
0.30
256.80
108.8
12
7.30
805
536
60.7
21.6
69.4
4.8
143.8
129
116.8
13.41
24.60
0.036
0.51
240.3
109.7
13
7.20
2295
1568
174.2
61.8
255.1
6.7
198.6
244
476.7
24.78
51.40
0.071
0.30
688.80
162.8
14
7.32
880
587
77.2
25.3
80.1
5.8
142.6
143
137.7
13.77
17.20
0.035
0.79
296.7
116.9
15
7.38
860
577
71.5
24.2
81.7
4.6
154.2
150
130.2
14.85
14.20
0.042
0.69
277.90
126.4
16
7.40
840
562
64.4
24.5
75.4
5.5
125.8
154
137.5
13.95
9.63
0.045
0.39
261.5
103.1
6.1 Water hardness
The high total concentrations of Ca2+ and Mg2+, are important factors, which increase the hardness of waters. Total hardness (TH) of the water samples of Wadi Marwani varies through a wide range between 181 mg CaCO3/l in the upstream part and 689 mg CaCO3/l in the downstream part (Table 1). This table shows that the groundwater ranges from slightly hard (150–250 mg CaCO3/l) to very hard (>420 mg CaCO3/l). It is also clear that most of the samples are slightly to moderately hard. Generally, these high values of total hardness could be related to lithology of the aquifer matrix, which is mostly formed of weathering products of the surrounding basic and intermediate igneous rocks of the Arabian Shield. These rocks are rich in calcium and magnesium which upon dissolution yield high concentrations of Ca and Mg that increase total hardness of the groundwater. However, dissolution of silicate minerals is a slow process (Appelo and Postma, 2005). An important component of the total hardness is temporary hardness, equivalent to water alkalinity, and is caused by a combination of calcium ions and bicarbonate ions in water (Table 1). Temporary hardness leads to precipitation of Ca and Mg carbonates in the soil zone when this water is used for irrigation in such dry area with high rate of evaporation. These precipitated salts will be further leached from the soil zone by CO2-bearing irrigation water and this will increase Ca and Mg concentrations in the underlying groundwater, which consequently will increase its hardness. The remaining component of total hardness is the permanent hardness, which is hardness (mineral content) that cannot be removed by boiling. It is usually caused by the presence of calcium and magnesium sulfates and/or chlorides in the water. This component is only of concern when assessing water for domestic and industrial uses. Generally, groundwater of Wadi Marwani is characterized by high temporary and permanent hardness.
6.2 Representation of hydrochemical data
To understand the spatial control of major ions concentration, the relationships between TDS (as a useful indicator of anthropogenic contamination) and major ions are illustrated in Fig. 4. For all major ions examined, concentrations tend to increase with increasing TDS, whereas an anomalous sample (no. 13) with the highest salinity value was observed. This positive correlation suggest the impact of agricultural activities on groundwater chemistry through leaching of readily soluble salts from the soil zone, where these salts are precipitated under the influence of high rate of evaporation. The leaching process leads usually to increase concentrations of most major ions.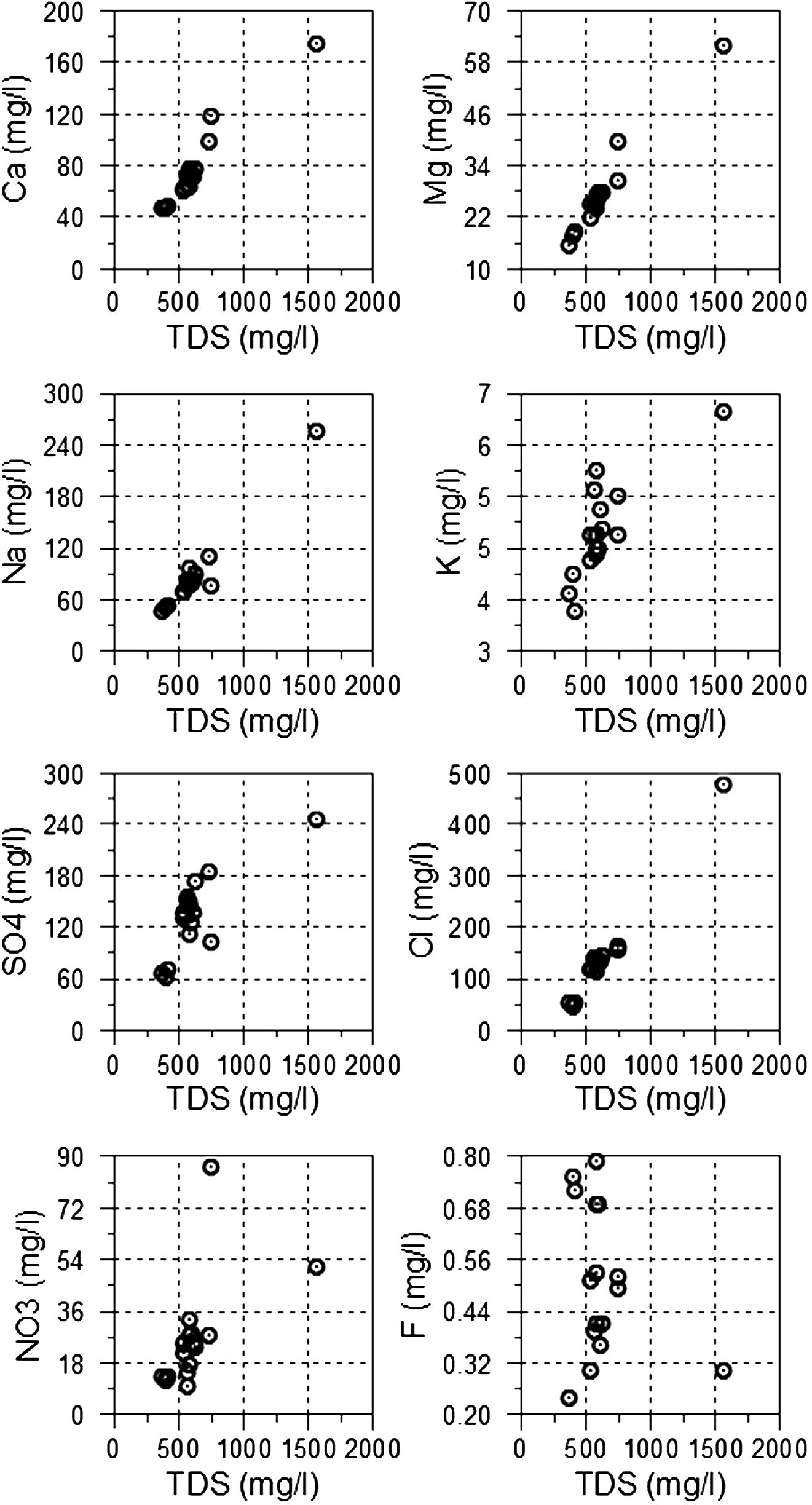
Relationships between TDS and the examined major and minor ions.
