Translate this page into:
Human gingiva marrow-derived stromal cells exert immunoprotection on rat liver transplantation model through regulating FAS/FASL pathway
⁎Corresponding author: Department of Pediatric Liver Transplantation, First Central Hospital and Key Laboratory of Organ Transplant of Tianjin, No. 24 Fukang Road, Nankai District, Tianjin 300192, China. caijinzhen@sina.com (Jin-zhen Cai)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Background
Marrow mesenchymal stem cells (MSCs), especially bone MSCs (BMSCs), play a vital role in immunomodulation of graft rejection but many shortcomings limit its application. In this study, we aimed to investigate the modulation effect of MSCs from gingiva (GMSCs) on the rejection of rat liver transplantation model and to explore the potential mechanisms.
Methods
GMSCs were obtained from human gingival tissues and BMSCs were harvested from Brown Norway (BN) rats. The siRNA that mediated knockdown of Fas ligand (FASL) expression in GMSCs (FASL-/-GMSCs) was transfected using lentivirus plasmid. Rat orthotopic liver transplantation model was established. The rats were divided randomly into four groups: normal saline (NS) group, FASL-/-GMSCs group, BMSCs group, and GMSCs group, all of which were injected the solution via dorsal vein of penis to construct the rat rejection model Graft survival and liver function indexes were measured. The immunological reactions of recipients including immune-cytokines, T-helper type 17 (Th17) and regulatory T cells (Treg) were also evaluated by flow cytometry.
Results
The graft survival of recipient rats in the GMSCs group was significantly prolonged in comparison with that of the NS, FASL-/-GMSCs and BMSCs group. GMSCs remarkably decreased the levels of AST, ALT and TBIL. As for immune-cytokines, serum levels of IL-2, IFN-γ and Th 17 in recipient rats from the GMSCs group reduced significantly compared with that of other three groups, while increased serum IL-10 and TGF-β expressions as well as peripheral serum Treg were also observed in GMSCs group.
Conclusion
GMSCs exert immunoprotection on liver transplants by regulating FAS-FASL pathway. The current study firstly provides basis for GMSCs to be used in clinical anti-rejection after liver transplantation.
Keywords
Gingiva mesenchymal stem cells
Liver transplantation
FAS/FASL
- MSCs
mesenchymal 1849cells
- BMSCs
bone mesenchymal stem cells
- GMSCs
gingiva mesenchymal stem cells
- BN
Brown Norway rats
- FASL
Fas ligand
- NS
normal saline
- Th17
T-helper type 17 cells
- Treg
regulatory T cells
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- TBIL
total bilirubin
- IL-10
interleukin
- TGF-β
transforming growth factor
- IFN-γ
interferon
- CD40
Cluster of differentiation 40
- PGE2
prostaglandin E2
- IDO
indoleamine 2, 3-dioxygenase
- NO
nitric oxide
- iNOS
inducible nitric oxide synthase
- MCP-1
chemoattractant protein 1
- PE
phycoerythrin
- FITC
fluorescein isothiocyanate
Abbreviations
1 Introduction
Orthotopic liver transplantation is the curative therapy for patients with end-stage liver disease. However, postoperative transplant rejection is still a major challenge that decrease patient’s survival. Therefore, it is urgently necessary to set up a safe and effective immunotolerance system for liver grafts to attenuate immunological rejection and avoid the existing side effects (Lodhi et al., 2011; Sasajima et al., 2018).
Mesenchymal stem cells (MSCs), widely applied in treating diabetes, autoimmune diseases and liver diseases, can differentiate into multiple mesenchymal cell lineages, with the self-renewal potential (Bianco et al., 2008; Sasajima et al., 2018). As we know, human gingiva maintains the oral health and develops a unique fetal-like scarless healing process after wounding. Recently, gingiva-derived mesenchymal stem cells (GMSCs) have been reported, and the multi-lineage differentiation capacity and immunomodulatory properties have also been confirmed (Xu et al., 2013). The immunoprotection was verified to be mediated by FAS/FASL coupling (Yang et al., 2018) and in GMSCs based therapy for collagen-induced arthritis, T-cell apoptosis induced by FASL is necessary (Gu and Shi, 2016). Therefore, FAS/FASL signaling pathway plays important role in GMSCs-based cell therapy. In addition to immunoprotection of GMSCs, it is extremely easy to collect gingival tissue by biopsy and GMSCs is feasible to isolate from gingival tissue based on their highly proliferative nature (Xu et al., 2013). GMSCs have displayed some potential advantages in controlling and treating disease such as autoimmune diseases, atherosclerosis and diabetes (Huang et al., 2018; Zhang et al., 2018). However, studies on the role of GMSCs in liver transplantation still remain scanty even by now.
Herein, we isolated GMSCs from human gingival tissue and cultured in vitro, then evaluated its effect on the rat liver transplantation model. In order to identify the advantage of GMSCs, bone marrow mesenchymal stem cells (BMSCs) was isolated from rat and used as the control. In addition, the immunotolerance mechanism of GMSCs during liver transplantation rejection was also investigated. To identify the role of FAS/FASL pathway in immunomodulation of GMSCs, the Fas ligand (FASL) gene was knocked down by siRNA in GMSCs (FASL-/-GMSCs) and then used in liver allograft model. The results not only provided novel enlightenments, but indicated the possibility of GMSCs in clinical prevention and treatment of liver allograft rejection, for the first time.
2 Materials and methods
2.1 Materials
Primary Antibodies in this study were purchased from Abcam (Cambridge, UK). The HRP-conjugated secondary antibody was acquired from Bioss Biotechnology (Beijing, China). The FASL siRNA (sc-29313) and ELISA kits for interleukin (IL)-10, IL-2, transforming growth factor (TGF)-β, and interferon (IFN)-γ were obtained from Santa Cruz Biotechnology (CA, USA). The DMEM medium and DMEM/F12 medium were both bought from Hyclone (UT, USA). The alanine aminotransferase (ALT), aspartate aminotransferase (AST) and total bilirubin (TBIL) assay kits were obtained from Sigma-Aldrich Co., Ltd. (MO, USA). The cell culture reagents were obtained from Gibco BRL Life Technologies (MD, USA). Other chemicals used in this research were of analytical grade.
2.2 Animals
Healthy adult male Lewis and Brown Norway (BN) rats (8–10 weeks, 210–250 g) were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). Animals were housed at a constant temperature and humidity with free access to food and water. Animals were permitted to acclimate to the environment for 7 days before the experiments began.
2.3 Isolation and expansion of GMSCs and BMSCs
Ten healthy individuals who needed crown lengthening surgery or extraction of the third mandibular molar incisors, aged between 20 and 50 without any evidence of dental caries, were recruited in this study. The criteria included: (1) no gingival bleeding and swelling, no loss of attachment, and the depth of probing was less than 3 mm; (2) no smoking history; (3) no systemic diseases. The gingival tissues collected in oral surgery unit of Tianjin Medical University Stomatological Hospital.
GMSCs were isolated as following procedures. After washing with PBS, gingival tissues were digested at 37 °C for 40 min in sterile PBS containing 4 mg/mL dispase II and 3 mg/mL collagenase I (Sigma, USA). The cells were separated by centrifugation for 6 min at 1000 rpm, and then cultured with DMEM containing 15% FBS, 100 U/mL penicillin, 100 μg/mL streptomycin and cultured at 37 °C in a culture incubator with 5% CO2. The cells were sub-cultured every 5 days.
BMSCs were isolated from femurs and bilateral fibula of 4 to 5 week old BN rats as previously described (Chen et al., 2018). The BMSCs were cultured in DMEM/F12 medium.
2.4 FASL knockdown in GMSCs
2 × 105 GMSCs were seeded in a 12-well culture plate with 1 mL complete medium per well. 3 µL FASL siRNA were blended with 3 µL lipofectamine 2000 and then, this mixture were dissolved in 200 µL FBS free medium, standing for 20 min. To knockdown FASL, 10 µL siRNA solutions were added into every well to incubating GMSCs. Seven days after puromycin screening, the levels of FASL mRNA and protein were detected according to the methods as previously described (Saad, 2011) and the Specific primers used for genes were as follows: FASL forward, 5′-GAACTGGCAGAACTCCGTGAGT-3′; and reverse, 5′-CAGAGATCAGAGCGGTTCCATA-3′; β-actin forward, 5′-CTGGGACGACATGGAGAAAA-3′; and reverse, 5′-AAGGAAGGCTGGAAGAGTGC-3′. The empty vector of siRNA had no influence on the expression of FASL.
2.5 Establishment of rat liver transplantation model
In this experiment, the liver organs for transplantation in male BN rats were obtained from male Lewis rats. Based on the “Two-Cuff Technique” proposed by Kamada and Calne (Kamada et al., 1979), we established an rat orthotopic liver transplantation model without hepatic rearterialization. After the donor liver was successfully implanted, BN rats were randomly divided into four groups: normal saline (NS) group, FASL-/-GMSCs group, BMSCs group, and GMSCs group. In NS group, each recipient was injected with 1 mL physiological saline through dorsal vein of penis. As FASL-/-GMSCs group, the recipients were injected with 1 mL physiological saline containing 5 × 106 FASL-/-GMSCs via vena dorsalis penis. Similarly, rats in BMSCs and GMSCs groups were respectively injected with an equal volume and cell number of BMSCs and GMSCs.