TDS is positively correlated to K concentrations of groundwater indicating the influence of agricultural activities and wastewater leakage, where it is known that potassium ion originates from agricultural fertilizer and wastewater (Trauth and Xanthopoulos, 1997). On the other hand, the relationship between NO3 and TDS show similar trend which suggested the leaching of nitrate fertilizers applied in the agricultural areas. Only one sample (no. 2) showed anomalously high nitrate concentration which is mainly attributed leakage of domestic wastewater from the residential area that is very close to this well. NO3 concentration may be further affected by complex hydrochemical processes such as nitrification or denitrification (Arnade, 1999; Graniel et al., 1999; Rosen et al., 1999; Sliva and Williams, 2001). Fluoride concentration varies from 0.24 to 0.79 mg/l and show indistinct trend with TDS, where its spatial distribution is independent of salinity of groundwater. This suggested different source of fluoride in groundwater rather than anthropogenic sources. The possible source of fluoride in groundwater is the dissolution of silicate minerals composing the aquifer matrix.
General distribution of groundwaters of Wadi Marwani is shown on a Piper diagram (Fig. 5). On the basis of this diagram, it is clear that in all waters, alkali earth elements (Ca + Mg) are higher than alkali elements (Na + K). The abundance of the alkali earth elements is attributed to dissolution of Ca and Mg-rich silicate minerals in the aquifer matrix. The removal of Ca, through the precipitation of carbonate minerals, may cause a disturbance in chemical equilibrium, resulting in the dissolution of minerals containing calcium such as calcite and gypsum (Freeze and Cherry, 1979). These minerals are possibly precipitated from irrigation water under the influence of high rate of evaporation and evapotranspiration. In few samples (nos. 1, 3, and 4), weak acids (HCO3) are higher than strong acids (Cl + SO4). These samples are representing the upstream part of Wadi Marwani, whereas the rest of the samples are characterized by the dominance of strong acids over weak acids. This change of the anion dominance is consistent with groundwater flow from the upstream to downstream, where the anthropogenic activities are the controlling factor. Irrigation practices involve dissolution of the readily soluble salts of chloride and sulfate ions precipitated in the soil zone due to high rate of evaporation. Generally, water chemistry is related to the different rocks and their interaction with water as well as impact of human activities which tend to alter the groundwater quality.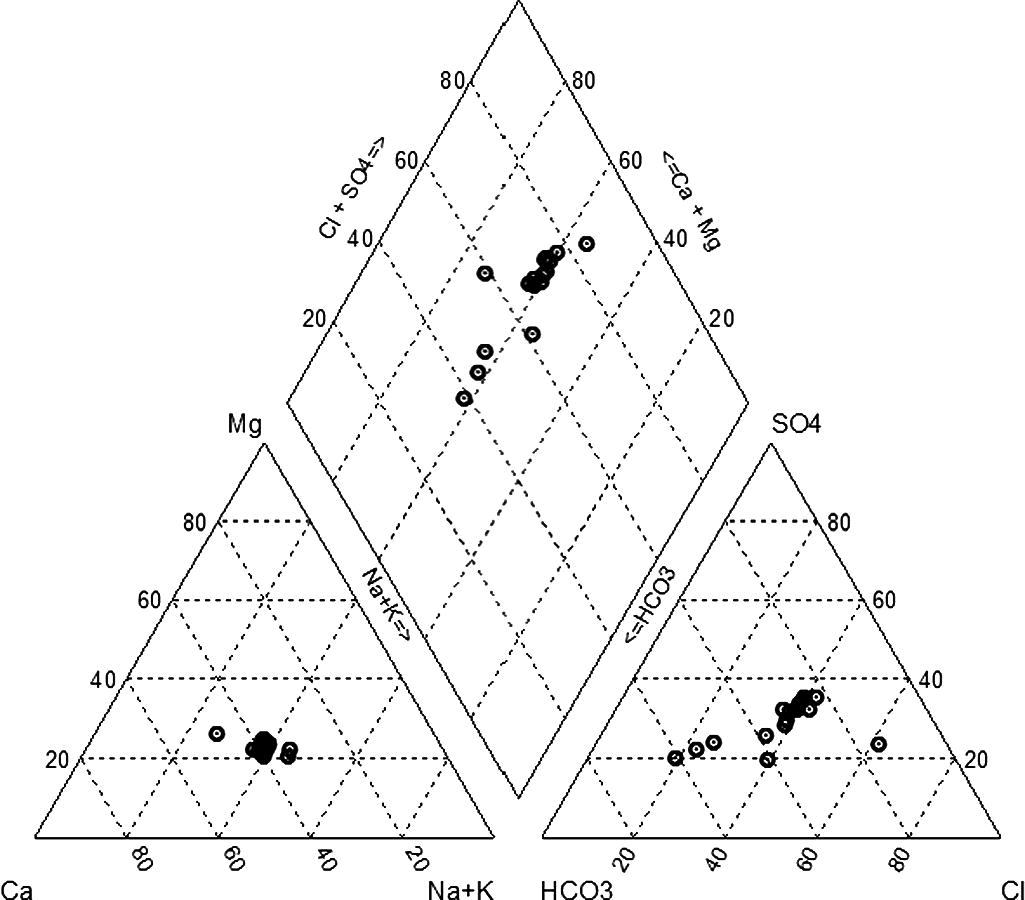
Piper diagram for the Wadi Marwani alluvial aquifer groundwaters.
The difference between ionic composition lines of groundwaters on the Schoeller diagram (Fig. 6) indicates that there are three major water types originating during water–rock interaction as well as impact of agricultural activities. The first group has the lower salinity values, which represents the upstream portion of Wadi Marwani and includes three samples (1, 3, and 4), where these samples are characterizing the recharge water composition. The second group includes clusters of most of samples having relatively higher salinity than the previous group. In this group, change of the anion dominance from HCO3 to Cl and SO4 is pronounced with groundwater flow. The third group includes only one sample (no. 13) of the highest salinity and dominance of Cl and SO4. This sample with anomalous high salinity represents an area of extensive agricultural development and irrigation practices.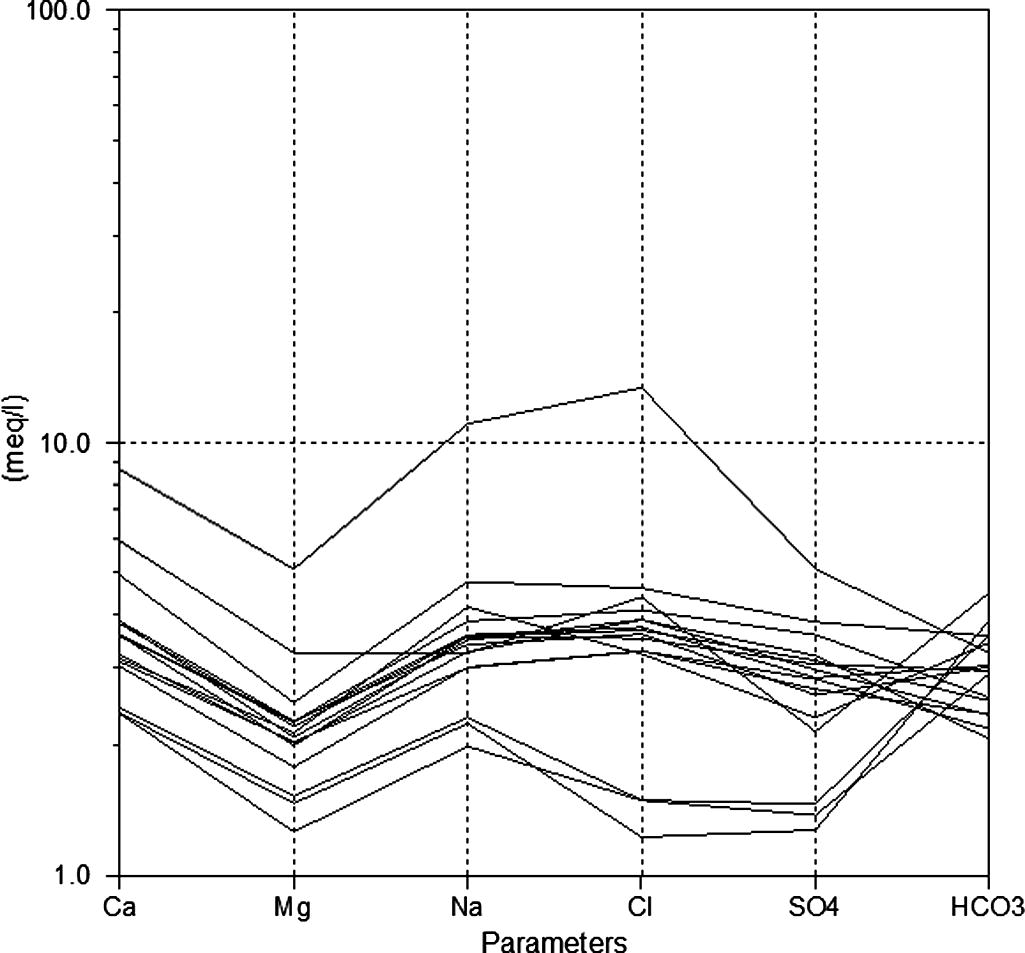
Schoeller diagram for the groundwaters of Wadi Marwani.
6.3 Hydrochemical facies
Groundwater facies of the unconfined aquifer in the study area have been examined separately on the basis of chemical analyses of 16 well waters (Table 2). This table indicates that Ca was found to be the most dominant cation in the hydrochemical facies of 11 water samples which is followed by Na and Mg. The dominance of Ca suggested its origin by dissolution of basic and intermediate silicate minerals forming the aquifer matrix. Sodium appeared as the dominant cation in the hydrochemical facies of five water samples, which is probably due dissolution of more acidic silicate minerals in the downstream part of Wadi Marwani and/or caused by cation exchange on clays which also increase in the downstream part. The removal of Ca from solution by exchange with Na causes the groundwater to become or remain undersaturated with respect to calcite and gypsum, thereby enabling calcite and gypsum dissolution to continue by return flow of irrigation water. HCO3 was found to be the most dominant anion in five upstream water samples followed by Cl and SO4 or NO3 in one sample (no. 2). The Cl and NO3 concentration tends to increase in samples collected from residential and agricultural areas. The dominance of HCO3 in the upstream samples reflected recharge water characteristic. In the remaining samples, Cl replaced HCO3 in their hydrochemical facies, which reflects geochemical evolution along flow path and the influence of agricultural activities and residential areas. Therefore, the hydrochemical change in relation to the hydrochemical processes and land-use in Wadi Marwani is predominantly in cation and anion distribution.