2.6 Tissue and specimen collection
To collect tissues, five recipients were randomly selected from every group. Other transplanted BN rats were maintained to acquire the information of their survival time. Serum was collected through the inferior vena cava, stored at −20 °C for usage, and the liver graft tissue was harvested on the postoperative days 3, 5, 7 and 14.
2.7 Survival of rats
The survival time of the rats was recorded for analysis and Kaplan-Meier survival curve was drawn. Log-rank test was used for statistical analysis.
2.8 Hematoxylin and eosin (H & E) staining and liver function test
The liver graft tissue was fixed in formalin for 24 h and H & E staining was performed to confirm histopathological features. The levels of peripheral serum ALT, AST and TBIL which are indicative of liver function in each group were detected by an automatic biochemical analyzer.
2.9 Enzyme-linked immunosorbent assay (ELISA)
The immunological response in rat liver transplantation model after GMSCs or BMSCs treatment was monitored by measuring the levels of IL-10, IL-2, TGF-β, and IFN-γ in peripheral serum, according to the ELISA kit manufacturer’s protocol.
2.10 Flow cytometry assay
Regulatory T cells (Treg) and T‐helper type 17 (Th17) cells were detected by flow cytometry. For analysis of Treg cells, the whole peripheral blood samples were first stained with PerCP-CD3, FITC-CD4 and APC-Cy7-CD25 for cell surface markers. Then cells were incubated with FoxP3 for intracellular staining. For Th17 detection, cells were stimulated with PMA and ionomycin, and then incubated with anti-CD4 and stained with PE-anti-IL17. The staining process of BMSCs and GMSCs were similar as above cells with antibodies of PE-CD29, -CD45, -RTIA; FITC-CD34, -CD90, -RT1B for BMSCs and FITC-OCT4, -SSEA4, −146 for GMSCs. All labeled cells were analyzed using BD FACSAria III (BD Biosciences, US).
2.11 Statistical analysis
GraphPad Prism 5.0 software and SPSS17.0 statistical software were applied for data analysis. All data was represented by mean ± standard deviation (SD). The student's t test was used for data comparison between two groups, and one-way ANOVA was used for data comparison between three or more groups. Survival analysis was drawn by Kaplan-Meier survival curve, and the survival rate was tested by Log-rank method. Differences were considered statistically significant when p ≤ 0.05.
2.12 Ethics