Well no.
Hydrochemical facies
1
Ca–Na–Mg–HCO3–Cl–SO4
2
Ca–Mg–Na–HCO3–Cl–NO3
3
Ca–Na–Mg–HCO3
4
Ca–Na–Mg–HCO3–Cl–SO4
5
Na–Ca–Mg–HCO3–Cl–SO4
6
Ca–Na–Mg–Cl–HCO3–SO4
7
Ca–Na–Cl–SO4–HCO3
8
Na–Ca–Mg–Cl–SO4–HCO3
9
Ca–Na–Mg–Cl–SO4–HCO3
10
Na–Ca–Mg–Cl–HCO3–SO4
11
Ca–Na–Mg–Cl–SO4–HCO3
12
Ca–Na–Mg–Cl–SO4–HCO3
13
Na–Ca–Mg–Cl–SO4
14
Ca–Na–Mg–Cl–SO4–HCO3
15
Ca–Na–Mg–Cl–SO4–HCO3
16
Na–Ca–Mg–Cl–SO4–HCO3
6.4 Groundwater recharge
The chloride mass-balance method of recharge estimation is commonly used for arid and semi-arid regions. This method provides a direct estimation procedure of the groundwater recharge based on the assumption that the chloride concentrations in the rainfall and recharge are in steady-state balance, i.e., input is equal to output without chloride storage change during a time period. The method has been shown to yield regional values of groundwater recharge comparable to those obtained by physically based methods, given that certain assumptions and conditions are met (Wood and Sandford, 1995). It requires only knowledge of annual precipitation, chloride concentration in precipitation and chloride concentration in groundwater. The basic equation of the chloride mass-balance method was given by Wood and Sandford (1995); where q = recharge flux (mm/y), P = average annual precipitation (mm/y), Clwap = weighted average chloride concentration in precipitation (mg/l), Clgw = average chloride concentration in groundwater (mg/l).
Bazuhair and Wood (1996) proposed that the regional precipitation-weighted chloride concentration of the mountainous area along the western edge of Saudi Arabia ranges from 6 to 12 mg/l; an average value of 9 mg/l was used for this study. The average annual precipitation for a period of 10 years (1987–1996) as recorded in Al-Kamil meteorological station is 60 mm, where this station is located in the upstream part of Wadi Marwani. Average chloride concentration in groundwater from the upstream part of the Wadi Marwani is 49.6 mg/l. It is worth to mention that some groundwater samples in the upstream part had high Cl concentrations, which is attributed to anthropogenic effects, were excluded from the calculations. This is consistent with the assumption that precipitation is the only source of chloride. The recharge rate of Wadi Marwani was estimated to be 18% of the annual precipitation. Since this value is based only on data from the upstream part, the recharge rate is expected to be more in the lower parts of the aquifer as more runoff is observed. However, calculation of recharge rate using Cl concentrations of anthropogenically contaminated groundwaters will yield very low values, such as the value of <1% for Khulais Basin calculated by Bazuhair and Wood (1996).
6.5 Geochemical modeling
The geochemical interactions lead to changes in water chemistry. The potential for a chemical reaction can be determined by calculating the chemical equilibrium of the water with the mineral phases. The equilibrium state of the water with respect to a mineral phase can be determined by calculating a saturation index (SI) using analytical data. The potential for mineral precipitation or dissolution is assessed using the saturation index (SI), which is based on the relation between analytic activities (the ion activity product, IAP) and the thermodynamic calculation of the solubility product (Ksp). The SI of a mineral is determined using the following equation (Parkhurst, 1995):
If SI > 0 the solution is theoretically oversaturated with respect to the mineral and precipitation may be expected. For SI = 0, the mineral and solution are in equilibrium and neither dissolution nor precipitation is predicted to occur. If SI < 0, the solution is theoretically undersaturated with respect to the mineral, and if present in the system, dissolution might be possible.
The geochemical modeling program PHREEQC (Parkhurst and Appelo, 1999) was used to evaluate the water chemistry. Table 3 shows the results of SI calculations for the groundwater with respect to various specific minerals. The values in Table 3 show that most of the groundwaters are slightly to moderately undersaturated with respect to calcite and dolomite, water sample no. 13 is slightly oversaturated with respect to calcite and slightly undersaturated with respect to dolomite. Sample no. 2 is the only sample that is slightly oversaturated with respect to these carbonate minerals. In these two samples, the influence of anthropogenic activities is manifested in high TDS as well as different dissolved ions. All groundwater samples are highly undersaturated with respect to sulfate minerals (gypsum and anhydrite) and fluorite, whereas they are moderately oversaturated with respect to quartz and slightly oversaturated with respect to chalcedony.
Well no.