All animal procedures conformed to the Council of Europe Convention Directive (2010/63/EU) for the protection of vertebrate animals used for scientific purposes. All individuals have been signed the informed consent form. All experiments were approved by the ethical committee of Tianjin First Central Hospital.
3 Results
3.1 Identification of BMSCs and GMSCs
As shown in Fig. 1, with passage and exchange, the BMSCs were purified, and the morphology became uniform. Most of them were slender spindle cells, and there were also flat-shaped cells with fissures (Fig. 1A). The cell surface MSC markers, CD29 and CD90 and the histocompatibility class I marker RT1A, were positively expressed, while hemacyte antigen, CD34 and CD45 and the histocompatibility class II marker RT1B, were negatively expressed (Fig. 1B). The primary GMSCs were arranged in a fusiform shape after being attached for 6 h (Fig. 1C). The differentiation capacity markers, OCT-4 and SSEA-4 and MSC markers, CD-146 and CD90, were detected with positive rate of 88.7%, 17.3%, 98.6%, and 99.9% (Fig. 1D). This result suggested that the cells obtained by the isolation and culture in this experiment were BMSCs and GMSC with higher purity.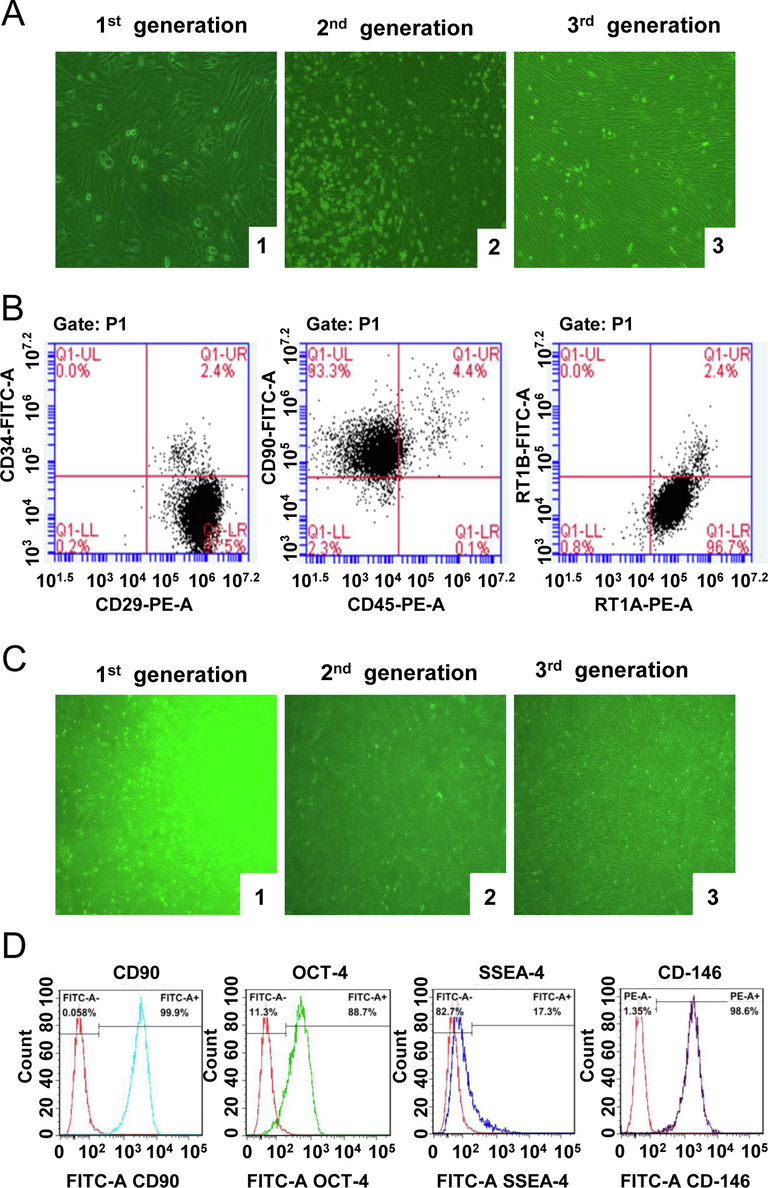
Morphological and molecular characterization of BMSCs and GMSCs. The morphological observation of BMSCs (A) and GMSCs (C) (magnification, 100×) and surface marker molecular detection of BMSCs (B) and GMSCs (D) by flow cytometry. 1, 2, 3 respectively represented the primary generation, the Second-generation and the third-generation of BMSCs.
3.2 GMSCs attenuate allograft rejection and prolong recipient survival
In order to explore the effect and mechanism of GMSCs on liver transplantation rejection, we successufully constructed FASL-/-GMSCs. As shown in Fig. 2 A and B, the expression of FASL was sharply decreased in FASL-/-GMSCs, which indcated the successful establishment of FASL-/-GMSCs. In rat liver transplantation model, most rats in NS and FASL-/-GMSCs groups, died within 21 days. When administrated with BMSCs and GMSC, the rats generally had better conditions with surviving for more than one month. The median survival time of rats in NS, FASL-/-GMSCs, BMSCs, and GMSCs groups were 15, 16, 30 and 50 days, respectively (Fig. 2C). The results suggested that GMSCs infusion had a beneficial effect on liver allograft survival.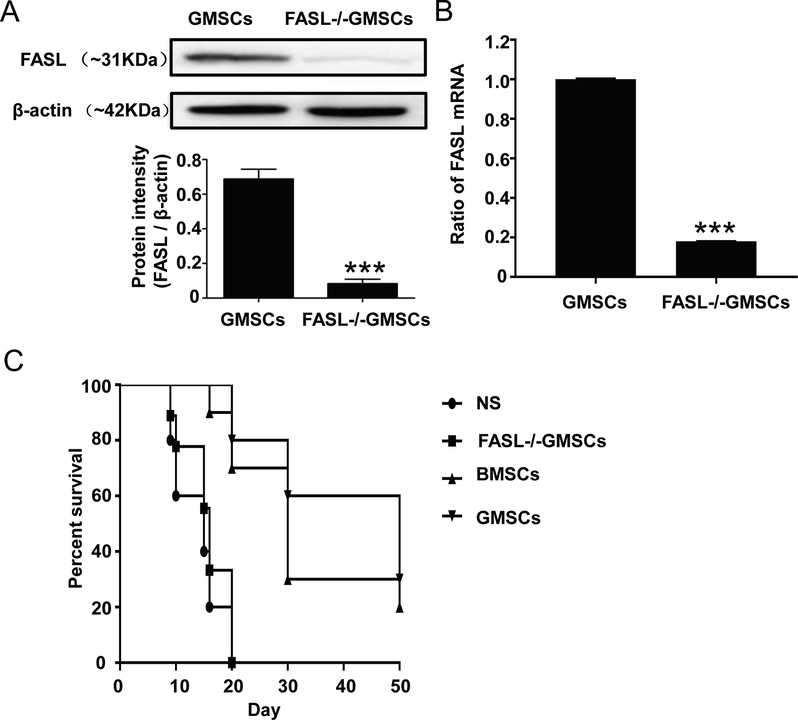
FASL protein (A) and mRNA (B) expression in GMSCs and the survival curve analysis of rats in each group (C). The Ratios of FasL mRNA were expressed as mean ± SD (n = 3). * p < 0.05, compared with control group.
AST, ALT and TBIL that are closely associated with injured liver cells, are important indicators for liver function evaluation. The ALT and AST levels of each group began to increase immediately after the operation. They greatly decreased on the 3rd day, then increased, and subsequently began to decrease after the 5th day (Fig. 3A). After surgery, the TBIL levels in each group increased gradiently from the 3th day to 14th day. There was no significant difference between the FASL-/-GMSCs group and NS group for the levels of ALT, AST, and TBIL at each time point. However, the BMSCs group gave a statistical difference when copmared with FASL-/-GMSCs group and NS group (p < 0.05). At the same time, the levels of AST, ALT and TBIL in GMSCs group were lower than those in BMSCs group (p < 0.05).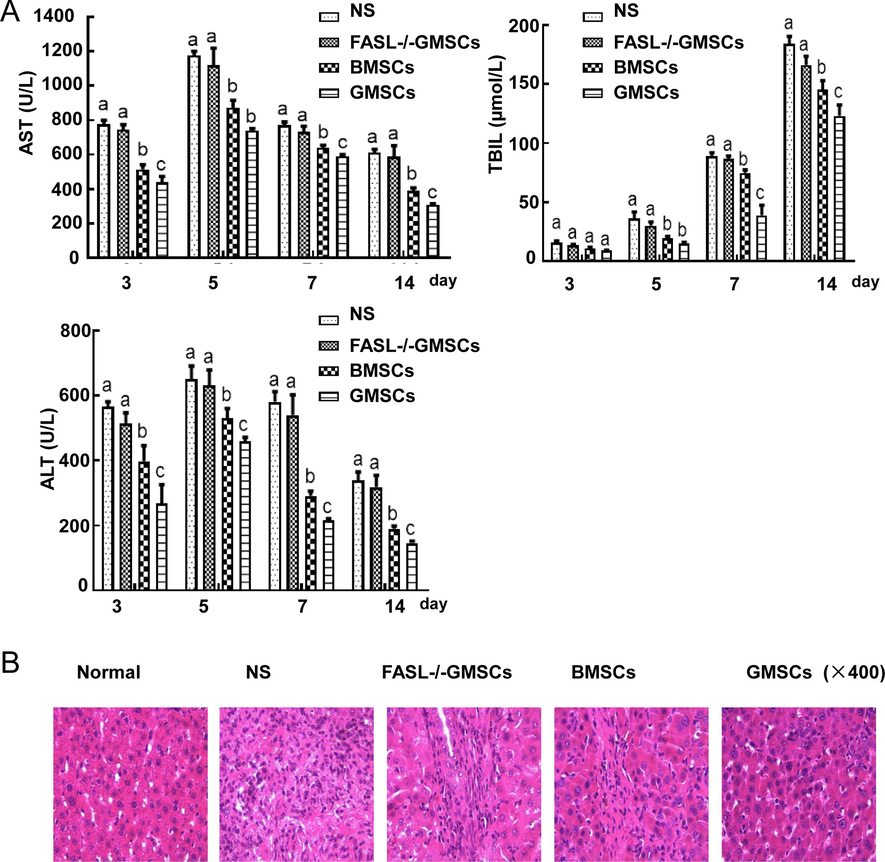
(A) The levels of ALT, AST and TBIL in each group from 3 to 14 days after surgery. The values of ALT, AST and TBIL were expressed as mean ± SD (n = 5). ap < 0.05 vs. Normal group; bp < 0.05 vs. FasL-/-GMSCs group; cp < 0.05 vs. BMSCs group. (B) Histologic examination (H & E staining) of liver grafts. In normal group, hepatic cells were arranged neatly and the structure of hepatocytes was intact; In model group, much inflammatory cell infiltration and complete disruption of structure of hepatic lobules; In FASL-/-GMSC group, the similar to model group, disturbance of structure of hepatic plate and inflammatory cell infiltration; In BMSCs and GMSCs groups, structural integrity of liver cells.