Calcite
Dolomite
Gypsum
Anhydrite
Fluorite
Quartz
Chalcedony
1
−0.33
−0.86
−1.93
−2.17
−2.37
0.55
0.110
2
0.16
0.12
−1.52
−1.76
−1.43
0.63
0.189
3
−0.21
−0.56
−1.97
−2.21
−1.39
0.60
0.156
4
−0.23
−0.60
−1.91
−2.15
−1.42
0.57
0.128
5
−0.18
−0.47
−1.67
−1.91
−1.85
0.58
0.138
6
−0.19
−0.51
−1.57
−1.81
−1.35
0.53
0.085
7
−0.12
−0.46
−1.33
−1.57
−1.56
0.60
0.156
8
−0.22
−0.61
−1.43
−1.66
−1.79
0.49
0.048
9
−0.26
−0.69
−1.49
−1.73
−1.56
0.50
0.054
10
−0.19
−0.52
−1.54
−1.78
−1.92
0.55
0.108
11
−0.28
−0.69
−1.58
−1.81
−2.12
0.55
0.110
12
−0.38
−0.93
−1.63
−1.87
−1.66
0.40
−0.041
13
0.02
−0.13
−1.11
−1.35
−1.85
0.67
0.229
14
−0.25
−0.70
−1.49
−1.72
−1.20
0.42
−0.029
15
−0.19
−0.57
−1.49
−1.73
−1.35
0.45
0.004
16
−0.30
−0.73
−1.52
−1.75
−1.88
0.42
−0.024
The carbonate chemistry of the groundwaters used for irrigation in agricultural areas can be produced by the addition of dissolved CO2 gas into the water. The source of the CO2 could be dissolution of atmospheric carbon dioxide, but it is more likely to be from the decay of organic matter in these areas. Regardless of the source, the added CO2 reduces the pH of the water and results in the dissolution of carbonate minerals in the soil zone. Additional silicate dissolution would be prompted by the lower pH, which is reflected in higher SiO2 concentrations in groundwater (13.41–24.78 mg/l).
6.6 Water quality and anthropogenic action
One of the aims of this study was to define the groundwater quality for drinking use and irrigation purposes. The presence of human activities such as agriculture, animal breeding and farming all exert anthropogenic pressure on the quality of the shallow alluvial groundwater in Wadi Marwani. In this area, there are several factors that cause contamination of groundwater. These are return flow caused by irrigation using the underlying groundwater and leakage of sewage from the residential areas where disposal of domestic wastewater are carried out in unlined septic tanks and latrines.
6.6.1 TDS distribution as a contamination indicator
The TDS values of groundwaters show a systematic control on land-use characteristics and tend to increase in the agricultural and residential areas. On the other hand, relatively low TDS values in the upstream part suggesting that increase of TDS downgradient is not significantly affected by water–rock interaction commonly occurring in the course of groundwater flow in the alluvial aquifer. This is because of the slow rates of dissolution of most silicate minerals composing the aquifer matrix. Therefore, the distribution of TDS in Wadi Marwani groundwaters likely reflects the dominant land-use characteristics around each well and indicates the effects of anthropogenic contamination. Thus, the TDS value can be effectively used as an indicator of groundwater contamination for planning sustainable management of this groundwater resource. The hydrochemistry of the samples from upstream part of Wadi Marwani is dominantly controlled by water–rock interaction within the aquifer and therefore can be used as a natural ‘background’ (sample nos. 1, 3, and 4). The general upper limits of the concentrations in this area are <49 mg/l for Ca, <18.5 mg/l for Mg, <53.1 mg/l for Na, <4.2 mg/l for K, <236 mg/l for HCO3, <53 mg/l for Cl, <70 mg/l for SO4, and <13.1 mg/l for NO3. These values can be effectively used as hydrochemical backgrounds for the evaluation of contamination status as the basis of a groundwater management plan for Wadi Marwani.
As the TDS of groundwater is strongly positively correlated with Cl concentration and due to conservative nature of Cl in the groundwater system, Cl in groundwater can also be used as useful indicator of anthropogenic contamination. Potential sources for Cl include domestic wastewater, septic effluent, agrichemicals and manure. Shallow groundwater is more likely to show effects of anthropogenic contamination from surficial activities than deep groundwater (Gillion et al., 1995). The highest Cl concentrations were recorded in wells no. 2 and 13 where intense agricultural activities are common at these sites as well as closeness to residential areas.
6.6.2 Nitrate
Nitrate contamination of groundwater is a common occurrence in many parts of the world. In drinking water, nitrate in excess of 50 mg NO3/l may be toxic for infants and may be responsible for increases in stomach cancer for others (Spalding and Exner, 1993). Possible sources of nitrate contamination include manure applied to land, agricultural fertilizer, industrial effluent, domestic wastewater, septic systems, human waste lagoons, animal feedlots, and native soil organic matter, as well as geologic sources (Helmut, 2000). The average human contribution to wastewater is about 5 kg N–NO3 P/person/year (Lewis et al., 1982) in organic forms which are subsequently mineralized to inorganic species during decomposition (in septic tanks or during percolation). Because nitrate is very mobile in most soils, it can be leached from all these sources into groundwater (Nolan, 1999). Furthermore, it is often difficult to ascertain which of these multiple sources in a watershed may be contributing to nitrate in the groundwater. The determination of the sources of nitrate in groundwater is an important first step in the process of improving groundwater quality, and perhaps reducing the amount of nitrate discharging to water bodies.
The NO3 concentration in the study area was within the range of 9.6–86.0 mg NO3/l. High nitrate levels were recorded in only two wells (nos. 2 and 13) and reached 86 and 51.4 mg NO3/l, respectively. These two wells were identified as being likely contaminated by point source pollution: a domestic septic tank. With the increasing distance from the septic tanks, the NO3 concentration became lower. The range of NO3 concentration in different areas reflected the influence of anthropogenic factors.
Low concentrations of NH4 (<0.01 mg/l in all samples) and nitrite (0.034–0.071 mg/l) indicates aerobic conditions of the shallow groundwater, which leads to oxidation of these nitrogen compounds into NO3. Generally, nitrogen as ammonia and in organic matter from domestic sewage is converted to nitrate in oxic groundwater. However, microbial activities associated with groundwater contamination with sewage may lead to increase NO2 in groundwater. On the other hand, increase of NO3 levels in groundwater in the study area with increasing TDS supports the idea of groundwater contamination by anthropogenic activities.
The nitrate concentrations of groundwater in Wadi Marwani decrease along flow path (from east towards west). Smith-Carrington et al. (1983) reported a similar case from a chalk aquifer in Britain and suggested that the downgradient decrease of nitrate in oxic groundwaters may be related to downgradient dispersion and dilution.