Histologic examination of liver allografts from recipients with nomal saline and FASL-/-GMSCs administration revealed severe acute rejection on 14 day. However, acute rejection of allografts was reduced in transplanted rats infused with BMSCs and GMSCs (Fig. 3B).
3.3 GMSCs regulated the peripheral serum cytokine and Th17/Treg levels
The levels of IL-10 and TGF-β in the peripheral blood of the GMSCs group were significantly increased, while IL-2 and IFN-γ levels were significantly reduced, compared with NS group (p < 0.05) (Fig. 4). The serum levels of immune regulatory Treg in the GMSCs group were also significantly improved, while inflammatory Th17 were decreased (p < 0.05) (Fig. 5A and B). The ratio of Th17/Treg reached to the highest level on the 5th day in each groups and this ratio was much higher in FASL-/-GMSCs group than that in other groups (Fig. 5C), suggesting that severe inflammation ocurred in FASL-/-GMSCs group.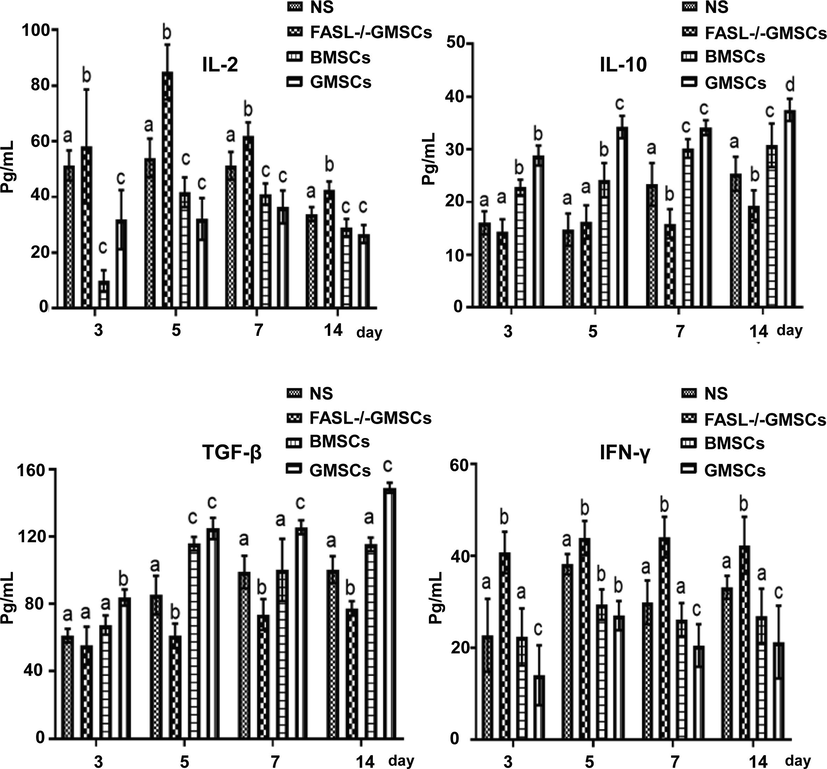
Peripheral serum cytokine levels of IL-10, IL-2, TGF-β and IFN-γ in each group, determined by specific ELISA kits. The values were expressed as mean ± SD (n = 5). ap < 0.05 vs. Normal group; bp < 0.05 vs. FasL-/-GMSCs group; cp < 0.05 vs. BMSCs group.