6.6.3 Fluoride
Fluoride-rich groundwater is well known in granite aquifers in India and the world. In general, apatite and fluorite, besides the replacement of hydroxyl by fluorine ions in mica, hornblende and soil that mostly consists of clay minerals, are the major sources of fluoride (F) in circulating water (Hem, 1989; Subba Rao et al., 1998; Saxena and Ahmed, 2001, 2003). Although its presence is necessary, chances of health risk become high if the fluoride concentration is more than the permissible limit of 1.5 mg/l (WHO, 1993). Fluoride concentration is an important aspect of hydrogeochemistry, because of its impact on human health.
In the present study, the aquifer matrix or alluvium represents weathering products of various types of igneous and metamorphic country rocks. These rocks are composed of the previously mentioned silicate minerals which could be the possible sources of F in groundwater. To examine the influence of such sources on F in groundwater, the relation between its content in groundwater and TDS is shown in Table 1 and is illustrated in Fig. 4. No distinct relation was observed, which indicated that fluoride in groundwater is not derived from anthropogenic activities and this suggested that it is derived from dissolution of silicate minerals compositing the aquifer matrix.
6.7 Groundwater quality assessment for different purposes
The main objective following the hydrochemical investigations of groundwater is to determine its suitability for different uses.
6.7.1 Drinking purposes
According to the WHO (1993), no standards have been established for TDS, chloride, sodium, potassium, hardness, and sulfate and their effects on health. The only standards that have been established are based on taste. The nitrate standard, however, is based on health effects. This also applies to the fluoride standard, but depending on local climatological conditions, and the amount of water consumed, it is sometimes difficult to meet this standard. The chloride standard is primarily based on the undesirable taste effect at a concentration higher than about 250 mg/l. However, people may get used to drinking water with a chloride concentration higher than 250 mg/l, and a concentration up to 600 mg/l is considered safe. Regarding sodium, it is believed that there is a relationship between sodium levels in drinking water and the occurrence of high blood pressure, however, no firm evidence supports this. Therefore, the sodium standard is only based on negative taste effects. These occur at a concentration higher than 200 mg/l. No data are available on the possible health effects of the TDS content. However, TDS may strongly influence the taste of drinking water. Water with a TDS lower than 1000 mg/l is usually acceptable for consumers, although this may strongly depend on local conditions. Water with a low TDS tastes flat and is often considered to be tasteless. There are indications that extremely soft water adversely affects the mineral balance. The public acceptance of the hardness of the water strongly depends on the local conditions. The taste limit for calcium is, somewhere, between 100 and 300 mg/l and for magnesium it is probably lower. In some cases, a hardness of 500 mg/l is tolerated by consumers. Sulfate is one of the least toxic anions. However, the presence of a high concentration in the drinking water may lead to dehydration, stomach complaints, and possibly diarrhea. Therefore, authorities are advised to be alert in cases of occurrence of water with a sulfate concentration higher than 500 mg/l. In general, the adverse affect on the taste is said to be minimal at levels lower than 250 mg/l. The 50 mg/l standard for nitrate is based on health effects. The WHO standard for fluoride of 1.5 mg/l is also based on health effects. A higher concentration increases the chance of skeletal deformations (fluorosis).
Comparison of the concentration of chemical constituents of well water of Wadi Marwani with respect to the drinking water standards of WHO (1993) showed that sample 2 (upstream part) and no. 13 (downstream part) had NO3 contents above the permissible limits. Generally, concentrations of the investigated ions in groundwater are below their limits, therefore, groundwater can be used safely for drinking. However, high NO3 content in most groundwater samples is alarming for further increase due to increase of the anthropogenic activities in the study area. In addition, microbiological investigation of these groundwaters should be carried out periodically to ensure absence of harmful microorganisms.
6.7.2 Irrigation purposes