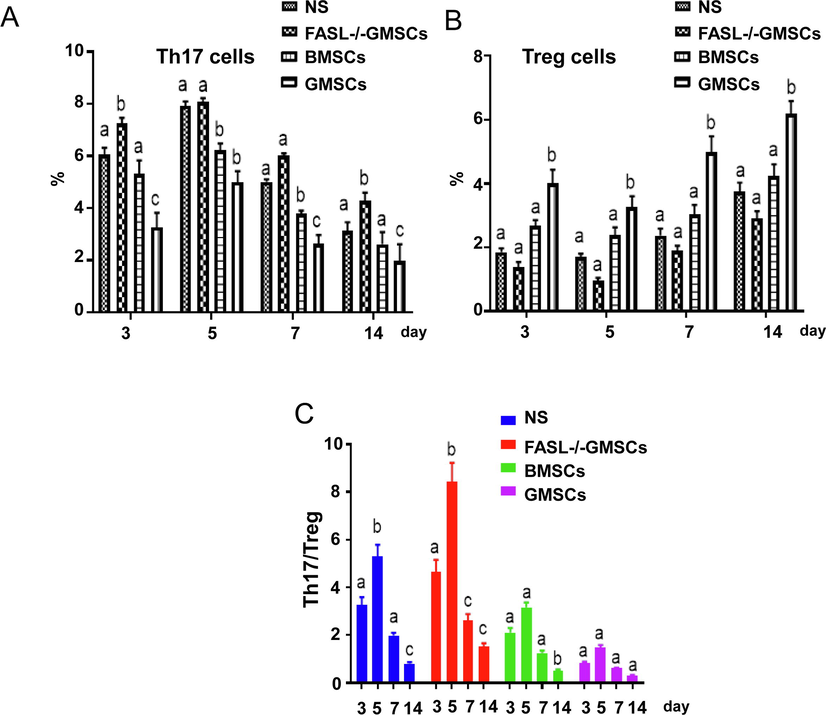
Treg and Th17 levels in peripheral serum from each group, detected by flow cytometry. (A) Th17 cells were decreased with days after surgery. (B) Treg cells in normal and FASL-/-GMSCs groups were significantly lower than that in BMSCs and GMSCs groups. (C) The balances of Th17/Treg were calculated according to the detection data. The values were expressed as mean ± SD (n = 5). ap < 0.05 vs. Normal group; bp < 0.05 vs. FasL-/-GMSCs group; cp < 0.05 vs. BMSCs group.
4 Discussion
Since the 1960s, organ transplantation has gradually become routine treatment for patients with end-stage organ failure. Although immuosuppressive drugs mainly control acute rejection, there is no effective therapies for chronic rejection which will lead to graft failure and severely affect the long-term survival of patients. Therefore, it is necessary to introduce an effective therapeutic approach to induce a long-lasting stable drug-free immune tolerance to prevent chronic rejection to organs (Lodhi et al., 2011). BMSCs can induce immune tolerance and have the characteristics of low immunogenicity, high survivability and specific persistence, which makes BMSCs became a promising therapeutic regimen in inhibiting rejection after transplantation (Chen et al., 2018; Pierog et al., 2018). However, the BMSCs need to be extracted from adults who will have to endure the painful cell-extracting process. Not many cells can be extracted, and they are usually associated with lower proliferation and insufficient immune tolerance (Li et al., 2018; Vanella et al., 2010; Yue et al., 2010). Therefore, the search for better sources of MSCs is one key to the development of MSCs-based cell therapy.
The gums are a unique soft tissue that serves as a biological barrier covering the upper jaw and lower jaw. Recently, some studies confirmed the existence of GMSCs as well as the potential of multi-directional differentiation and immunomodulatory properties (Liu et al., 2012; Su et al., 2011; Tang et al., 2011). Xu found that 90% of GMSCs were derived from cranial neural crest cells and those cells exerted good immunomodulation associated with high FASL expression, which indicated that GMSCs have more optential to induction of immune tolerance (Xu et al., 2013). Therefore, compared with BMSCs, the extraction of GMSCs is relatively easier and less painful to the donor, and is associated with remarkable benefits of stronger induction of immune tolerance.
FAS is one of the apoptosis-related factors whose extracellular domain can bind to the ligand FASL, and the cytoplasmic zone transmits death signals (Itoh et al., 1991). When FASL and FAS were combined, FAS formed into an active trimer capable of transmitting apoptotic signals to caspase 8. Activation of caspase 8 will cause a series of enzyme-linked reactions in the caspase family and finally activate caspase 3 (Wolf et al., 1999). Caspase 3 caused the DNA fragmentation, chromosome condensation, nucleosome formation, mitochondrial permeability changes, and cytoplasmic condensation, which then completed the FAS mediated apoptotic process.
It was confirmed that MSCs from various sources could induce T cell apoptosis and promote Treg production through FAS/FASL pathway, and ultimately lead to immune tolerance (Pittenger et al., 1999). This immune tolerance formation involves following progresses that firstly MSCs control the secretion of monocyte chemoattractant protein 1 (MCP-1) through FAS, while MCP-1 can adsorb activated T cells. Subesequently, MSCs induce the apoptosis of activated T cells through FASL and then, apoptotic T cells trigger macrophages to produce high levels of TGF-β. Consequently, high levels of TGF-β up-regulate Treg, inducing immune tolerance (Chen et al., 2001). In the present study, compared with other groups, the GMSCs group showed longer survival times and better liver functions. In addition, the current study proved that GMSCs were more effective than BMSCs in exerting protective effects on grafts of liver transplantation rejection models.
As reported, IL-2 is a Th1-associated cytokine, while IL-10 is a Th2-associated cytokine. The ratio of IL-2/IL-10 is closely related to the occurrence of rejection and induction of transplant tolerance (Park et al., 2019). Both of IFN-γ and TGF-β are Th17/Treg axis-associated cytokines, which determine whether the T cells will differentiate into inflammatory Th17 or immune regulatory Treg. The pro-inflammatory cytokine TNF-α is closely related to immune regulation, apoptosis and T cell proliferation, and is positively correlated with the severity of transplant rejection. Our results showed that all of IL-2 and IFN-γ was significantly reduced, and both of IL-10 and TGF-β were significantly increased after GMSCs infusion. The balance of Th17/Treg cells showed no changing in GMSCs group. This further suggested that GMSCs have a stronger immunoprotective effect on grafts of the liver transplantation rejection model compared with BMSCs, and that the FAS-FASL pathway mediated the involvement of GMSCs in inducing immune tolerance in rat liver transplantation.
5 Conclusion
GMSCs exerted an efficient immunosuppressive effect and better protective effects on grafts of liver transplantation model than BMSCs, via regulating FAS-FASL pathway. This study provides an experimental basis and theoretical basis for the use of GMSCs for clinical immune rejection after liver transplantation.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant no. 81670600), the Natural Science Foundation of Tianjin city (17JCYBJC27500) and the Elite Program of China Organ Transplant Development Foundation (2019JYJH03).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Mesenchymal stem cells: revisiting history, concepts and assays. Cell Stem Cell. 2008;2:313-319.
- [Google Scholar]
- TGF-beta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715-725.
- [Google Scholar]
- Bone marrow mesenchymal stromal cells attenuate liver allograft rejection may via upregulation PD-L1 expression through downregulation of miR-17-5p. Transpl. Immunol.. 2018;51:21-29.
- [Google Scholar]
- Transplantation of gingiva-derived mesenchymal stem cells ameliorates collagen-induced arthritis. Arthritis Res. Ther.. 2016;18:262.
- [Google Scholar]
- Updates on GMSCs treatment for autoimmune diseases. Curr. Stem Cell Res. Ther.. 2018;13:345-349.
- [Google Scholar]
- The polypeptide encoded by the cDNA for human cell surface antigen Fas can mediate apoptosis. Cell. 1991;66:233-243.
- [Google Scholar]
- Comparison of the biological characteristics of human mesenchymal stem cells derived from exfoliated deciduous teeth, bone marrow, gingival tissue, and umbilical cord. Mol. Med. Rep.. 2018;18:4969-4977.
- [Google Scholar]
- Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J. Clin. Periodontol.. 2012;39:1174-1182.
- [Google Scholar]
- Solid organ allograft survival improvement in the United States: the long-term does not mirror the dramatic short-term success. Am. J. Transplant.. 2011;11:1226-1235.
- [Google Scholar]
- Ischemic time of graft liver forces Th1-to-Th2 activity toward Th1 activity in patients who underwent living donor liver transplantation. Eur. Cytokine Netw.. 2019;30:23-28.
- [Google Scholar]
- Bone marrow stem cells modified with human interleukin 10 attenuate acute rejection in rat lung allotransplantation. Eur. J. Cardiothorac. Surg.. 2018;53:194-200.
- [Google Scholar]
- Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143-147.
- [Google Scholar]
- Apoptotic genes expression in mice hepatocytes during malaria infection. J. King Saud Univ.-Sci.. 2011;23:63-68.
- [Google Scholar]
- Cytoprotective effects of mesenchymal stem cells during liver transplanation from donors after cardiac death in rats. Transplant Proc.. 2018;50:2815-2820.
- [Google Scholar]
- Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin E2-dependent mechanisms. Stem Cells. 2011;29:1849-1860.
- [Google Scholar]
- Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J. Cell. Physiol.. 2011;226:832-842.
- [Google Scholar]
- HO-1 expression increases mesenchymal stem cell-derived osteoblasts but decreases adipocyte lineage. Bone. 2010;46:236-243.
- [Google Scholar]
- Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem.. 1999;274:30651-30656.
- [Google Scholar]
- Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J. Dent. Res.. 2013;92:825-832.
- [Google Scholar]
- Hydrogen sulfide promotes immunomodulation of gingiva derived mesenchymal stem cells via the Fas/FasL coupling pathway. Stem Cell Res. Ther.. 2018;9:62.
- [Google Scholar]
- Effect of fusion protein TAT and heme oxygenase-1 on liver sinusoidal endothelial cells apoptosis during preservation injury. Chin. Med. J. (Engl). 2010;123:68-73.
- [Google Scholar]
- Human gingiva-derived mesenchymal stem cells modulate monocytes/macrophages and alleviate atherosclerosis. Front. Immunol.. 2018;9:878.
- [Google Scholar]