In order to identify the suitability of groundwaters for irrigation use, the Wilcox classification diagram (1955) in Fig. 7 has been used. This graph is based on the electrical conductivity (EC) and on the sodium adsorption ratio (SAR). This parameter is of particular importance because high Na contents in irrigation waters may increase soil hardness and reduce its permeability (Tijani, 1994). The use of saline waters in permeable rocks may also increase the salinity of groundwaters. In Fig. 7, only one sample (no. 13) was plotted in C4-S2 field indicating very high salinity hazard and medium sodium hazard. Irrigation using groundwater from this site should be assessed in terms of selection of salt-tolerant crops and good drainage especially on clay soils. The remaining samples were clustered in two fields (C2-S1 and C3-S1) indicating medium to high salinity hazard and low sodium hazard. These groundwaters can be used safely for irrigation on clay soil, however, selection of crops depending on salt tolerance should be carried out prior to cultivation. Boron content in groundwater should be periodically determined because of its toxic effect on plants over certain limits.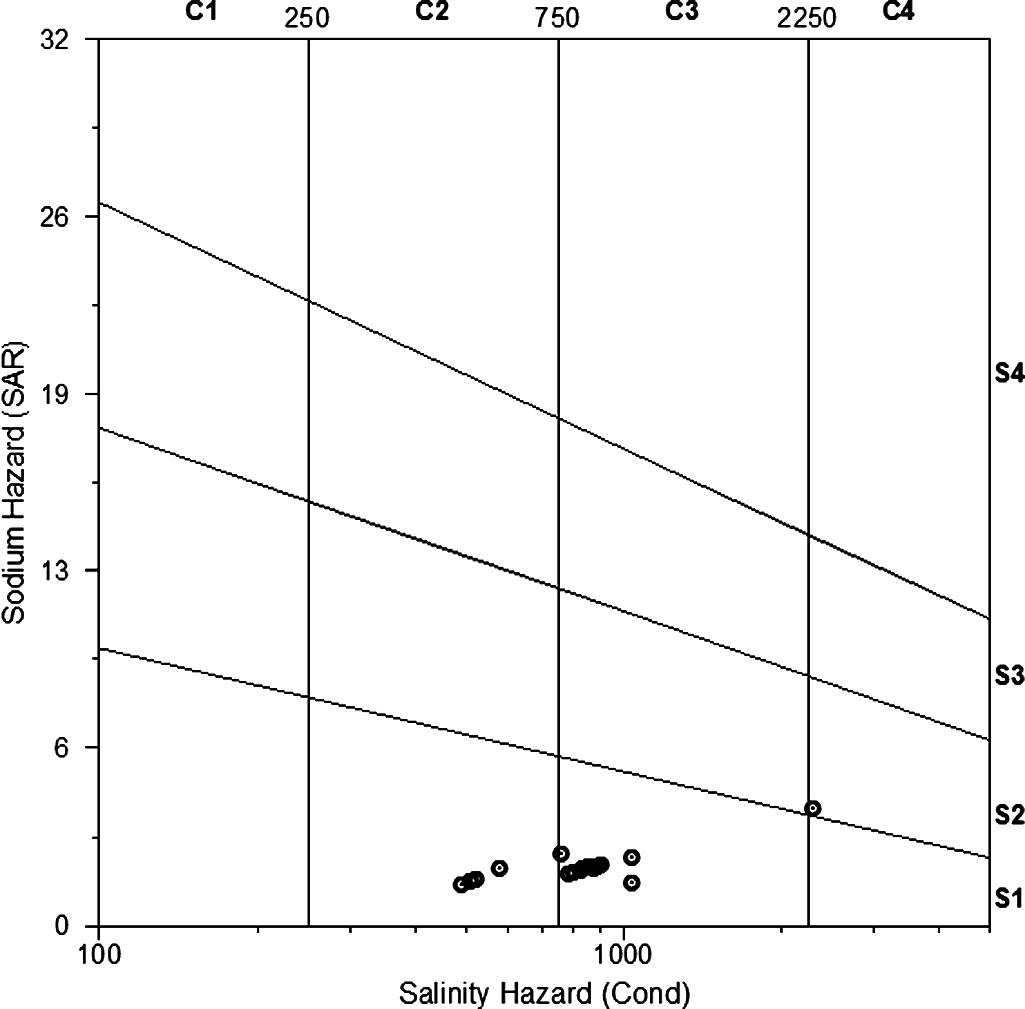
Wilcox diagram showing salinity and sodium hazards of groundwaters of Wadi Marwani.
7 Conclusions and recommendations
Hydrochemical studies of shallow groundwater from Wadi Marwani, central western Saudi Arabia indicated the presence of anthropogenic sources of contamination. The TDS concentration appears to be a useful indicator of this contamination and generally increases in agricultural and residential areas. The groundwater chemistry in Piper’s diagram indicated that Ca + Mg are the dominant cations in the hydrochemical facies, however, it changes widely from a HCO3–Cl–(NO3)–SO4 type (in the upstream part) to a Cl–SO4–HCO3 type (downgradient), suggesting that increase in Cl and NO3 concentrations is typical of anthropogenically contaminated groundwaters. However, the concentrations of all the major ions examined increase with the degree of anthropogenic contamination. The hydrochemistry of groundwaters encountered in the upstream part can be used as the background water quality criteria for the evaluation of groundwater quality in Wadi Marwani. Careful examination of the distribution of major ions showed that the contamination of groundwaters in this area is affected by direct input of various contaminants in relation to land-use type. Slow dissolution of the aquifer matrix had little contribution in addition of solutes to groundwater. Results of this study showed that appropriate control of the specific pollutant source(s) in relation to land-use characteristics, necessarily based on long-term monitoring of spatial variation of major ions, will be most effective for protection of Wadi Marwani groundwaters from anthropogenic contamination. The water quality information presented in this paper will be useful for sustainable management of this important groundwater resource. The following recommendations should be taken into consideration for proper management and sustainable development of the groundwater resource in the study area:
Subsurface drip irrigation method should be applied and generalized in this area to achieve water conservation approach and to prevent aquifer depletion caused by overdraft of groundwater. This irrigation technique will help in decreasing the chance of precipitation of Ca and Mg carbonate salts in the soil zone.
Estimation of water requirements for each crop should be performed to help in water conservation practices.
Complete quality assessment of irrigation water should be carried out especially for boron content.
Complete and detailed quality assessment program including detection of the possible contaminants, which are possibly leached from the soil of the agricultural areas. Nitrate, phosphate and pesticides are of concern in such program. In addition, microbiological examination of groundwater samples should be carried out to monitor possible contamination from the wastewater disposal system.
Disposal of domestic wastewater from the residential areas in unlined septic tanks should be prohibited and replaced by properly designed and lined septic tanks.
References
- Abdulrazzak, M.J., Sorman, A.U., Aborizaiza, O.S., 1992. Estimation of Natural Groundwater Recharge. Final Report of Project No. ARP-6-170, v. 1, Submitted to KACST, King Abdulaziz City, Saudi Arabia.
- Al-Nujaidi, H.A., 1978. Hydrogeology of Wadi Marwani–Khulais. M. Sc. Thesis, Institute of Applied Geology, King Abdulaziz University, Jeddah, Kingdom of Saudi Arabia, 259p.
- Hydrogeology and hydrochemistry of a shallow alluvial aquifer, western Saudi Arabia. Hydrogeol. J.. 2008;16:155-165.
- [Google Scholar]
- Geochemistry, Groundwater and Pollution (second ed.). Rotterdam: Balkema; 2005. 649p
- Seasonal correlation of well contamination and septic tank distance. Ground Water. 1999;37:920-923.
- [Google Scholar]
- Chloride mass-balance method for estimating ground water recharge in arid areas: examples from western Saudi Arabia. J. Hydrol.. 1996;186:153-159.
- [Google Scholar]
- Bazuhair, A.S., Hussein, T.M., Ibrahim, K.E., Al-Yamani, M.S., 1992. Hydrogeophysical Studies of Khulais Basin, Western Region, Saudi Arabia. Unpublished Report No. 85/411 King Abdulaziz University, Jeddah, Saudi Arabia, 207p.
- Hydrogeochemistry of the upper Ganges River, India. J. Geol. Soc. India. 1996;48:171-182.
- [Google Scholar]
- Physical and Chemical Hydrogeology (second ed.). New York: Wiley; 1998. 506p
- Groundwater. Englewood Cliffs, NJ: Prentice-Hall Inc.; 1979. 553p
- Gillion, R.J., Alley, W.M., Gurtz, M.E., 1995. Design of the National Water-Quality Assessment Program: Occurrence and Distribution of Water-Quality Conditions. US Geol. Surv. Circ. 1112, 33p.
- Effects of urbanization on groundwater resources of Merida, Yucatan, Mexico. Environ. Geol.. 1999;37:303-312.
- [Google Scholar]
- Soil and groundwater contamination and remediation technology in Europe. In: Sato K., ed. Groundwater Updates. Hong Kong: Springer, Best-set Typesetter Ltd.; 2000. p. :3-8.
- [Google Scholar]
- Hem, J.D., 1989. Study and Interpretation of the Chemical Characteristics of Natural Water, third ed. US Geological Survey Water-Supply Paper 2254, Washington, United States Government Printing Office, 263p.
- Italoconsult, 1967. Final Report on Water Supply Survey on J.M.T. Area. Ministry of Agriculture and Water, Riyadh, Saudi Arabia.
- Italoconsult, 1976. Detailed Investigations of the Wadi Khulais Basin. Unpublished Report, Ministry of Agriculture and Water, Riyadh, Saudi Arabia.
- Groundwater Assessment, Development and Management. New Delhi: McGraw-Hill Publishers; 1997.
- Lewis, W.J., Foster, S.S.D., Drasar, B., 1982. The Risk of Groundwater Pollution by On-Site Sanitation in Developing Countries. WHO-1RCWD Rep 01-82, Dubendorf, Switzerland.
- Nitrate behavior in groundwater of southeastern USA. J. Environ. Qual.. 1999;28:1518-1527.
- [Google Scholar]
- Parkhurst, D.L., 1995. User’s Guide to PHREEQC – A Computer Program for Speciation, Reaction-Path, Advective-Transport, and Inverse Geochemical Calculations. US Geological Survey Water-Resources Investigations Report 95-4227, 143p.
- Parkhurst, D.L., Appelo, A.A.J., 1999. User’s Guide to PHREEQC (Version 2) – A Computer Program for Speciation, Batch-Reaction, One-Dimensional Transport, and Inverse Geochemical Modeling. US Geological Survey, Water-Resources Investigations 99–4259, 312p.
- American Public Health Association (APHA), 1998. Standard Methods for the Examination of Water and Wastewater, 20th ed. APHA-AWWA-WET, Washington, DC.
- Ramsay, C.R., 1986. Geologic Map of the Rabigh Quadrangle, Sheet 22 D, Kingdom of Saudi Arabia, Scale 1: 250 000.
- Estimating rainfall recharge and soil water residence times in Pukekohe, New Zealand, by combining geophysical, chemical, and isotopic methods. Ground Water. 1999;37:836-844.
- [Google Scholar]
- Dissolution of fluoride in groundwater: a water-reaction study. Environ. Geol.. 2001;40:1084-1087.
- [Google Scholar]
- Inferring the chemical parameters for the dissolution of fluoride in groundwater. Environ. Geol.. 2003;43:731-736.
- [Google Scholar]
- Skiba, W.J., 1980. The form and evolution of late Precambrian plutonic masses in the Jiddah-Rabigh-Wadi al Quaha area, Saudi Arabia. In: Evolution and Mineralization of the Arabian–Nubian Shield (Al-Shanti, A.M.S. convenor) Institute of Applied Geology, King Abdulaziz University, Jeddah, Bulletin 3(3), pp. 106–120.
- Skiba, W.J., Gilboy, C.F., 1975. Geology of the Rabigh-Khulays Quadrangle, 2/39, Kingdom of Saudi Arabia (Unpublished Manuscript in DGMR Technical Library), 2 volumes, 597p.
- Buffer area versus whole catchment approaches to studying land-use impact on river water quality. Water Res.. 2001;35:3462-3472.
- [Google Scholar]
- Smith-Carrington, A.K., Bridge, L.R., Robertson, A.S., Foster, S.D., 1983. The Nitrate Pollution Problem in Groundwater Supplies from Jurassic Limestones in Central Lincolnshire. Institute Geological Sciences Report 83-3. Natural Environmental Research Council, London, UK.
- Occurrence of nitrate in groundwater – a review. J. Environ. Qual.. 1993;22:392-402.
- [Google Scholar]
- Variation of fluoride in groundwaters of crystalline terrain. J. Environ. Hydrol.. 1998;6:1-5.
- [Google Scholar]
- Hydrochemical assessment of groundwater in Moro area, Kwara State, Nigeria. Environ. Geol.. 1994;24:194-202.
- [Google Scholar]
- WHO, 1993. Guidelines for Drinking-Water Quality, vol. 1. Recommendations. WHO, Geneva.
- Wilcox, L.V., 1955. Classification and Use of Irrigation Waters. US Dept. Agric. Circ., p. 969.
- Chemical and isotopic method for quantifying ground-water recharge in a regional, semiarid environment. Ground Water. 1995;33(3):458-486.
- [Google Scholar]







